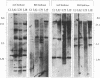
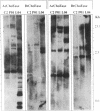
Abstract
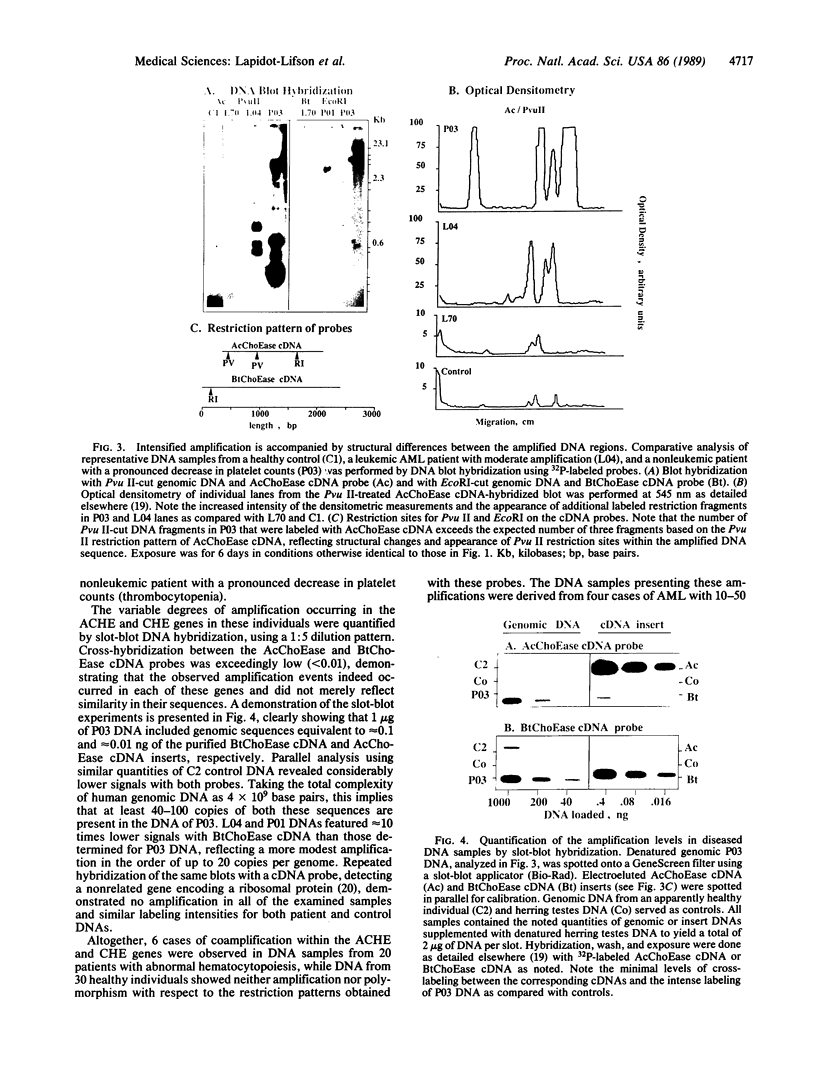
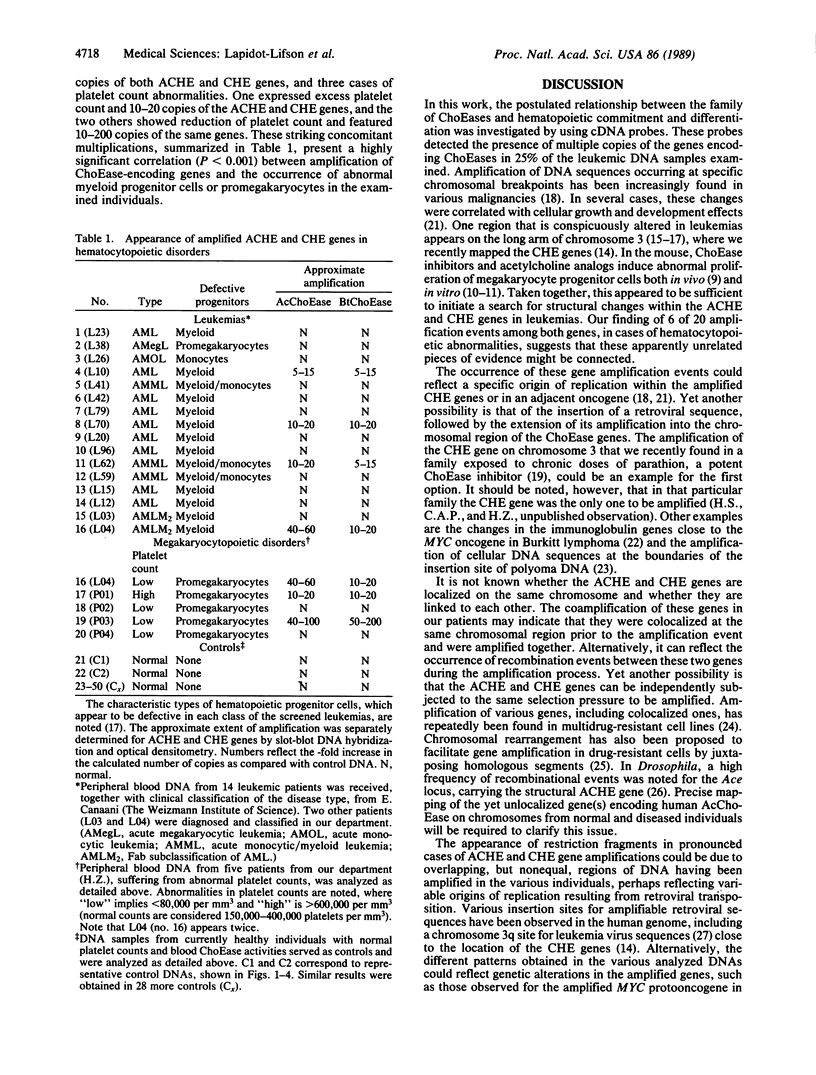
To study the yet unknown role of the ubiquitous family of cholinesterases (ChoEases) in developing blood cells, the recently isolated cDNAs encoding human acetylcholinesterase (AcChoEase; acetylcholine acetylhydrolase, EC 3.1.1.7) and butyrylcholinesterase (BtChoEase; cholinesterase; acylcholine acylhydrolase, EC 3.1.1.8) were used in blot hybridization with peripheral blood DNA from various leukemic patients. Hybridization signals (10- to 200-fold intensified) and modified restriction patterns were observed with both cDNA probes in 4 of the 16 leukemia DNA preparations examined. These reflected the amplification of the corresponding AcChoEase and BtChoEase genes (ACHE and CHE) and alteration in their structure. Parallel analysis of 30 control samples revealed nonpolymorphic, much weaker hybridization signals for each of the probes. In view of previous reports on the effect of acetylcholine analogs and ChoEase inhibitors in the induction of megakaryocytopoiesis and production of platelets in the mouse, we further searched for such phenomena in nonleukemic patients with platelet production disorders. Amplifications of both ACHE and CHE genes were found in 2 of the 4 patients so far examined. Pronounced coamplification of these two related but distinct genes in correlation with pathological production of blood cells suggests a functional role for members of the ChoEase family in megakaryocytopoiesis and raises the question whether the coamplification of these genes could be causally involved in the etiology of hemocytopoietic disorders.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baran N., Lapidot A., Manor H. Unusual sequence element found at the end of an amplicon. Mol Cell Biol. 1987 Jul;7(7):2636–2640. doi: 10.1128/mcb.7.7.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein R., Pinto M. R., Behr A., Mendelow B. Chromosome 3 abnormalities in acute nonlymphocytic leukemia (ANLL) with abnormal thrombopoiesis: report of three patients with a "new" inversion anomaly and a further case of homologous translocation. Blood. 1982 Sep;60(3):613–617. [PubMed] [Google Scholar]
- Biedler J. L., Chang T. D., Scotto K. W., Melera P. W., Spengler B. A. Chromosomal organization of amplified genes in multidrug-resistant Chinese hamster cells. Cancer Res. 1988 Jun 1;48(11):3179–3187. [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Burstein S. A., Adamson J. W., Harker L. A. Megakaryocytopoiesis in culture: modulation by cholinergic mechanisms. J Cell Physiol. 1980 May;103(2):201–208. doi: 10.1002/jcp.1041030205. [DOI] [PubMed] [Google Scholar]
- Burstein S. A., Boyd C. N., Dale G. L. Quantitation of megakaryocytopoiesis in liquid culture by enzymatic determination of acetylcholinesterase. J Cell Physiol. 1985 Jan;122(1):159–165. doi: 10.1002/jcp.1041220124. [DOI] [PubMed] [Google Scholar]
- Burstein S. A., Harker L. A. Control of platelet production. Clin Haematol. 1983 Feb;12(1):3–22. [PubMed] [Google Scholar]
- Drews U. Cholinesterase in embryonic development. Prog Histochem Cytochem. 1975;7(3):1–52. [PubMed] [Google Scholar]
- Escot C., Theillet C., Lidereau R., Spyratos F., Champeme M. H., Gest J., Callahan R. Genetic alteration of the c-myc protooncogene (MYC) in human primary breast carcinomas. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4834–4838. doi: 10.1073/pnas.83.13.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flintoff W. F., Livingston E., Duff C., Worton R. G. Moderate-level gene amplification in methotrexate-resistant Chinese hamster ovary cells is accompanied by chromosomal translocations at or near the site of the amplified DHFR gene. Mol Cell Biol. 1984 Jan;4(1):69–76. doi: 10.1128/mcb.4.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J. F., Wasmuth J. J. Report of the committee on the genetic constitution of chromosomes 3 and 4. Cytogenet Cell Genet. 1987;46(1-4):131–146. doi: 10.1159/000132474. [DOI] [PubMed] [Google Scholar]
- Layer P. G., Alber R., Sporns O. Quantitative development and molecular forms of acetyl- and butyrylcholinesterase during morphogenesis and synaptogenesis of chick brain and retina. J Neurochem. 1987 Jul;49(1):175–182. doi: 10.1111/j.1471-4159.1987.tb03411.x. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Tanaka N., Lamb P. W., Gilmer T. M., Barrett J. C. Induction of gene amplification by arsenic. Science. 1988 Jul 1;241(4861):79–81. doi: 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Nusbaum H. R., Razon N., Kris R., Lax I., Soreq H., Whittle N., Waterfield M. D., Ullrich A., Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985 Jan 10;313(5998):144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Razon N., Bartal A. D., Yarden Y., Schlessinger J., Soreq H. Expression of epidermal growth factor receptors in human brain tumors. Cancer Res. 1984 Feb;44(2):753–760. [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Nagoshi R. N., Gelbart W. M. Molecular and recombinational mapping of mutations in the Ace locus of Drosophila melanogaster. Genetics. 1987 Nov;117(3):487–502. doi: 10.1093/genetics/117.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Linney E., Ruoslahti E. Molecular cloning and nucleotide sequence of a cDNA clone coding for the cell attachment domain in human fibronectin. J Biol Chem. 1983 Sep 10;258(17):10193–10196. [PubMed] [Google Scholar]
- Paulus J. M., Maigne J., Keyhani E. Mouse megakaryocytes secrete acetylcholinesterase. Blood. 1981 Dec;58(6):1100–1106. [PubMed] [Google Scholar]
- Pintado T., Ferro M. T., San Román C., Mayayo M., Laraña J. G. Clinical correlations of the 3q21;q26 cytogenetic anomaly. A leukemic or myelodysplastic syndrome with preserved or increased platelet production and lack of response to cytotoxic drug therapy. Cancer. 1985 Feb 1;55(3):535–541. doi: 10.1002/1097-0142(19850201)55:3<535::aid-cncr2820550311>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Prody C. A., Dreyfus P., Zamir R., Zakut H., Soreq H. De novo amplification within a "silent" human cholinesterase gene in a family subjected to prolonged exposure to organophosphorous insecticides. Proc Natl Acad Sci U S A. 1989 Jan;86(2):690–694. doi: 10.1073/pnas.86.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prody C. A., Zevin-Sonkin D., Gnatt A., Goldberg O., Soreq H. Isolation and characterization of full-length cDNA clones coding for cholinesterase from fetal human tissues. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3555–3559. doi: 10.1073/pnas.84.11.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakonczay Z., Brimijoin S. Biochemistry and pathophysiology of the molecular forms of cholinesterases. Subcell Biochem. 1988;12:335–378. doi: 10.1007/978-1-4899-1681-5_10. [DOI] [PubMed] [Google Scholar]
- Razon N., Soreq H., Roth E., Bartal A., Silman I. Characterization of activities and forms of cholinesterases in human primary brain tumors. Exp Neurol. 1984 Jun;84(3):681–695. doi: 10.1016/0014-4886(84)90215-2. [DOI] [PubMed] [Google Scholar]
- Roberts W. L., Kim B. H., Rosenberry T. L. Differences in the glycolipid membrane anchors of bovine and human erythrocyte acetylcholinesterases. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7817–7821. doi: 10.1073/pnas.84.22.7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Varmus H. E., Bishop J. M. Human N-myc gene contributes to neoplastic transformation of mammalian cells in culture. Nature. 1985 Jul 11;316(6024):160–162. doi: 10.1038/316160a0. [DOI] [PubMed] [Google Scholar]
- Scotto K. W., Biedler J. L., Melera P. W. Amplification and expression of genes associated with multidrug resistance in mammalian cells. Science. 1986 May 9;232(4751):751–755. doi: 10.1126/science.2421411. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Soreq H., Gnatt A. Molecular biological search for human genes encoding cholinesterases. Mol Neurobiol. 1987 Spring-Summer;1(1-2):47–80. doi: 10.1007/BF02935264. [DOI] [PubMed] [Google Scholar]
- Soreq H., Zamir R., Zevin-Sonkin D., Zakut H. Human cholinesterase genes localized by hybridization to chromosomes 3 and 16. Hum Genet. 1987 Dec;77(4):325–328. doi: 10.1007/BF00291419. [DOI] [PubMed] [Google Scholar]
- Stark G. R. DNA amplification in drug resistant cells and in tumours. Cancer Surv. 1986;5(1):1–23. [PubMed] [Google Scholar]
- Taub R., Kelly K., Battey J., Latt S., Lenoir G. M., Tantravahi U., Tu Z., Leder P. A novel alteration in the structure of an activated c-myc gene in a variant t(2;8) Burkitt lymphoma. Cell. 1984 Jun;37(2):511–520. doi: 10.1016/0092-8674(84)90381-7. [DOI] [PubMed] [Google Scholar]
- Turchini M. F., Travade P., De Larocque A., Geneix A., Perissel B., Malet P. Translocation t(3;20) associated with thrombocythemia in Ph-positive CML. Cancer Genet Cytogenet. 1986 Feb 1;20(1-2):1–4. doi: 10.1016/0165-4608(86)90101-9. [DOI] [PubMed] [Google Scholar]
- Wong A. J., Bigner S. H., Bigner D. D., Kinzler K. W., Hamilton S. R., Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut H., Even L., Birkenfeld S., Malinger G., Zisling R., Soreq H. Modified properties of serum cholinesterases in primary carcinomas. Cancer. 1988 Feb 15;61(4):727–737. doi: 10.1002/1097-0142(19880215)61:4<727::aid-cncr2820610416>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Zakut H., Matzkel A., Schejter E., Avni A., Soreq H. Polymorphism of acetylcholinesterase in discrete regions of the developing human fetal brain. J Neurochem. 1985 Aug;45(2):382–389. doi: 10.1111/j.1471-4159.1985.tb03999.x. [DOI] [PubMed] [Google Scholar]