Abstract
Herpes simplex virus infection of mammalian hosts involves lytic replication at a primary site, such as the cornea, translocation by axonal transport to sensory ganglia and replication, and latent infection at a secondary site, ganglionic neurons. The virus-encoded thymidine kinase, which is a target for antiviral drugs such as acyclovir, is not essential for lytic replication yet evidently is required at the secondary site for replication and some phase of latent infection. To determine the specific stage in viral pathogenesis at which this enzyme is required, we constructed virus deletion mutants that were acyclovir resistant and exhibited no detectable thymidine kinase activity. After corneal inoculation of mice, the mutants replicated to high titers in the eye but were severely impaired for acute replication in trigeminal ganglia and failed to reactivate from ganglia upon cocultivation with permissive cells. Nevertheless, latency-associated transcripts were expressed in neuronal nuclei of ganglia from mutant-infected mice and superinfection of the ganglia with a second virus rescued the latent mutant virus. Thus, contrary to a widely accepted hypothesis, the thymidine kinase-negative mutants established latent infections, implying that neither thymidine kinase activity nor ganglionic replication is necessary for establishment of latency. Rather, thymidine kinase appears to be necessary for reactivation from latency. These results suggest that acyclovir-resistant viruses could establish latent infections in clinical settings and have implications for the use of genetically engineered herpesviruses to deliver foreign genes to neurons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns W. H., Saral R., Santos G. W., Laskin O. L., Lietman P. S., McLaren C., Barry D. W. Isolation and characterisation of resistant Herpes simplex virus after acyclovir therapy. Lancet. 1982 Feb 20;1(8269):421–423. doi: 10.1016/s0140-6736(82)91620-8. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Miller C. H., Schrager L. E., Crumpacker C. S. Successful treatment with foscarnet of an acyclovir-resistant mucocutaneous infection with herpes simplex virus in a patient with acquired immunodeficiency syndrome. N Engl J Med. 1989 Feb 2;320(5):297–300. doi: 10.1056/NEJM198902023200507. [DOI] [PubMed] [Google Scholar]
- Chiou H. C., Weller S. K., Coen D. M. Mutations in the herpes simplex virus major DNA-binding protein gene leading to altered sensitivity to DNA polymerase inhibitors. Virology. 1985 Sep;145(2):213–226. doi: 10.1016/0042-6822(85)90155-2. [DOI] [PubMed] [Google Scholar]
- Christophers J., Sutton R. N., Noble R. V., Anderson H. Clinical resistance to acyclovir of herpes simplex virus infections in immunocompromised patients. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):121–125. doi: 10.1093/jac/18.supplement_b.121. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Fleming H. E., Jr, Leslie L. K., Retondo M. J. Sensitivity of arabinosyladenine-resistant mutants of herpes simplex virus to other antiviral drugs and mapping of drug hypersensitivity mutations to the DNA polymerase locus. J Virol. 1985 Feb;53(2):477–488. doi: 10.1128/jvi.53.2.477-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Irmiere A. F., Jacobson J. G., Kerns K. M. Low levels of herpes simplex virus thymidine- thymidylate kinase are not limiting for sensitivity to certain antiviral drugs or for latency in a mouse model. Virology. 1989 Feb;168(2):221–231. doi: 10.1016/0042-6822(89)90261-4. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Weinheimer S. P., McKnight S. L. A genetic approach to promoter recognition during trans induction of viral gene expression. Science. 1986 Oct 3;234(4772):53–59. doi: 10.1126/science.3018926. [DOI] [PubMed] [Google Scholar]
- Collins P., Oliver N. M. Sensitivity monitoring of herpes simplex virus isolates from patients receiving acyclovir. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):103–112. doi: 10.1093/jac/18.supplement_b.103. [DOI] [PubMed] [Google Scholar]
- Corey L., Spear P. G. Infections with herpes simplex viruses (1). N Engl J Med. 1986 Mar 13;314(11):686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- Corey L., Spear P. G. Infections with herpes simplex viruses (2). N Engl J Med. 1986 Mar 20;314(12):749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
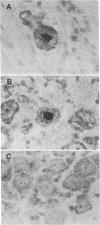
- Croen K. D., Ostrove J. M., Dragovic L. J., Smialek J. E., Straus S. E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene "anti-sense" transcript by in situ hybridization. N Engl J Med. 1987 Dec 3;317(23):1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Marlowe S. I., Kowalsky P. N., Hershey B. J., Levin M. J. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N Engl J Med. 1982 Feb 11;306(6):343–346. doi: 10.1056/NEJM198202113060606. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., Fraser N. W. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987 May;84(10):3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich K. S., Mills J., Chatis P., Mertz G. J., Busch D. F., Follansbee S. E., Grant R. M., Crumpacker C. S. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989 Feb 2;320(5):293–296. doi: 10.1056/NEJM198902023200506. [DOI] [PubMed] [Google Scholar]
- Field H. J., Darby G., Wildy P. Isolation and characterization of acyclovir-resistant mutants of herpes simplex virus. J Gen Virol. 1980 Jul;49(1):115–124. doi: 10.1099/0022-1317-49-1-115. [DOI] [PubMed] [Google Scholar]
- Field H. J. Development of clinical resistance to acyclovir in herpes simplex virus-infected mice receiving oral therapy. Antimicrob Agents Chemother. 1982 May;21(5):744–752. doi: 10.1128/aac.21.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Lay E. Characterization of latent infections in mice inoculated with herpes simplex virus which is clinically resistant to acyclovir. Antiviral Res. 1984 Apr;4(1-2):43–52. doi: 10.1016/0166-3542(84)90024-x. [DOI] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focher F., Hildebrand C., Freese S., Ciarrocchi G., Noonan T., Sangalli S., Brown N., Spadari S., Wright G. N2-phenyldeoxyguanosine: a novel selective inhibitor of herpes simplex thymidine kinase. J Med Chem. 1988 Aug;31(8):1496–1500. doi: 10.1021/jm00403a004. [DOI] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Geller A. I., Breakefield X. O. A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science. 1988 Sep 23;241(4873):1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y. J., Johnson B., Romanowski E., Araullo-Cruz T. RNA complementary to herpes simplex virus type 1 ICP0 gene demonstrated in neurons of human trigeminal ganglia. J Virol. 1988 May;62(5):1832–1835. doi: 10.1128/jvi.62.5.1832-1835.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y., Gilden D. H., Shtram Y., Asher Y., Tabor E., Wellish M., Devlin M., Snipper D., Hadar J., Becker Y. A low thymidine kinase-producing mutant of herpes simplex virus type 1 causes latent trigeminal ganglia infections in mice. Arch Virol. 1983;76(1):39–49. doi: 10.1007/BF01315702. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Schooley R. T. Resistance to antiviral drugs: the end of innocence. N Engl J Med. 1989 Feb 2;320(5):313–314. doi: 10.1056/NEJM198902023200510. [DOI] [PubMed] [Google Scholar]
- Irmiere A. F., Manos M. M., Jacobson J. G., Gibbs J. S., Coen D. M. Effect of an amber mutation in the herpes simplex virus thymidine kinase gene on polypeptide synthesis and stability. Virology. 1989 Feb;168(2):210–220. doi: 10.1016/0042-6822(89)90260-2. [DOI] [PubMed] [Google Scholar]
- Jacobson J. G., Martin S. L., Coen D. M. A conserved open reading frame that overlaps the herpes simplex virus thymidine kinase gene is important for viral growth in cell culture. J Virol. 1989 Apr;63(4):1839–1843. doi: 10.1128/jvi.63.4.1839-1843.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Coen D. M., Bogard C. L., Hicks K. A., Yager D. R., Knipe D. M., Tyler K. L., Schaffer P. A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989 Feb;63(2):759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A. M., McMahan L., Schaffer P. A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989 Jan;63(1):18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter L. M., Grill S. P., Dutschman G. E., Sharma R. A., Bobek M., Cheng Y. C. Demonstration of viral thymidine kinase inhibitor and its effect on deoxynucleotide metabolism in cells infected with herpes simplex virus. Antimicrob Agents Chemother. 1987 Mar;31(3):368–374. doi: 10.1128/aac.31.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella T. D., Silverman L. J., Schroll C. T., Homa F. L., Levine M., Kelley W. N. Herpes simplex virus-mediated human hypoxanthine-guanine phosphoribosyltransferase gene transfer into neuronal cells. Mol Cell Biol. 1988 Jan;8(1):457–460. doi: 10.1128/mcb.8.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. W. Herpes simplex virus latency: adaptation to the peripheral nervous system. I. Cancer Invest. 1985;3(3):285–292. doi: 10.3109/07357908509039789. [DOI] [PubMed] [Google Scholar]
- Price R. W. Herpes simplex virus latency: adaptation to the peripheral nervous system. II. Cancer Invest. 1985;3(4):389–403. doi: 10.3109/07357908509039799. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F., del Bene V., Scott R. T., Dudley-Thorpe J. B. Characterization of acyclovir-resistant and -sensitive herpes simplex viruses isolated from a patient with an acquired immune deficiency. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):127–134. doi: 10.1093/jac/18.supplement_b.127. [DOI] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears A. E., Meignier B., Roizman B. Establishment of latency in mice by herpes simplex virus 1 recombinants that carry insertions affecting regulation of the thymidine kinase gene. J Virol. 1985 Aug;55(2):410–416. doi: 10.1128/jvi.55.2.410-416.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibrack C. D., Gutman L. T., Wilfert C. M., McLaren C., St Clair M. H., Keller P. M., Barry D. W. Pathogenicity of acyclovir-resistant herpes simplex virus type 1 from an immunodeficient child. J Infect Dis. 1982 Nov;146(5):673–682. doi: 10.1093/infdis/146.5.673. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Latent characteristics of selected herpesviruses. Adv Cancer Res. 1978;26:227–256. doi: 10.1016/s0065-230x(08)60089-5. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Stroop W. G., Rock D. L., Fraser N. W. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest. 1984 Jul;51(1):27–38. [PubMed] [Google Scholar]
- Svennerholm B., Vahlne A., Löwhagen G. B., Widell A., Lycke E. Sensitivity of HSV strains isolated before and after treatment with acyclovir. Scand J Infect Dis Suppl. 1985;47:149–154. [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Edris W. A. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus after in vivo complementation. J Virol. 1987 Jul;61(7):2171–2174. doi: 10.1128/jvi.61.7.2171-2174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Ressel S., Dunstan M. E. Herpes simplex virus thymidine kinase expression in trigeminal ganglion infection: correlation of enzyme activity with ganglion virus titer and evidence of in vivo complementation. Virology. 1981 Jul 15;112(1):328–341. doi: 10.1016/0042-6822(81)90638-3. [DOI] [PubMed] [Google Scholar]
- Wade J. C., McLaren C., Meyers J. D. Frequency and significance of acyclovir-resistant herpes simplex virus isolated from marrow transplant patients receiving multiple courses of treatment with acyclovir. J Infect Dis. 1983 Dec;148(6):1077–1082. doi: 10.1093/infdis/148.6.1077. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Aschman D. P., Sacks W. R., Coen D. M., Schaffer P. A. Genetic analysis of temperature-sensitive mutants of HSV-1: the combined use of complementation and physical mapping for cistron assignment. Virology. 1983 Oct 30;130(2):290–305. doi: 10.1016/0042-6822(83)90084-3. [DOI] [PubMed] [Google Scholar]
- Whitby A. J., Blyth W. A., Hill T. J. The effect of DNA hypomethylating agents on the reactivation of herpes simplex virus from latently infected mouse ganglia in vitro. Brief report. Arch Virol. 1987;97(1-2):137–144. doi: 10.1007/BF01310742. [DOI] [PubMed] [Google Scholar]