Abstract
Background:
Streptococcus pneumoniae is a leading and serious co-infection of HIV-infected adults, particularly in Africa. Prevention of disease by vaccination with the current 23-valent polysaccharide vaccine is sub-optimal. Protein conjugate vaccines offer a further option for protection but no data exist on their clinical efficacy in any adult population.
Methods:
We conducted a double-blind randomized placebo-controlled clinical efficacy trial of the seven-valent conjugate pneumococcal vaccine in predominantly HIV-infected Malawian adults who had recovered from documented invasive pneumococcal disease (IPD). Vaccine was given as a two dose schedule four weeks apart. The primary end-point was a further episode of IPD caused by a vaccine-serotype or serotype-6A (VST/6A) pneumococcus.
Results:
Between February 2003 and October 2007, 496 individuals (44% male, 88% HIV seropositive) were followed for 798 person years of observation. There were 67 IPD events in 52 individuals, all in the HIV infected sub-group. There were 24 VST/6A events (19 VST, five 6A) in 24 participants, 5 in vaccine and 19 in the placebo recipients, a vaccine efficacy of 74% (95% CI 30% - 90%). There were 73 deaths in the vaccine arm and 63 in the placebo arm, Hazard Ratio 1.18 (95% confidence intervals 0.84 -1.66). Compared to placebo, serious adverse events were significantly lower (3 vs 17, p = 0.002) and minor adverse events significantly higher (41 vs 13, p = 0.003 ) in vaccine recipients.
Conclusions:
The seven-valent pneumococcal conjugate vaccine protects HIV infected adults from recurrent IPD of vaccine serotype or serotype 6A.
Streptococcus pneumoniae, is a leading cause of morbidity and mortality in HIV-infected adults particularly in sub-Saharan Africa.1,2 The risk of invasive pneumococcal disease (IPD) is 30 to 100 times higher than in age matched HIV-uninfected controls.3,4 Recurrent IPD is common, with up to 25% of individuals experiencing a further IPD event, predominantly re-infection, in the subsequent 12 months.1,5 Case-fatality from IPD even with access to timely and effective care is at least 8%,6 rising to 50% in African populations with meningitis.7 The frequency and serious nature of IPD makes prevention by vaccination desirable.
The 23-valent pneumococcal polysaccharide vaccine (PPV) has sub-optimal activity in HIV-infected adults and is not recommended in Africa.8,5 In regions where PPV is used it is recommended early in the course of HIV disease.9 Pneumococcal conjugate vaccines (PCV) offer an alternative approach to preventing pneumococcal disease. Seven and nine valent PCVs have been shown to be highly efficacious.10-13 PCV is effective at preventing IPD in HIV-infected children,11 although with a reduced efficacy and duration of effect compared to HIV-uninfected children.14 There are no definitive data on the clinical efficacy of PCV in adult populations. Studies in HIV-infected adults show the vaccine to be immunogenic with similar quantitative antibody responses to those seen with PPV.15,16 Improved qualitative responses, evidence of boosting with two dose schedules16 and mucosal responses17 suggest that PCV is processed in an immunologically different way from PPV in HIV-infected adults. With no validated serological correlate of protection for adults, a clinical trial is required to provide information on the efficacy of this vaccine and its potential role in the management of HIV infection.
We report the results of a randomised, placebo controlled trial of a seven-valent PCV (7PCV) to prevent recurrent IPD (secondary prophylaxis), in a cohort of predominantly HIV-infected Malawian adults with a history of documented IPD. We first considered a primary prophylaxis trial, but since the available 7PCV has low serotype coverage,18 this would have required a large sample size with a costly, complex, multisite study. We chose to conduct a secondary prophylaxis study, as it would require a smaller sample size with more rapid accumulation of end-points. Furthermore demonstration of efficacy in a population with HIV infection, documented previous IPD, and at substantial risk of subsequent IPD, would make a plausible clinical case for the use of PCV as primary prophylaxis.
Methods
The study took place at the Queen Elizabeth Central Hospital in Blantyre, Malawi. This is the main public hospital serving a population of approximately one million people. Enrolment started in February 2003 and ceased in May 2007. Follow-up continued until October 2007. We identified potential participants on the adult medical wards by review of blood culture and cerebrospinal fluid results from which S. pneumoniae had been isolated. Individuals who had survived an IPD event were invited to return to the study clinic one week post-discharge, if they were 15 years or older, were able to give informed consent, resided within Blantyre district and were willing to have an HIV test.
To avoid possible stigmatisation of the study as an HIV trial, we offered enrolment to all patients who had suffered an IPD event, irrespective of HIV status. We recognized that the HIV-uninfected group would be small, approximately 10% of enrollees,19 and unlikely to experience enough recurrent IPD events for a meaningful sub-group analysis. Thus, as pre-specified in the protocol and analysis plan, the group of primary interest would be the HIV-infected.
Written consent, enrolment and laboratory investigations followed standard procedures (supplementary material). Two doses of vaccine were given four weeks apart after which participants were requested to attend regular three monthly appointments. Individuals who moved out of the study area, who could not be traced by virtue of inaccurate address or who declined further follow-up were treated as lost to follow-up. We encouraged individuals to attend the hospital medical wards for assessment by the study clinical team in the event of any illness occurring between scheduled visits. Participants with HIV infection were encouraged to enrol in public-sector HIV care and ART centres as these were set up in Blantyre over the course of the trial.
Participants received two doses of either 7PCV (Prevenar®, Wyeth pharmaceuticals, Collegeville, PA) or matching placebo (Nova Laboratories Ltd, Leicester, UK) The randomization list was generated by the Data and Safety Monitoring Board (DSMB) statistician and took place in permuted groups of random size up to 20. Product presentation is described in supplementary materials.
An IPD event was defined by the isolation of Streptococcus pneumoniae from a normally sterile site i.e. blood, cerebro-spinal fluid or pleural fluid, in the context of a consistent clinical presentation. S. pneumoniae was identified by standard methods (supplementary material). The primary study end point was an IPD episode caused by one of the vaccine-serotypes (VST), 4, 6B, 9V, 14, 18C, 19F and 23F. During the course of the study and prior to unblinding, the Trial Steering Group (TSG) agreed to the broadening of the primary end-point to VST or serotype 6A (VST/6A) based on published data showing cross-protection to serotype 6A.20,21
The secondary end points were vaccine serogroup IPD, all IPD, death and all cause pneumonia. Pneumonia was defined as a respiratory illness of 28 days or less duration with new pulmonary parenchymal shadowing on chest x-ray. X-rays were reported independently by two clinicians, prior to unblinding. Discrepant results were reviewed by an independent physician.
Adverse events were defined and recorded according to the ICH Harmonised Tripartite Guidelines for Clinical Safety Data Management (1994). Grade 3 or 4 events in the 14 days following vaccination were defined as serious adverse events (SAE). SAEs were captured at the time of hospitalisation, a study clinic attendance or, if an out of hospital death occurred, by a relatives report. To investigate possible harmful effects of vaccine as seen with the use of PPV in Uganda5 additional safety end-points were included. All deaths were reviewed by the senior study clinician and classified in terms of perceived association with S. pneumoniae into four categories; definite/probable, possible, unlikely or unclassifiable owing to inadequate information. Since many deaths took place outside of medical care, as is typical in this region, several sources of information including verbal autopsy reports and individual health records retained by relatives were synthesized to reach a final decision, prior to unblinding.
To show a 60% reduction in vaccine-serotype IPD (β = 0.2, α = 0.05), we planned to accumulate 42 first-event primary endpoints requiring an expected 402 person years of observation (pyo). This used a 1:1 allocation ratio and an assumed invasive disease rate of 250/1000 pyo of which 60% were vaccine serotype, and loss to follow-up and death rates of 100 and 250/1000pyo respectively. Insufficient endpoint accumulation prior to the planned end date of March 2006 led the DSMB to recommend continuing the study until October 2007 or until the target of 42 endpoints had been achieved, whichever came earlier.
The TSG approved an analysis plan, and this was lodged with the chair of the TSG along with a cleaned dataset prior to unblinding. The primary analysis was on the intention to treat basis. A per-protocol analysis was performed for the primary end-point for which the second dose of vaccine must have been received between 4 and 8 weeks after the first dose and the at-risk period commenced two weeks after the second vaccination. Hazard ratios (HR) for the first event in vaccine versus placebo recipients were estimated from a Cox proportional hazards regression model. Vaccine efficacy was calculated as 1-HR × 100%. Adjusted hazard ratios were estimated by incorporating terms representing WHO clinical stage (3 or 4), enrolment CD4 T-cell count (<200, ≤200-500, >500), viral load (<100,000, ≥100,000 copies/ml), sex and age (15-24, 25-34, 35-75) as the most important modifiers of IPD risk. When the proportional hazards assumption was violated the Cox model was stratified by year of recruitment and baseline CD4 count and data were analysed separately for the first 12 months. Individual records were censored at the 31st October 2007, date of death, or loss to follow-up. We used negative binomial regression models to estimate incidence rate ratios for multiple event analyses. All tests of statistical significance were two-sided using the 5% significance level (α). One interim analysis was planned and performed after two years by SW for the DSMB using α= 0.1%. Three additional analyses were requested and performed using α= 0.1%; the Haybittle-Peto approach to significance levels was used.22 There was no adjustment of the overall significance level.
We report baseline findings for the whole cohort and efficacy results for HIV-infected participants, unless otherwise indicated. One post-hoc analysis, of efficacy in the CD4<200 subgroup, was performed; the purpose was to confirm efficacy in this higher risk subgroup. CD4 category-by-treatment group interaction is reported
A placebo controlled efficacy trial was deemed ethically acceptable following the failure of PPV in the earlier Ugandan trial.5 The study was approved by the College of Medicine Research and Ethics Committee, College of Medicine, Malawi and by the Liverpool School of Tropical Medicine institutional review board. NF and CFG conceived the study. MEM SBG and EZ provided intellectual input into the design of the study. NF, SBG, TM, HL, MM and GM undertook the data collection. SW oversaw data management. All authors vouch for the completeness and accuracy of the data presented. SW and NF undertook the primary analysis and vouch for the data. NF produced the first draft of the article and chose to publish the paper. The funders of the study (The Wellcome Trust), the vaccine suppliers (Wyeth pharmaceuticals) and the study sponsors (The University of Liverpool) had no part in the design, running, analysis or reporting of the study. The study was registered as a clinical trial registration number ISRCTN54494731.
Results
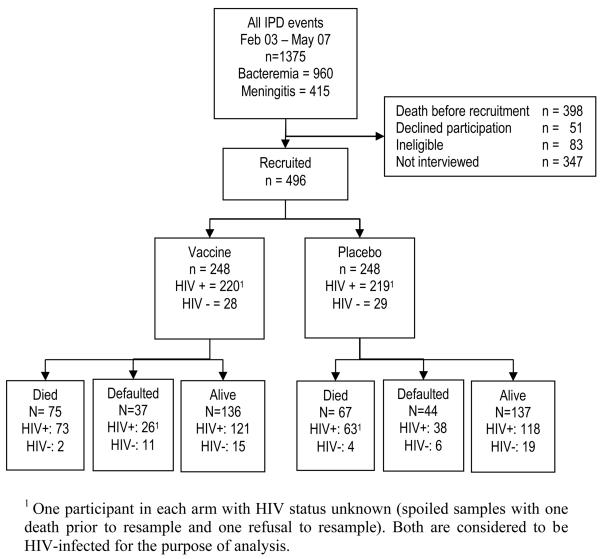
During the study enrolment period, 977 adults surviving a confirmed invasive pneumococcal event were identified, figure 1. Of this group 496 (50.7%) were enrolled, of whom 88% were HIV-infected, 465 (93.7%) received two doses of vaccine and 445 (89.7%) received vaccine within the 28-56 day period of the protocol, table 1. The reasons for failing to receive two doses were death (19), withdrawal of consent (5) and loss from follow-up (7). Trial arms were well balanced except for an excess of placebo recipients reporting past treatment for tuberculosis.
Figure 1.
Trial profile: Of those recruited, 44% were male with a median age of 32 [range 15-75]. Those not recruited were 44% male (missing data n=14), with a median age of 32 [range 14-78] (missing data n=80). Individuals would not have been interviewed if they were not found on the ward or could not be traced to a home address in Blantyre.
Table 1.
Baseline demographic, clinical and laboratory parameters for all randomised participants, loss to follow up and uptake of HIV care (antiretroviral therapy and cotrimoxazole prophylaxis). Extended version available in supplementary material.
| Variable | Category | Treatment group | P1 | |
|---|---|---|---|---|
|
|
||||
| Vaccine | Placebo | |||
| Number of patients | 248 | 248 | ||
| Gender: | Male | 106 (43%) | 111 (45%) | 0.92 |
| Age (years) | Median [Range] | 31 [16 - 72] | 33 [15 – 75] | 0.74 |
|
|
||||
|
|
||||
| Previous IPD | Bacteraemic pneumonia | 187 (75.4%) | 196 (79.0%) | 0.74 |
| Meningitis | 60 (24.2%) | 51 (20.6%) | ||
| Other invasive syndrome | 1 (0.4%) | 1 (0.4%) | ||
| Days since previous IPD |
Median [Range] | 19 [7 - 1946]2 |
20 [7 – 1715]2 |
0.70 |
| Previous TB? | Yes | 60 (24.2%) | 83 (33.5%) | 0.02 |
| Previous pneumonia?3 | Yes | 99 (39.9%) | 116 (46.8%) | 0.25 |
|
|
||||
|
|
||||
| HIV | Positive | 219 (88.3%) | 218 (87.9%) | 0.70 |
| WHO clinical stage 4 | 42 (16.9%) | 45 (18.2%) | ||
| Unknown | 1 (0.4%) | 1 (0.4%) | ||
| Enrolment CD4 count | Median [Range] | 212 [1,1342] | 214 [1,1200] | 0.60 |
| Enrolment Viral load | Median [Range] – Log10 | 4.9 [2.5,5.9] 4 | 5.0 [2.5,6.8] | 0.16 |
|
|
||||
|
|
||||
| Antiretroviral use6 | ARVs at enrolment | 32 (14.5%)4 | 26 (11.9%) | 0.48 |
| Median duration (days) [Range] | 91 [3,337] | 84 [2,652] | ||
| Cotrimoxazole use | Cotrimoxazole at enrolment | 20 (9.1%) | 20 (9.2%) | 1.00 |
| Combined ART & CTX use at any time during study | 89 (40.6%) | 95 (43.6%) | 0.56 | |
|
|
||||
|
|
||||
| Number (%) who received 2nd dose between 28 and 56 days | 221 (89.1%) | 224 (90.3%) | 0.62 | |
|
|
||||
|
|
||||
| Lost to follow-up | Declined participation | 37 (14.9% | 44 (17.7%) | 0.28 |
For comparison within the HIV-infected sub-group obtained using Fisher's exact, Pearson's χ2 or Wilcoxon's rank sum test..
13 recruits had more than twelve months from IPD event to recruit
Other than the recruitment event
For HIV-infected participants only
All participants used Triomune® (Cipla pharmaceuticals, Mumbai, India) consisting of Stavudine, Lamivudine and Nevirapine. Two participants substituted Nevirapine with Efavirenz, and two participants substituted Stavudine with Zidovudine, because of intolerance.
At study termination there were 273 individuals under follow-up, 239 HIV-infected, with similar numbers in each arm and an accumulated 797.8 pyo, 682.4 in HIV-infected. Median follow-up time was 1.24 years [range 2 days to 4.7years). Eighty one (16.3%) individuals were lost to follow-up at a rate of 102/1000 pyo, figure 1. More placebo than vaccine recipients were lost to follow-up.
All IPD and pneumonia events occurred in HIV-infected participants, with no identified events in the HIV-uninfected. Fifty-two participants had a total of 67 IPD events (rate 98/1000 pyo), 10 cases of meningitis, 32 bacteraemic pneumonia, 24 bacteraemic cases with unconfirmed focus and 1 empyema. Twenty four (36%) were of VST/6A and occurred in 24 participants, table 2. Five occurred in vaccine recipients and 19 in the placebo recipients, table 3. The unadjusted vaccine efficacy in the HIV-infected participants to prevent VST/6A is 74% (95% confidence intervals [CI] 30%-90%), table 3, 73% (CI 23%-89%) for the whole cohort including the HIV-uninfected.
Table 2.
Breakdown of the 67 invasive pneumococcal isolates by serotype vaccine assignment.
| Treatment arm | |||
|---|---|---|---|
| Serotype | Vaccine | Placebo | Total |
| Vaccine serotypes & 6A | |||
| 4 | 1 | 2 | 3 |
| 6A1 | 1 | 4 | 5 |
| 6B | 1 | 2 | 3 |
| 9V | 2 | 3 | 5 |
| 14 | 0 | 5 | 5 |
| 19F | 0 | 2 | 2 |
| 23F | 0 | 1 | 1 |
|
|
|||
| Sub-total | 5 | 19 | 24 |
|
|
|||
| Vaccine serogroups | |||
| 9L | 1 | 1 | 2 |
| 23 | 12 | 0 | 1 |
|
|
|||
| Sub-total | 2 | 1 | 3 |
|
|
|||
| Non-vaccine serotypes | |||
| 0 | 0 | 42 | 4 |
| 1 | 5 | 2 | 7 |
| 3 | 5 | 0 | 5 |
| 7A | 1 | 0 | 1 |
| 10B | 0 | 1 | 1 |
| 12F | 0 | 5 | 5 |
| 12B | 2 | 0 | 2 |
| 15 | 0 | 12 | 1 |
| 15A | 3 | 0 | 3 |
| 163 | 3 | 1 | 4 |
| 223 | 2 | 0 | 2 |
| 253 | 0 | 1 | 1 |
| 33F | 0 | 1 | 1 |
| 35B | 0 | 1 | 1 |
| 46 | 1 | 1 | 2 |
|
|
|||
| Sub-total | 22 | 18 | 40 |
|
| |||
| Total | 29 | 38 | 67 |
Confirmed as 6A by PCR characterisation of the wciN region
Isolates lost viability prior to completion of serotyping
Isolates not factor typed.
Table 3.
Primary and secondary end-point numbers and loss to follow-up in the 437 HIV-infected participants. There were no pneumococcal or pneumonia events recorded in the 57 HIV-uninfected individuals participating in the study. Secondary end-point and loss to follow-up numbers by CD4 group are available in supplementary material.
| End-point | Number of participants (events) | First event | Recurrent events | |||
|---|---|---|---|---|---|---|
| Vaccine | Placebo | Unadjusted HR (95%CI) |
Adjusted HR1 | Unadjusted IRR | Adjusted IRR | |
| Primary end-point | ||||||
| Vaccine serotype or 6A | 5 (5) | 19 (19) | 0.26 (0.10-0.70) | 0.31 (0.11-0.84)2 | - | - |
| CD4≤200 | 2(2) | 16 (16) | ||||
| 200<CD4≤500 | 3 (3) | 1 (1) | ||||
| >500 | 0 (0) | 1 (1) | ||||
| CD4 missing | 0 (0) | 1 (1) | ||||
|
Vaccine serotype or 6A (Per protocol analysis) |
4 (4) | 18 (18) | 0.22 (0.08-0.66) | 0.26 (0.08-0.78) | - | - |
| Secondary end-point | ||||||
| Vaccine serogroup | 7 (7) | 19 (20) | 0.37 (0.15-0.87) | 0.41 (0.17-1.02) | 0.19 (0.06-0.66) | 0.30 (0.09-1.02) |
| Any IPD | 22 (29)3 | 30 (38) | 0.72 (0.42-1.25) | 0.80 (0.45-1.44) | 0.35 (0.12-1.01) | 0.34 (0.11-1.02) |
| All cause pneumonia | 32 (44) | 41 (58) | 0.75 (0.47-1.19) | 0.71 (0.43-1.17) | 0.54 (0.26-1.14) | 0.49 (0.20-1.21) |
| Minor adverse events | 27 (41) | 9 (13) | P=0.0034 | - | - | - |
| Serious adverse events | 3 (3) 5 | 17 (17)6 | P=0.002 | - | - | - |
| Number of participants | ||||||
| (Number on ART) | ||||||
| Death | 73 (11) | 63 (14) | 1.18 (0.84-1.66) | 1.24 (0.88-1.75) | - | - |
| Definite, probable and possible pneumococcal |
35 (6) | 35 (7) | 1.02 (0.64-1.63) | 1.14 (0.71-1.85) | - | - |
| related death7 | ||||||
| Loss to follow-up | 26 | 38 | - | - | - | - |
Adjusted for age, sex, enrolment viral load, clinical stage and enrolment CD4
Proportional hazards assumption violated model stratified by enrolment CD4 count and year of recruitment.
In vaccine arm 3 individuals had 2, 2, 6 recurrent events and in placebo arm 7 individuals had 2, 2, 2, 2, 2, 2, 3 recurrent events respectively.
P values derived from a Fisher's exact test
Consisting of 2 deaths, 1 hospitalisation.
Consisting of 7 deaths 10 hospitalisations – 5 hospitalisations from IPD, 1 vaccine serotype.
Made up of 9 definite, 4 probable and 57 possible pneumococcal deaths
During the first 12 months post-enrolment there were 17 VST/6A events, 2 in vaccine and 15 in placebo recipients with an estimated efficacy of 85%. In the period after the first 12 months there were 7 events, 3 in vaccine and 4 in placebo recipients with an estimated efficacy of 25%. The difference between the two periods was not statistically significant (p=0.12, likelihood ratio test for heterogeneity). IPD was most common in individuals with CD4 T-cell counts below 200 at enrolment. Two VST/6A IPD events occurred in vaccine recipients in this sub-group versus 16 in placebo recipients, table 3, a vaccine efficacy in this sub-group of 86% (CI 41%-97%), likelihood ratio test for heterogeneity of effect across the three CD4 categories: p=0.06. Nineteen of 220 individuals in this CD4 stratum were taking antiretrovirals at the time of enrolment, 9 in the vaccine arm.
Minor adverse events were infrequent with 10.9% of vaccine, and 3.6% of placebo, recipients reporting an event, predominantly self-limiting injection site pain (35% of reported symptoms) and self-reported fever (28%),(table 5 supplementary material). Serious adverse events were significantly more common in the placebo recipients, (table 6 supplementary material). Mortality rates were 199/1000 pyo in the HIV-infected participants and 52/1000 pyo in the HIV-uninfected. Of the 136 deaths in HIV-infected, 111 (82%) occurred in persons not taking antiretrovirals (ART) at a rate of 270/1000 pyo versus 92/1000 pyo in those taking ART. There was a non-significant excess of deaths in vaccine recipients. This excess occurred in the HIV-infected ART-untreated patients, two or more years post vaccination (figure 2 supplementary material).
There was no difference between the arms in total number of deaths classified as definite/probable or possible pneumococcal, table 3.
Discussion
In this secondary prophylaxis trial, 7-PCV prevented 74% of recurrent invasive pneumococcal disease events caused by vaccine serotype or serotype 6A in participants with underlying HIV infection. The efficacy tended to be highest during the first 12 months post vaccination. Vaccine prevented disease when given to subjects with CD4 counts below 200 cells/μl.
There was no overall effect on mortality, with the proportions alive and under follow-up at study termination similar in both arms. There was a non-significant excess of reported deaths in the vaccine recipients specifically in the subgroup with CD4 counts below 500 at enrolment and who never received ART. These were not categorized as deaths attributable to pneumococcal disease. Up-regulation of HIV by vaccines has been postulated and could theoretically manifest as an increase in mortality in those without ART-induced HIV control.23 However the excess of those lost to follow-up in the placebo group is likely to include concealed mortality. Other groups have highlighted the problems of loss-to-follow up in trials in low-income settings and have emphasized the frequency of death in these groups.24-27
The study spanned a period of change in HIV care in Malawi with the commencement of the national ART programme and promotion of co-trimoxazole prophylaxis. Modification of the primary end-point and prolongation of the study were in part a consequence of these changes. The study lacked power to investigate the interaction with ART, and refinements to the use of PCV with ART should be investigated further; specifically whether a two dose vaccine schedule, based on evidence from immunogenicity studies carried out in non ART treated HIV-infected Ugandans16 is optimal; and how primary and repeat doses of vaccine should best be used in relation to ART to maximize efficacy and duration of protection.
This was a secondary prophylaxis trial undertaken in a population of predominantly HIV-infected adults. The HIV-uninfected recruits experienced no primary or pneumonia end-points and no conclusions can be made about vaccine efficacy in this sub-group. By extension from our results in HIV infected adults, it seems likely that PCV will also work as primary prophylaxis. The pre-enrolment invasive event may have primed for a response to a matching PCV serotype, but with a recognized low risk of recurrent disease caused by the same serotype,1,5 the contribution of any potential priming to efficacy is probably small.
Vaccine serotype pneumococci made up 50% of the IPD events in the placebo arm and broader serotype coverage would be desirable. Higher valency PCVs will soon be available, but responses to these vaccines should be evaluated, in particular those with different carrier proteins. HIV-infected adults maintain responses to toxoid vaccines such as diphtheria but this may not be true of other carrier proteins. The use of PCV in HIV-infected adults will also now have to be considered in the context of paediatric vaccination programmes, and how these might alter the distribution of disease-causing serotypes in adults.28,29
We have demonstrated the ability of HIV-infected adults to produce clinically relevant responses to a PCV that leads to protection against a common and serious co-infection. The ability of a conjugate vaccine to generate protective responses at low CD4 counts is of particular note and merits further work to elucidate the immune mechanisms involved, and how this may be used to produce other vaccines for this population. PCV provides an additional therapeutic intervention for improving care of HIV-infected adults that is both simple and safe to administer, and highly relevant to Africa.
Supplementary Material
Table 4.
Hazard Ratios derived from multivariable proportional hazards regression model and including antiretroviral and cotrimoxazole use as time-dependent covariates for the first event end-points vaccine serotype and 6A invasive pneumococcal disease, all invasive pneumococcal disease, pneumonia and death for HIV-infected participants. Thirty one participants had no enrolment CD4 count (15 vaccine, 16 placebo) and are excluded from the analysis.
| Hazard Ratio | ||||
|---|---|---|---|---|
| Primary end point1 | Any Invasive Pneumococcal disease |
All cause Pneumonia | Death | |
| Vaccine | 0.25 (0.08-0.71) | 0.74 (0.41-1.35) | 0.70 (0.42-1.16) | 1.38 (0.97-1.97) |
| Age 15-24 (ref) | - | |||
| 25-34 | 1.17 (0.28-4.78) | 1.95 (0.65-5.89) | 1.61 (0.62-4.22) | 1.57 (0.81-3.06) |
| 35-74 | 0.65 (0.14-3.13) | 1.37 (0.43-4.35) | 1.00 (0.37-2.73) | 1.43 (0.72-2.84) |
| Sex (male) | 1.41 (0.52-3.85) | 1.21 (0.65-2.25) | 1.57 (0.94-2.64) | 0.82 (0.55-1.19) |
| CD4 ≤200 (ref) | - | |||
| 200-500 | - | 0.61 (0.34-1.13) | 0.43 (0.24-0.78) | 0.34 (0.22-0.53) |
| >500 | - | 0.14 (0.02-1.06) | 0.29 (0.07-1.23) | 0.47 (0.21-1.04) |
| Viral load ≥100,000 copies/ml | 1.30 (0.50-3.38) | 1.40 (0.75-2.62) | 2.68 (1.51-4.74) | 1.75 (1.20-2.57) |
| WHO Stage 4 | 0.94 (0.32-2.76) | 1.08 (0.51-2.27) | 1.30 (0.73-2.32) | 2.11 (1.43-3.11) |
| Antiretroviral use | 0.49 (0.12-2.01) | 0.73 (0.31-1.73) | 1.09 (0.55-2.19) | 0.34 (0.19-0.58) |
| Cotrimoxazole use | 0.60 (0.13-2.76) | 0.52 (0.20-1.34) | 0.81 (0.43-1.51) | 0.92 (0.59-1.43) |
Proportional hazards assumption violated model stratified by enrolment CD4 count and year of recruitment.
Acknowledgements
The Wellcome Trust, UK, funded the project through fellowship support to NF (grant no. 061230). SBG (grant no.061231) and MM (grant no. 058390) also received Wellcome Trust support. Wyeth pharmaceuticals provided Prevenar through an Investigator originated protocol agreement and donated vaccine for placebo recipients following release of results.
We would like to thank Keith Klugman (chair), James Whitworth (former chair) Helena Makela, John Macfarlane, Di Gibb, Ed Wilkins and Henry Mwandumba for participation on the Trial Steering group and Tim Peto, Johnstone Kumwenda and Andrew Nunn for their role as the Data and Safety Monitoring Board. We also thank Rose Malamba, Marie Kunkeyani, Neema Mtunthama, Mary Matemba and the other staff of the adult wards of the Queen Elizabeth Central Hospital for their essential contributions. Further thanks to Anne von Gottberg at the Respiratory and Meningeal Pathogens Research unit, Johannesburg, South Africa; Steve Graham and Michael Boele van Hensbroek for vaccine/ placebo blinding; Helen Tolmie at the Liverpool School of Tropical Medicine and Pontiano Kaleebu at the Medical Research Council/Uganda Virus Research Institute, Entebbe, Uganda.
Footnotes
Disclosure statement
Dr French has sat on adult vaccine advisory panels for Glaxo Smith Kline and Novartis and received honoraria for this work. Drs Gordon, Mwalukomo, White, Mwaiponya, Ziljstra, Molyneux, Gilks and Messrs Longwe and Mwafulirwa have no financial conflicts.
Presented in part at the 6th International Symposium on the Pneumococcus and Pneumococcal disease, Reykjavik, Iceland, June 2008.
References
- 1.Gilks CF, Ojoo SA, Ojoo JC, et al. Invasive pneumococcal disease in a cohort of predominantly HIV-1 infected female sex-workers in Nairobi, Kenya. Lancet. 1996;347:718–723. doi: 10.1016/s0140-6736(96)90076-8. [DOI] [PubMed] [Google Scholar]
- 2.Klugman KP, Madhi SA, Feldman C. HIV and pneumococcal disease. Curr Opin Infect Dis. 2007;20:11–15. doi: 10.1097/QCO.0b013e328012c5f1. [DOI] [PubMed] [Google Scholar]
- 3.Janoff EN, Breiman RF, Daley CL, Hopewell PC. Pneumococcal disease during HIV infection. Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992;117:314–324. doi: 10.7326/0003-4819-117-4-314. [DOI] [PubMed] [Google Scholar]
- 4.Heffernan RT, Barrett NL, Gallagher KM, et al. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995-2000. J Infect Dis. 2005;191:2038–2045. doi: 10.1086/430356. [DOI] [PubMed] [Google Scholar]
- 5.French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355:2106–2111. doi: 10.1016/s0140-6736(00)02377-1. [DOI] [PubMed] [Google Scholar]
- 6.Grau I, Pallares R, Tubau F, et al. Epidemiologic changes in bacteremic pneumococcal disease in patients with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Intern Med. 2005;165:1533–1540. doi: 10.1001/archinte.165.13.1533. [DOI] [PubMed] [Google Scholar]
- 7.Scarborough M, Gordon SB, Whitty CJ, et al. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med. 2007;357:2441–2450. doi: 10.1056/NEJMoa065711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec. 2008;83:373–384. [PubMed] [Google Scholar]
- 9.Masur H, Kaplan JE, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons--2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Ann Intern Med. 2002;137:435–478. doi: 10.7326/0003-4819-137-5_part_2-200209031-00002. [DOI] [PubMed] [Google Scholar]
- 10.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien KL, Moulton LH, Reid R, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003;362:355–361. doi: 10.1016/S0140-6736(03)14022-6. [DOI] [PubMed] [Google Scholar]
- 13.Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–1146. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 14.Madhi SA, Adrian P, Kuwanda L, et al. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine. 2006 doi: 10.1016/j.vaccine.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine. 2001;20:545–553. doi: 10.1016/s0264-410x(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 16.Miiro G, Kayhty H, Watera C, et al. Conjugate pneumococcal vaccine in HIV-infected Ugandans and the effect of past receipt of polysaccharide vaccine. J Infect Dis. 2005;192:1801–1805. doi: 10.1086/497144. [DOI] [PubMed] [Google Scholar]
- 17.Gordon SB, Kayhty H, Molyneux ME, et al. Pneumococcal conjugate vaccine is immunogenic in lung fluid of HIV-infected and immunocompetent adults. J Allergy Clin Immunol. 2007;120:208–210. doi: 10.1016/j.jaci.2007.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon SB, Kanyanda S, Walsh AL, et al. Poor potential coverage for 7-valent pneumococcal conjugate vaccine, Malawi. Emerg Infect Dis. 2003;9:747–749. doi: 10.3201/eid0906.030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon SB, Chaponda M, Walsh AL, et al. Pneumococcal disease in HIV-infected Malawian adults: acute mortality and long-term survival. AIDS. 2002;16:1409–1417. doi: 10.1097/00002030-200207050-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–1502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 21.Klugman KP, Cutts FT, Adegbola RA, Black S, Madhi SA, O'Brien KL, et al. Meta-analysis of the efficacy of conjugate vaccines against invasive pneumococcal disease. In: Siber G, Klugman KP, Makela H, editors. Pneumococcal vaccines: the impact of conjugate vaccine. American Society for Microbiology; Washington: 2008. pp. 317–326. [Google Scholar]
- 22.Piantadosi S. Clinical Trials: A Methodologic Perspective. New York: 1997. p. 248. [Google Scholar]
- 23.Brichachek B, Swindells S, Janoff EN, Pirrucello S, Stevenson M. Increased plasma Human Immunodeficiency Virus type 1 burden following antigenic challenge with pneumococcal vaccine. J Infect Dis. 1996;174:1191–1199. doi: 10.1093/infdis/174.6.1191. [DOI] [PubMed] [Google Scholar]
- 24.Anglaret X, Toure S, Gourvellec G, et al. Impact of vital status investigation procedures on estimates of survival in cohorts of HIV-infected patients from Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2004;35:320–323. doi: 10.1097/00126334-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 25.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 26.Yu JK, Chen SC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flannery B, Heffernan RT, Harrison LH, et al. Changes in invasive Pneumococcal disease among HIV-infected adults living in the era of childhood pneumococcal immunization. Ann Intern Med. 2006;144:1–9. doi: 10.7326/0003-4819-144-1-200601030-00004. [DOI] [PubMed] [Google Scholar]
- 29.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.