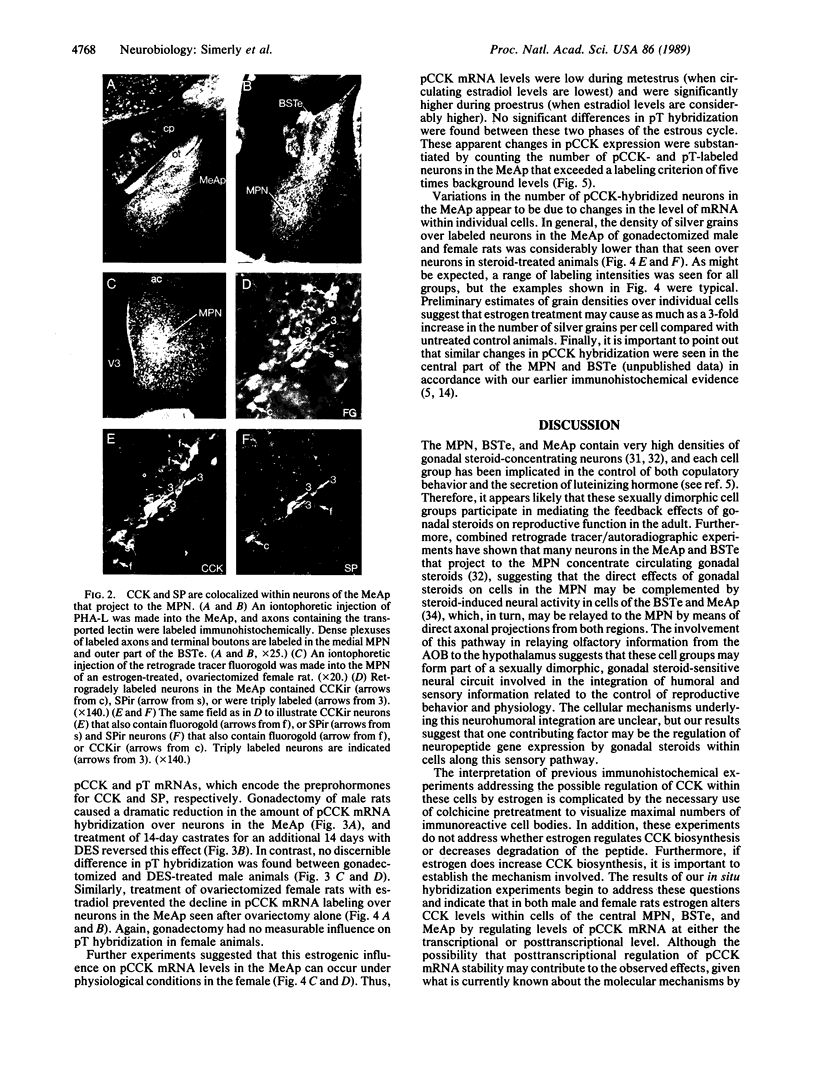
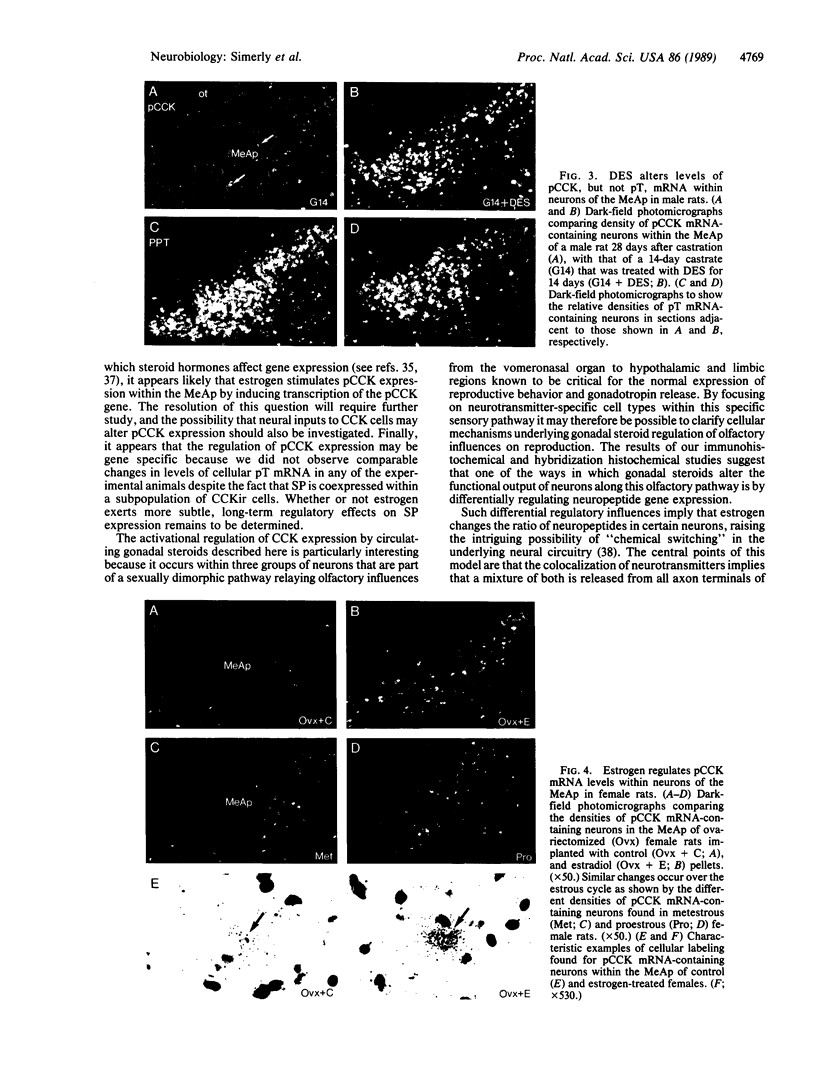
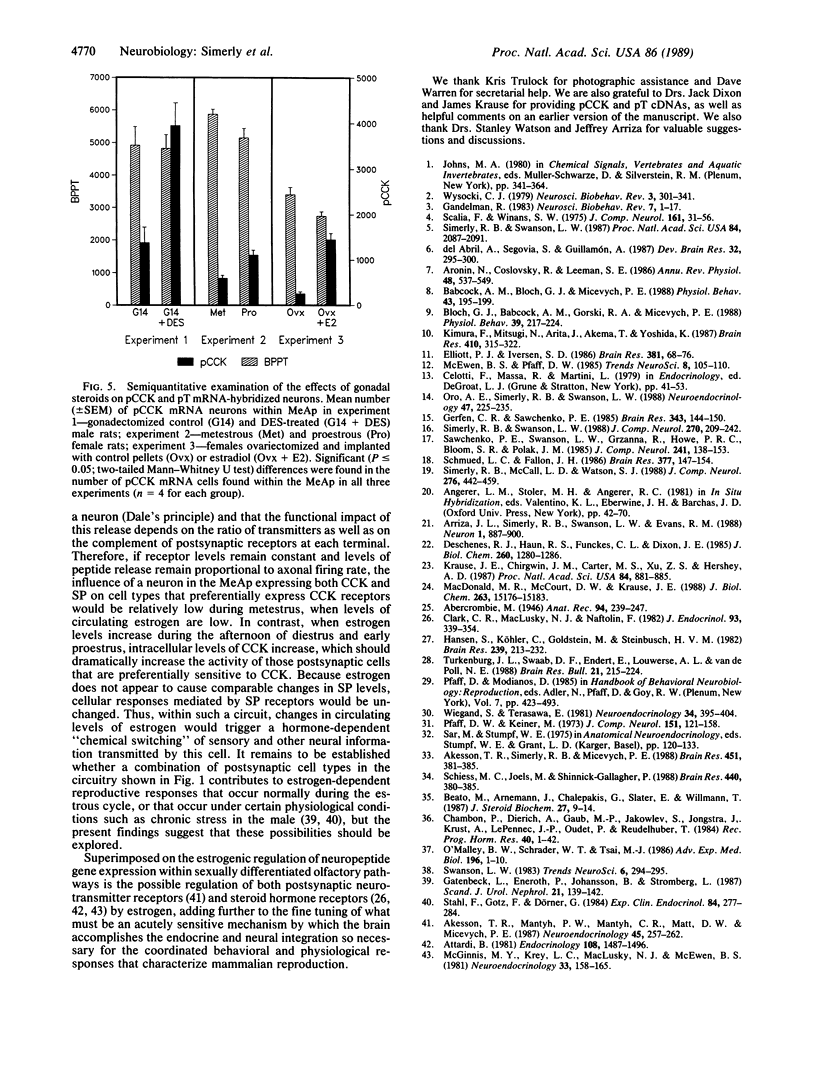
Abstract
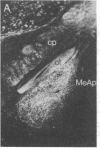
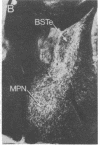
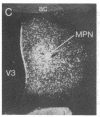
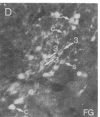
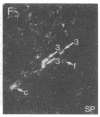
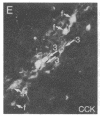
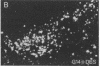
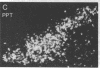
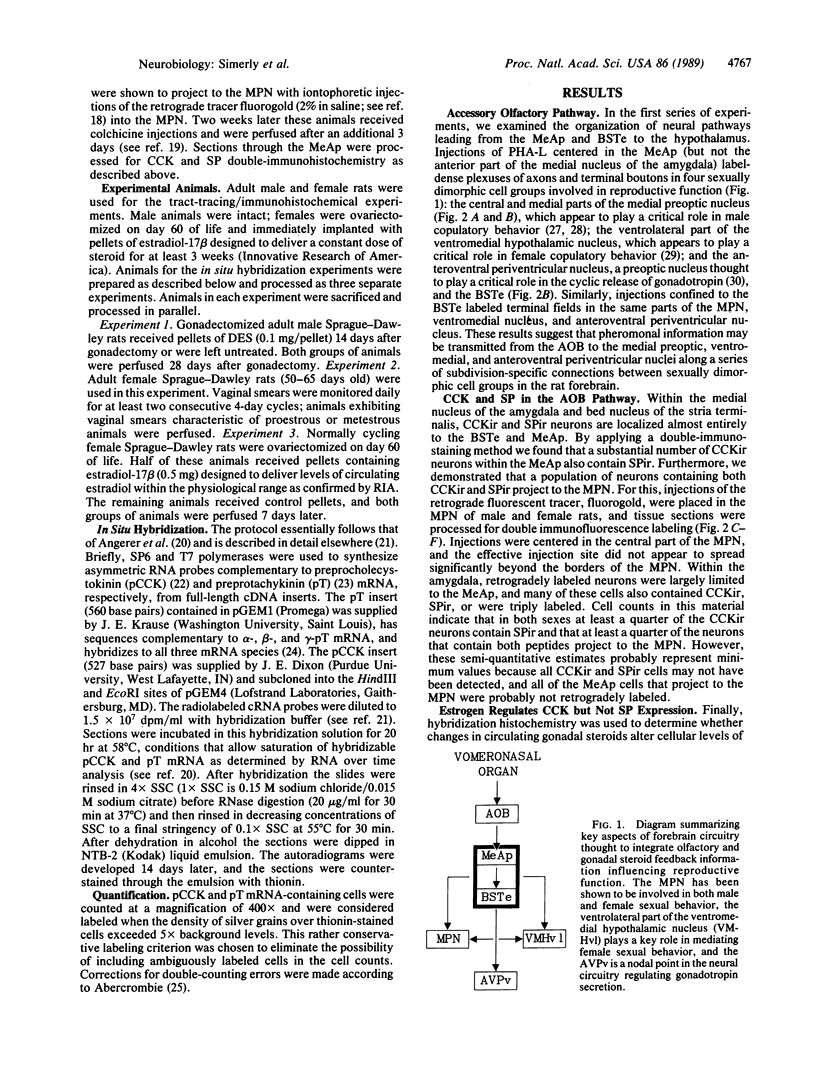
The posterodorsal part of the medial nucleus of the amygdala (MeAp) receives its major sensory input from the accessory olfactory bulb and projects massively to the medial preoptic nucleus and other sexually dimorphic hypothalamic nuclei thought to play key roles in mediating steroid-sensitive reproductive functions. A combined axonal transport/double-immunohistochemical method was used to show that at least one-quarter of the cholecystokinin-immunoreactive cells in the MeAp cocontain substance P and that a substantial proportion of these cells project to the medial preoptic nucleus. In situ hybridization histochemistry was then used to demonstrate that estrogen regulates the expression of preprocholecystokinin in these cells at the mRNA level in male and female rats. In contrast, levels of preprotachykinin mRNA within the MeAp do not appear to be sensitive to acute changes in circulating gonadal steroids in either sex. Although posttranscriptional regulation of mRNA stability may contribute to the observed effects, it appears likely that estrogen stimulates preprocholecystokinin expression within the MeAp by selectively inducing transcription of the corresponding gene, thereby altering the relative amounts of cholecystokinin and substance P coexpressed within individual neurons of the MeAp that project to the hypothalamus.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akesson T. R., Mantyh P. W., Mantyh C. R., Matt D. W., Micevych P. E. Estrous cyclicity of 125I-cholecystokinin octapeptide binding in the ventromedial hypothalamic nucleus. Evidence for downmodulation by estrogen. Neuroendocrinology. 1987 Apr;45(4):257–262. doi: 10.1159/000124737. [DOI] [PubMed] [Google Scholar]
- Akesson T. R., Simerly R. B., Micevych P. E. Estrogen-concentrating hypothalamic and limbic neurons project to the medial preoptic nucleus. Brain Res. 1988 Jun 7;451(1-2):381–385. doi: 10.1016/0006-8993(88)90789-5. [DOI] [PubMed] [Google Scholar]
- Aronin N., Coslovsky R., Leeman S. E. Substance P and neurotensin: their roles in the regulation of anterior pituitary function. Annu Rev Physiol. 1986;48:537–549. doi: 10.1146/annurev.ph.48.030186.002541. [DOI] [PubMed] [Google Scholar]
- Arriza J. L., Simerly R. B., Swanson L. W., Evans R. M. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron. 1988 Nov;1(9):887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Attardi B. Facilitation and inhibition of the estrogen-induced luteinizing hormone surge in the rat by progesterone: effects on cytoplasmic and nuclear estrogen receptors in the hypothalamus-preoptic area, pituitary, and uterus. Endocrinology. 1981 Apr;108(4):1487–1496. doi: 10.1210/endo-108-4-1487. [DOI] [PubMed] [Google Scholar]
- Babcock A. M., Block G. J., Micevych P. E. Injections of cholecystokinin into the ventromedial hypothalamic nucleus inhibit lordosis behavior in the rat. Physiol Behav. 1988;43(2):195–199. doi: 10.1016/0031-9384(88)90237-5. [DOI] [PubMed] [Google Scholar]
- Beato M., Arnemann J., Chalepakis G., Slater E., Willmann T. Gene regulation by steroid hormones. J Steroid Biochem. 1987;27(1-3):9–14. doi: 10.1016/0022-4731(87)90288-3. [DOI] [PubMed] [Google Scholar]
- Bloch G. J., Babcock A. M., Gorski R. A., Micevych P. E. Cholecystokinin stimulates and inhibits lordosis behavior in female rats. Physiol Behav. 1987;39(2):217–224. doi: 10.1016/0031-9384(87)90012-6. [DOI] [PubMed] [Google Scholar]
- Chambon P., Dierich A., Gaub M. P., Jakowlev S., Jongstra J., Krust A., LePennec J. P., Oudet P., Reudelhuber T. Promoter elements of genes coding for proteins and modulation of transcription by estrogens and progesterone. Recent Prog Horm Res. 1984;40:1–42. doi: 10.1016/b978-0-12-571140-1.50005-0. [DOI] [PubMed] [Google Scholar]
- Clark C. R., MacLusky N. J., Naftolin F. Oestrogen induction of progestin receptors in the rat brain and pituitary gland: quantitative and kinetic aspects. J Endocrinol. 1982 Jun;93(3):339–353. doi: 10.1677/joe.0.0930339. [DOI] [PubMed] [Google Scholar]
- Deschenes R. J., Haun R. S., Funckes C. L., Dixon J. E. A gene encoding rat cholecystokinin. Isolation, nucleotide sequence, and promoter activity. J Biol Chem. 1985 Jan 25;260(2):1280–1286. [PubMed] [Google Scholar]
- Elliott P. J., Iversen S. D. Behavioural effects of tachykinins and related peptides. Brain Res. 1986 Aug 27;381(1):68–76. doi: 10.1016/0006-8993(86)90691-8. [DOI] [PubMed] [Google Scholar]
- Gandelman R. Gonadal hormones and sensory function. Neurosci Biobehav Rev. 1983 Spring;7(1):1–17. doi: 10.1016/0149-7634(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Gatenbeck L., Eneroth P., Johansson B., Strömberg L. Plasma testosterone concentrations in male rats during short and long-term stress stimulation. Scand J Urol Nephrol. 1987;21(2):139–142. doi: 10.3109/00365598709180309. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Sawchenko P. E. A method for anterograde axonal tracing of chemically specified circuits in the central nervous system: combined Phaseolus vulgaris-leucoagglutinin (PHA-L) tract tracing and immunohistochemistry. Brain Res. 1985 Sep 16;343(1):144–150. doi: 10.1016/0006-8993(85)91168-0. [DOI] [PubMed] [Google Scholar]
- Hansen S., Köhler C., Goldstein M., Steinbusch H. V. Effects of ibotenic acid-induced neuronal degeneration in the medial preoptic area and the lateral hypothalamic area on sexual behavior in the male rat. Brain Res. 1982 May 6;239(1):213–232. doi: 10.1016/0006-8993(82)90843-5. [DOI] [PubMed] [Google Scholar]
- Kimura F., Mitsugi N., Arita J., Akema T., Yoshida K. Effects of preoptic injections of gastrin, cholecystokinin, secretin, vasoactive intestinal peptide and PHI on the secretion of luteinizing hormone and prolactin in ovariectomized estrogen-primed rats. Brain Res. 1987 May 5;410(2):315–322. doi: 10.1016/0006-8993(87)90330-1. [DOI] [PubMed] [Google Scholar]
- Krause J. E., Chirgwin J. M., Carter M. S., Xu Z. S., Hershey A. D. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci U S A. 1987 Feb;84(3):881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M. R., McCourt D. W., Krause J. E. Posttranslational processing of alpha-, beta-, and gamma-preprotachykinins. Cell-free translation and early posttranslational processing events. J Biol Chem. 1988 Oct 15;263(29):15176–15183. [PubMed] [Google Scholar]
- McGinnis M. Y., Krey L. C., MacLusky N. J., McEwen B. S. Steroid receptor levels in intact and ovariectomized estrogen-treated rats: an examination of quantitative, temporal and endocrine factors influencing the efficacy of an estradiol stimulus. Neuroendocrinology. 1981 Sep;33(3):158–165. doi: 10.1159/000123222. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Schrader W. T., Tsai M. J. Molecular actions of steroid hormones. Adv Exp Med Biol. 1986;196:1–10. doi: 10.1007/978-1-4684-5101-6_1. [DOI] [PubMed] [Google Scholar]
- Oro A. E., Simerly R. B., Swanson L. W. Estrous cycle variations in levels of cholecystokinin immunoreactivity within cells of three interconnected sexually dimorphic forebrain nuclei. Evidence for a regulatory role for estrogen. Neuroendocrinology. 1988 Mar;47(3):225–235. doi: 10.1159/000124916. [DOI] [PubMed] [Google Scholar]
- Pfaff D., Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973 Sep 15;151(2):121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Grzanna R., Howe P. R., Bloom S. R., Polak J. M. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985 Nov 8;241(2):138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- Scalia F., Winans S. S. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975 May 1;161(1):31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Schiess M. C., Joëls M., Shinnick-Gallagher P. Estrogen priming affects active membrane properties of medial amygdala neurons. Brain Res. 1988 Feb 9;440(2):380–385. doi: 10.1016/0006-8993(88)91012-8. [DOI] [PubMed] [Google Scholar]
- Schmued L. C., Fallon J. H. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986 Jul 2;377(1):147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., McCall L. D., Watson S. J. Distribution of opioid peptides in the preoptic region: immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. J Comp Neurol. 1988 Oct 15;276(3):442–459. doi: 10.1002/cne.902760309. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W. Castration reversibly alters levels of cholecystokinin immunoreactivity within cells of three interconnected sexually dimorphic forebrain nuclei in the rat. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2087–2091. doi: 10.1073/pnas.84.7.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988 Apr 8;270(2):209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Stahl F., Götz F., Dörner G. Plasma testosterone levels in rats under various conditions. Exp Clin Endocrinol. 1984 Dec;84(3):277–284. doi: 10.1055/s-0029-1210399. [DOI] [PubMed] [Google Scholar]
- Turkenburg J. L., Swaab D. F., Endert E., Louwerse A. L., van de Poll N. E. Effects of lesions of the sexually dimorphic nucleus on sexual behavior of testosterone-treated female Wistar rats. Brain Res Bull. 1988 Aug;21(2):215–224. doi: 10.1016/0361-9230(88)90234-1. [DOI] [PubMed] [Google Scholar]
- Wiegand S. J., Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982 Jun;34(6):395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wysocki C. J. Neurobehavioral evidence for the involvement of the vomeronasal system in mammalian reproduction. Neurosci Biobehav Rev. 1979 Winter;3(4):301–341. doi: 10.1016/0149-7634(79)90015-0. [DOI] [PubMed] [Google Scholar]
- del Abril A., Segovia S., Guillamón A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res. 1987 Apr;429(2):295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]