Abstract
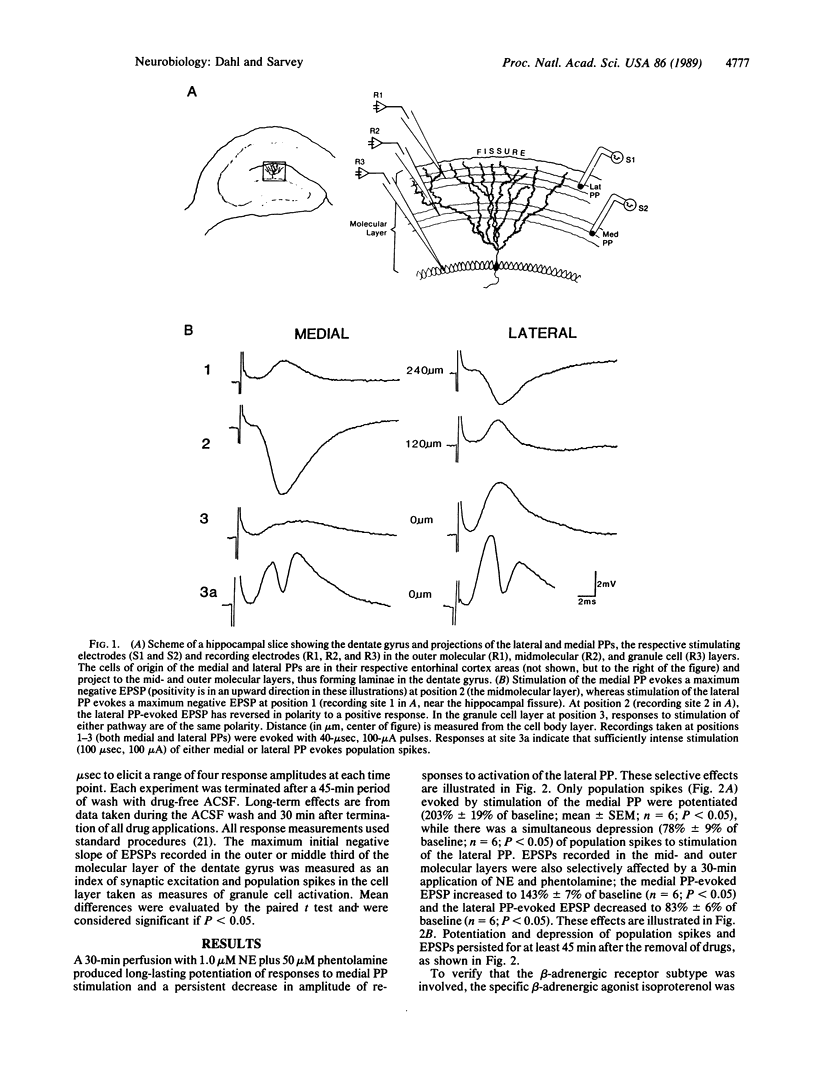
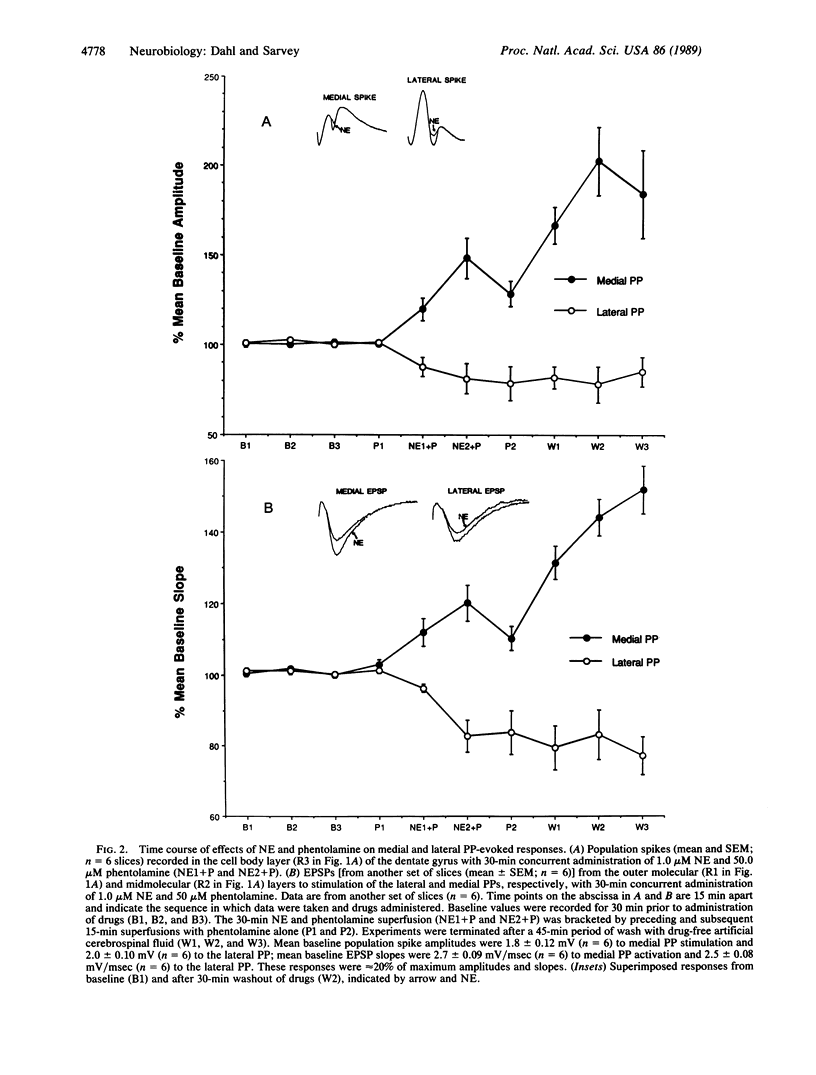
The study presented here indicates that norepinephrine (NE) selectively induces long-lasting modifications of synaptically mediated responses in the dentate gyrus of the rat hippocampal slice. A low concentration of NE (1.0 microM; in the presence of 50 microM phentolamine, an alpha-adrenergic antagonist) or a 1.0 microM concentration of the specific beta-adrenergic agonist isoproterenol induced long-lasting pathway-specific alterations of granule cell electrophysiological responses. Excitatory postsynaptic potentials and population spikes evoked by stimulation of the medial perforant pathway (PP) were potentiated for more than 45 min. In contrast, responses to lateral PP stimulation were depressed for the same period. Both potentiation and depression were blocked by the beta-adrenergic antagonist propranolol (1.0 microM). These results indicate that NE can act differentially on projections to the dentate gyrus arising in the entorhinal cortex. Such selective persistent modifications of cortical circuits may be involved in processes in the mammalian brain underlying attention, learning, and memory.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. C., Goddard G. V. Asymmetric relationships between homosynaptic long-term potentiation and heterosynaptic long-term depression. Nature. 1983 Oct 20;305(5936):717–719. doi: 10.1038/305717a0. [DOI] [PubMed] [Google Scholar]
- Andersen P., Holmqvist B., Voorhoeve P. E. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966 Apr;66(4):448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Bloom F. E. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981 Aug;1(8):887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear M. F., Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986 Mar 13;320(6058):172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Beckstead R. M. Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell-labeling with horseradish peroxidase. Brain Res. 1978 Aug 25;152(2):249–264. doi: 10.1016/0006-8993(78)90254-8. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Goddard G. V., Riives M. Reduction of long-term potentiation in the dentate gyrus of the rat following selective depletion of monoamines. J Physiol. 1983 Jan;334:475–491. doi: 10.1113/jphysiol.1983.sp014507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard E. C., Decker G., Sarvey J. M. NMDA receptor antagonists block norepinephrine-induced long-lasting potentiation and long-term potentiation in rat dentate gyrus. Brain Res. 1989 Mar 20;482(2):351–355. doi: 10.1016/0006-8993(89)91199-2. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G. Olfactory and other afferent connections of the hippocampus in the rabbit, rat, and cat. Exp Neurol. 1961 Jun;3:588–600. doi: 10.1016/s0014-4886(61)80007-1. [DOI] [PubMed] [Google Scholar]
- Collier T. J., Gash D. M., Sladek J. R., Jr Transplantation of norepinephrine neurons into aged rats improves performance of a learned task. Brain Res. 1988 May 10;448(1):77–87. doi: 10.1016/0006-8993(88)91103-1. [DOI] [PubMed] [Google Scholar]
- Dahl D., Bailey W. H., Winson J. Effect of norepinephrine depletion of hippocampus on neuronal transmission from perforant pathway through dentate gyrus. J Neurophysiol. 1983 Jan;49(1):123–133. doi: 10.1152/jn.1983.49.1.123. [DOI] [PubMed] [Google Scholar]
- Dahl D., Winson J. Action of norepinephrine in the dentate gyrus. I. Stimulation of locus coeruleus. Exp Brain Res. 1985;59(3):491–496. doi: 10.1007/BF00261339. [DOI] [PubMed] [Google Scholar]
- Deadwyler S. A., West M. O., Robinson J. H. Entorhinal and septal inputs differentially control sensory-evoked responses in the rat dentate gyrus. Science. 1981 Mar 13;211(4487):1181–1183. doi: 10.1126/science.7466392. [DOI] [PubMed] [Google Scholar]
- Deadwyler S. A., West M. O., Robinson J. H. Evoked potentials from the dentate gyrus during auditory stimulus generalization in the rat. Exp Neurol. 1981 Mar;71(3):615–624. doi: 10.1016/0014-4886(81)90036-4. [DOI] [PubMed] [Google Scholar]
- Everitt B. J., Robbins T. W., Gaskin M., Fray P. J. The effects of lesions to ascending noradrenergic neurons on discrimination learning and performance in the rat. Neuroscience. 1983 Oct;10(2):397–410. doi: 10.1016/0306-4522(83)90142-2. [DOI] [PubMed] [Google Scholar]
- Harley C. W. A role for norepinephrine in arousal, emotion and learning?: limbic modulation by norepinephrine and the Kety hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11(4):419–458. doi: 10.1016/0278-5846(87)90015-7. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A. Projection of the lateral part of the entorhinal area to the hippocampus and fascia dentata. J Comp Neurol. 1972 Oct;146(2):219–232. doi: 10.1002/cne.901460206. [DOI] [PubMed] [Google Scholar]
- Hopkins W. F., Johnston D. Frequency-dependent noradrenergic modulation of long-term potentiation in the hippocampus. Science. 1984 Oct 19;226(4672):350–352. doi: 10.1126/science.6091272. [DOI] [PubMed] [Google Scholar]
- Jones B. E., Yang T. Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985 Dec 1;242(1):56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T. Norepinephrine hypothesis for visual cortical plasticity: thesis, antithesis, and recent development. Curr Top Dev Biol. 1987;21:367–389. doi: 10.1016/s0070-2153(08)60144-1. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A., Bear M. F., Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Koerner J. F., Cotman C. W. Micromolar L-2-amino-4-phosphonobutyric acid selectively inhibits perforant path synapses from lateral entorhinal cortex. Brain Res. 1981 Jul 6;216(1):192–198. doi: 10.1016/0006-8993(81)91288-9. [DOI] [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol. 1977 Apr 15;172(4):723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- Lacaille J. C., Harley C. W. The action of norepinephrine in the dentate gyrus: beta-mediated facilitation of evoked potentials in vitro. Brain Res. 1985 Dec 9;358(1-2):210–220. doi: 10.1016/0006-8993(85)90965-5. [DOI] [PubMed] [Google Scholar]
- Lanthorn T. H., Cotman C. W. Baclofen selectively inhibits excitatory synaptic transmission in the hippocampus. Brain Res. 1981 Nov 23;225(1):171–178. doi: 10.1016/0006-8993(81)90326-7. [DOI] [PubMed] [Google Scholar]
- Mason S. T., Fibiger H. C. Noradrenaline and spatial memory. Brain Res. 1978 Nov 10;156(2):382–386. doi: 10.1016/0006-8993(78)90524-3. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Barnes C. A. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J Comp Neurol. 1977 Oct 15;175(4):439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- Rainbow T. C., Parsons B., Wolfe B. B. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Wester K. Long-lasting facilitation of a synaptic potential following tetanization in the in vitro hippocampal slice. Brain Res. 1975 May 16;89(1):107–119. doi: 10.1016/0006-8993(75)90138-9. [DOI] [PubMed] [Google Scholar]
- Smejda Haug F. M. Heavy metals in the brain. A light microscope study of the rat with Timm's sulphide silver method. Methodological considerations and cytological and regional staining patterns. Adv Anat Embryol Cell Biol. 1973;47(4):1–71. [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. Blockade of norepinephrine-induced long-lasting potentiation in the hippocampal dentate gyrus by an inhibitor of protein synthesis. Brain Res. 1985 Dec 30;361(1-2):276–283. doi: 10.1016/0006-8993(85)91299-5. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J Neurosci. 1985 Aug;5(8):2169–2176. doi: 10.1523/JNEUROSCI.05-08-02169.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. Norepinephrine regulates long-term potentiation of both the population spike and dendritic EPSP in hippocampal dentate gyrus. Brain Res Bull. 1987 Jan;18(1):115–119. doi: 10.1016/0361-9230(87)90039-6. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. The effect of high-frequency electrical stimulation and norepinephrine on cyclic AMP levels in normal versus norepinephrine-depleted rat hippocampal slices. Brain Res. 1985 Dec 9;358(1-2):343–348. doi: 10.1016/0006-8993(85)90981-3. [DOI] [PubMed] [Google Scholar]
- Stein L., Belluzzi J. D., Wise C. D. Memory enhancement by central administration of norepinephrine. Brain Res. 1975 Feb 7;84(2):329–335. doi: 10.1016/0006-8993(75)90989-0. [DOI] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976 Jun 1;167(3):285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B., Huang Y. Y. Mode of action of excitatory amino acid receptor antagonists on hippocampal long-lasting potentiation. Neuroscience. 1986 Apr;17(4):1105–1115. doi: 10.1016/0306-4522(86)90080-1. [DOI] [PubMed] [Google Scholar]
- Wilson R. C., Steward O. Polysynaptic activation of the dentate gyrus of the hippocampal formation: an olfactory input via the lateral entorhinal cortex. Exp Brain Res. 1978 Nov 15;33(3-4):523–534. doi: 10.1007/BF00235572. [DOI] [PubMed] [Google Scholar]
- Winson J., Dahl D. Long-term potentiation in dentate gyrus: induction by asynchronous volleys in separate afferents. Science. 1986 Nov 21;234(4779):985–988. doi: 10.1126/science.3775372. [DOI] [PubMed] [Google Scholar]
- Winson J. Influence of raphe nuclei on neuronal transmission from perforant pathway through dentate gyrus. J Neurophysiol. 1980 Nov;44(5):937–950. doi: 10.1152/jn.1980.44.5.937. [DOI] [PubMed] [Google Scholar]



