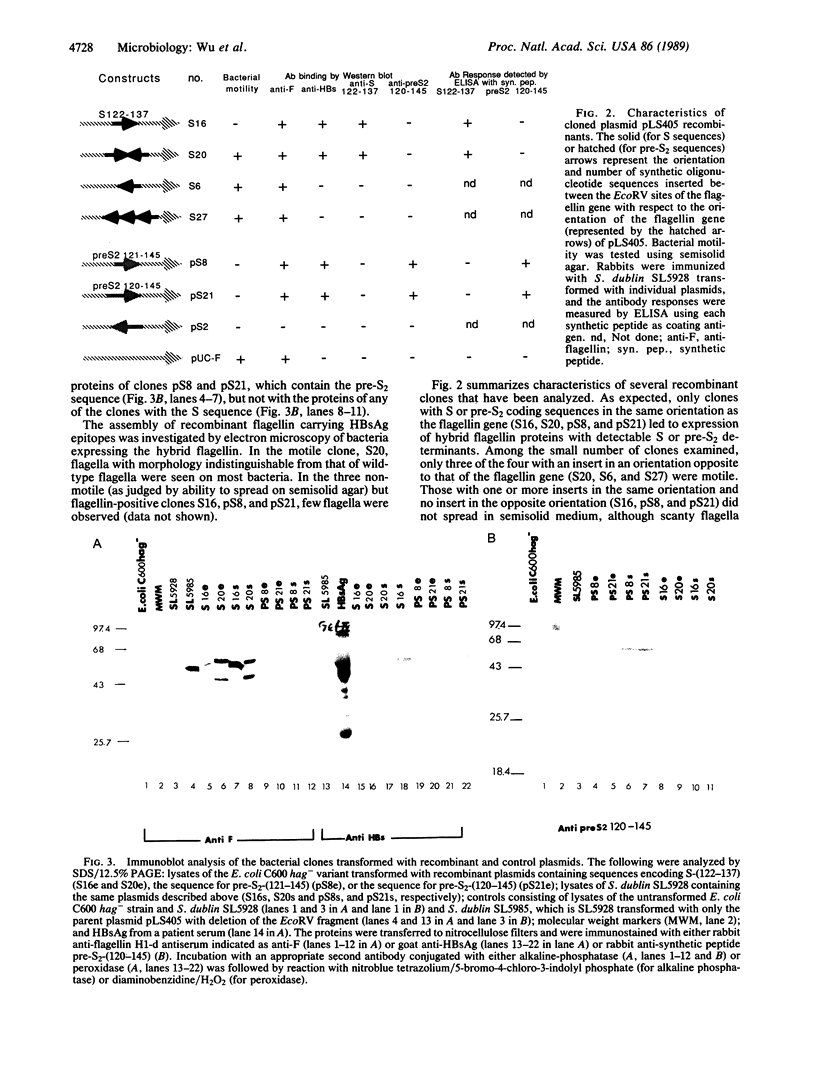
Abstract
A nonvirulent Salmonella dublin flagellin-negative, aromatic-dependent live vaccine strain has been used to express hepatitis B virus surface antigen epitopes in an immunogenic form. The envelope proteins of the virion are encoded by the S gene, which contains the pre-S1, pre-S2, and S coding regions. Synthetic oligonucleotides corresponding to amino acid residues S-(122-137) and pre-S2-(120-145) were inserted in-frame into the hypervariable region of a cloned Salmonella flagellin gene, and the recombinant plasmids were introduced into a flagellin-negative aroA mutant live vaccine strain of S. dublin, SL5928. The flagellin gene was expressed in bacteria carrying the plasmids as detected by immunoblotting with anti-flagellin (H1-d) serum. Both the S and pre-S2 epitopes were detected in bacteria carrying the relevant plasmid by immunoblotting with anti-HBs (antibody to hepatitis B virus surface antigen) and anti-peptide antisera. Animals immunized intramuscularly or orally with the live recombinant bacteria developed antibodies specific to these hepatitis B virus epitopes as detected by ELISA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dreesman G. R., Sanchez Y., Ionescu-Matiu I., Sparrow J. T., Six H. R., Peterson D. L., Hollinger F. B., Melnick J. L. Antibody to hepatitis B surface antigen after a single inoculation of uncoupled synthetic HBsAg peptides. Nature. 1982 Jan 14;295(5845):158–160. doi: 10.1038/295158a0. [DOI] [PubMed] [Google Scholar]
- Gerin J. L., Alexander H., Shih J. W., Purcell R. H., Dapolito G., Engle R., Green N., Sutcliffe J. G., Shinnick T. M., Lerner R. A. Chemically synthesized peptides of hepatitis B surface antigen duplicate the d/y specificities and induce subtype-specific antibodies in chimpanzees. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2365–2369. doi: 10.1073/pnas.80.8.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Takai E., Ohnuma H., Kitajima K., Tsuda F., Machida A., Mishiro S., Nakamura T., Miyakawa Y., Mayumi M. A synthetic peptide vaccine involving the product of the pre-S(2) region of hepatitis B virus DNA: protective efficacy in chimpanzees. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9174–9178. doi: 10.1073/pnas.83.23.9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. C., Dreesman G. R., Sparrow J. T., Culwell A. R., Sanchez Y., Ionescu-Matiu I., Hollinger F. B., Melnick J. L. Inhibition of a common human anti-hepatitis B surface antigen idiotype by a cyclic synthetic peptide. J Virol. 1983 May;46(2):653–655. doi: 10.1128/jvi.46.2.653-655.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugman S. The newly licensed hepatitis B vaccine. Characteristics and indications for use. JAMA. 1982 Apr 9;247(14):2012–2015. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly J. A., Kaplan J. M., Janeway C. A., Jr Two distinct antigen-specific suppressor factors induced by the oral administration of antigen. J Exp Med. 1980 Sep 1;152(3):545–554. doi: 10.1084/jem.152.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Kent S. B., Thorton G. B. Immune response to the pre-S(1) region of the hepatitis B surface antigen (HBsAg): a pre-S(1)-specific T cell response can bypass nonresponsiveness to the pre-S(2) and S regions of HBsAg. J Immunol. 1986 Jul 1;137(1):315–322. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Thornton G. B. Nonoverlapping T and B cell determinants on an hepatitis B surface antigen pre-S(2) region synthetic peptide. J Exp Med. 1986 Aug 1;164(2):532–547. doi: 10.1084/jem.164.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Thornton G. B., Neurath A. R., Kent S. B., Michel M. L., Tiollais P., Chisari F. V. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science. 1985 Jun 7;228(4704):1195–1199. doi: 10.1126/science.2408336. [DOI] [PubMed] [Google Scholar]
- Mukkur T. K., McDowell G. H., Stocker B. A., Lascelles A. K. Protection against experimental salmonellosis in mice and sheep by immunisation with aromatic-dependent Salmonella typhimurium. J Med Microbiol. 1987 Aug;24(1):11–19. doi: 10.1099/00222615-24-1-11. [DOI] [PubMed] [Google Scholar]
- Murray K., Bruce S. A., Hinnen A., Wingfield P., van Erd P. M., de Reus A., Schellekens H. Hepatitis B virus antigens made in microbial cells immunise against viral infection. EMBO J. 1984 Mar;3(3):645–650. doi: 10.1002/j.1460-2075.1984.tb01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Parker K., Prince A. M., Strick N., Brotman B., Sproul P. Antibodies to a synthetic peptide from the preS 120-145 region of the hepatitis B virus envelope are virus neutralizing. Vaccine. 1986 Mar;4(1):35–37. doi: 10.1016/s0264-410x(86)80001-9. [DOI] [PubMed] [Google Scholar]
- Richman L. K., Graeff A. S., Yarchoan R., Strober W. Simultaneous induction of antigen-specific IgA helper T cells and IgG suppressor T cells in the murine Peyer's patch after protein feeding. J Immunol. 1981 Jun;126(6):2079–2083. [PubMed] [Google Scholar]
- Robertsson J. A., Lindberg A. A., Hoiseth S., Stocker B. A. Salmonella typhimurium infection in calves: protection and survival of virulent challenge bacteria after immunization with live or inactivated vaccines. Infect Immun. 1983 Aug;41(2):742–750. doi: 10.1128/iai.41.2.742-750.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Stocker B. A., Hoiseth S. K., Johnson E. Aromatic-dependent Salmonella dublin as a parenteral modified live vaccine for calves. Am J Vet Res. 1984 Nov;45(11):2231–2235. [PubMed] [Google Scholar]
- Tabor E., Gerety R. J. Possible role of immune responses to hepatitis B core antigen in protection against hepatitis B infections. Lancet. 1984 Jan 21;1(8369):172–172. doi: 10.1016/s0140-6736(84)90114-4. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Wei L. N., Joys T. M. Covalent structure of three phase-1 flagellar filament proteins of Salmonella. J Mol Biol. 1985 Dec 20;186(4):791–803. doi: 10.1016/0022-2836(85)90397-3. [DOI] [PubMed] [Google Scholar]