Abstract
There is currently no means of primary prevention for prostate cancer. Increased exposure to ultraviolet-radiation may be protective, but the literature is inconclusive. We investigated associations of life-course exposure to sunlight with prostate cancer. The study design was a UK-wide nested case-control study, based on 1,020 PSA-detected cases and 5,044 matched population controls and a systematic review with meta-analysis. Men with olive/brown skin (OR= 1.47; 95% CI: 1.00 to 2.17), men who burnt rarely/never (OR= 1.11; 0.95 to 1.29) and men with the lowest levels of intense sun exposure in the 2 years prior to diagnosis (OR = 1.24; 1.03 to 1.50) had an increased prostate cancer risk. However, amongst men with prostate cancer, spending less time outside was associated with a reduced risk of advanced cancer (OR = 0.49; 0.27 to 0.89) and high Gleason grade (OR = 0.62; 0.43 to 0.91), and men who burnt rarely/never had a reduced risk of advanced cancer (OR = 0.71; 0.47 to 1.08). The meta-analysis provided weak evidence that men with the lowest (versus highest) sunlight exposure had an increased prostate cancer risk (4 studies, random-effects pooled relative risk = 1.13; 0.98 to 1.29) and higher advanced or fatal prostate cancer risk (6 studies, random-effects pooled relative risk = 1.14; 0.98 to 1.33). Our data and meta-analyses provide limited support for the hypothesis that increased exposure to sunlight may reduce prostate cancer risk. The findings warrant further investigation because of their implications for vitamin D chemoprevention trials.
Keywords: Prostate cancer, sun exposure, pigmentation
Introduction
Worldwide over 220,000 men die annually from prostate cancer (1;2). Known risk factors are age, ethnicity and family history (3;4) but as these risk factors are not modifiable, control through primary prevention is not an option. It is, therefore, important to identify modifiable lifestyle or environmental factors that may decrease the risk of prostate cancer.
A number of ecological studies have reported an inverse correlation between sunlight levels and prostate cancer mortality (5;6). This association may reflect an inverse association of vitamin D with prostate cancer (7-10), because vitamin D status is strongly determined by the amount of sun exposure and the capacity of the skin to synthesise it and, in turn, vitamin D regulates cellular growth and differentiation (7;11). There is evidence from individual-based epidemiological studies that risk of cancer, including of the prostate, is decreased in men with high sun exposure (10;12;13), and that pigmentary characteristics which may inhibit vitamin D synthesis, such as dark skin (10;14) or tanning easily (15), are positively associated with prostate cancer. However, there is also contradictory ecological evidence that high levels of exposure to ultra-violet radiation are associated with an increased risk of prostate cancer mortality (16).
We tested the hypothesis that increased sun exposure during the life-course is associated with reduced prostate cancer risk and that the association would be stronger for advanced versus localised disease. We also tested the hypothesis that characteristics which would lead to increased sun exposure, having fair skin, light hair or a tendency to burn easily, would be associated with reduced prostate cancer risk. The analysis is based on a case-control study nested within the UK-wide population-based phase of prostate cancer detection for a multi-centre randomised controlled trial of treatments for localised disease: the Prostate Testing for Cancer and Treatment (ProtecT) study (17). As well as being large (n = 1,020 men with prostate cancer eligible for the current analysis) and with nationwide, population-based coverage, a novel perspective provided by this study is that we have measures of reported skin pigmentation at age 20 and sun exposure in both childhood and adulthood, representing a life-course assessment of exposure. In addition, given the absence of an up-to-date review of the existing evidence, we carried out a systematic review and meta-analysis of comparable studies in order to place our results in the context of the worldwide literature.
Materials and Methods
Case Control Analysis
During recruitment to the ProtecT study (between 2001 and 2008), men aged 50-69 years at 400 general practices in nine UK centres (Birmingham, Bristol, Cambridge, Cardiff, Edinburgh, Leeds, Leicester, Newcastle, Sheffield) were offered a PSA test at a community-based ‘prostate check clinic’, and those with raised levels (≥ 3 ng/ml) were offered diagnostic biopsy. Detected tumours were all histology-confirmed and clinically staged using the TNM system (18). Cancer stages T1-T2 and NXM0 were categorised as ‘localised’; and T3-T4 and N1 or M1 as ‘advanced’. ‘High grade’ tumours were defined as a Gleason score ≥ 7, after review by a pathologist. All participants in ProtecT who had no evidence of prostate cancer were eligible for selection as controls; that is, men with a PSA test < 3ng/ml or a raised PSA (≥ 3 ng/ml) combined with at least one negative biopsy and no subsequent prostate cancer diagnosis during the follow-up protocol for negative biopsies. Six randomly selected controls were stratum-matched to each case by five-year age band (age at PSA test) and GP/recruitment centre. All men provided written informed consent prior to inclusion in the study. Trent Multicentre Research Ethics Committee approved the study.
Exposure Data
A self-administered diet, health and lifestyle questionnaire, administered at the prostate check clinic (i.e. typically completed prior to knowledge of the PSA level or diagnosis), included questions about pigmentation (skin reaction to sun, and skin and hair colour) and behaviours affecting the level of sun exposure, such as time spent in the sun and type of sun protection used. These questions were based on questionnaires used in the South Wales Skin Cancer study (19;20), the Nurses Health Study Sun Exposure Questionnaire (21), the Queensland study of childhood melanoma (22;23) and the Queensland melanoma case control study (24;25). The questions split the life-course into three segments: childhood (0-19 years), mid-adulthood (20-49 years) and exposure in the two years prior to attending the prostate check clinic. The variables derived are described briefly below. Further details of individual questions and the variables derived from the original questions are shown in Appendix 1.
‘Time spent outside in summer’ was derived by summing the weighted number of hours spent outside in the summer across different ages (5-12, 13-19, 20-29, 30-39, 40-49 and 50-69 years) (weights: < 1hour/day = 0.5 hours; 1-4hours = 2 hours; > 4hours = 6 hours) (26). Missing answers were assigned to zero. The distribution of this score was then split into thirds and labelled ‘low’, ‘medium’ and ‘high’, where ‘high’ indicates increased sun exposure. Separate scores for childhood (5-19 years) and adult (20-69) exposure were also calculated using the same method. Sensitivity analyses on the following categories of men made very little difference to results and are not reported here: a) men with complete data; b) all men, but using imputed missing values if more than half the data on hours spent outside for the man were recorded; c) all men, but using imputed missing values so long as at least one data item on hours spent outside for the man was recorded. For the sensitivity analyses reported above, the method of deriving imputed values was to replace missing answers to questions with the mean of the non-missing answers.
A measure of ‘intense sun exposure’ was derived by summing time spent sunbathing, time away from home on holiday and time spent in foreign countries, in childhood (0-19), adulthood (20-49), the two years prior to prostate clinic, and overall. ‘High’ intense sun exposure indicates increased sun exposure. A missing answer was equal to zero; however scores were not calculated if more than half of the answers were missing. Separate questions to capture the number of times men had, at different ages, been severely sunburned, or how often on average they had used a sun-bed in an average year, were also analysed.
The men were asked to self-report which of the following categories best described their ethnic group: white, African-Caribbean, Black British, Indian, Pakistani, Bangladeshi, Chinese, Other. Measures of physical activity, smoking, family history of prostate cancer and occupational social class were derived from a questionnaire, administered at the prostate check clinic.
Statistical Analysis
To allow for the matched sets of cases and controls, conditional logistic regression, controlling for exact age (years) at prostate clinic attendance, was used to estimate odds ratios (OR) and 95% confidence intervals (CI) quantifying the association between sun exposures and all prostate cancers. Analyses of the association between pigmentation and prostate cancer were further adjusted for sunscreen use throughout life, and analyses of the association between sun exposure and prostate cancer had additional adjustment for sunscreen use, usual reaction of skin to sunlight, skin colour and hair colour, as these factors can affect the amount of vitamin D absorbed during sun exposure.
A subanalysis using unconditional logistic regression assessed the associations of life course sun exposure and usual reaction of skin to sunlight with stage and Gleason grade in men with prostate cancer. Analyses were carried out in Stata 10 (StataCorp, 2007. College Station, TX).
Systematic Literature Review
A systematic literature review was carried out using a MEDLINE search from 1966 to 10th April 2008 that combined the key words “sunlight”, “ultraviolet rays”, “sun exposure” or “sunburn” with “prostate cancer” or “prostatic neoplasms”. The abstracts of papers identified using this search were read and all epidemiological studies and review papers relating a measure of sun exposure to prostate cancer, published in English, were identified. Papers that were either prostate-only or where prostate cancer was one of multiple cancers analysed were both considered for inclusion. Additional reports of epidemiological studies were identified from a manual search of the reference lists of retrieved papers. Epidemiological studies with measures comparable to our ‘time spent outside’ variable, and which related these variables to prostate cancer risk, were included in a meta-analysis. As there was evidence of statistical heterogeneity between studies, effect-estimates from the maximally-adjusted models presented from each individual study were pooled using random-effects models (although results from fixed-effects models were similar). We calculated the I2 value as a quantitative measure of the degree of inconsistency across studies that is not dependent on the number of studies (27), where 0% indicates no observed heterogeneity, and larger percentages show increasing heterogeneity. We identified instances where more than one publication arose from the same study by reviewing study name, authors, location, study population, dates and study design.
Results
Case Control Analysis
By November 2006, 55,172 men had received a PSA test; 5,873 had a raised PSA and 1,933 men had prostate cancer. The questionnaire was given to 39,856 men, of whom 9,274 were selected for potential inclusion in a ProtecT-based case-control series (1,374 men with prostate cancer). The questionnaire was completed by 1,153 (84% response) histology-confirmed cases and 5,821 (74% response) controls. The mean age of responders was 62.4 years versus 61.3 years amongst non-responders, 48.0% of responders were in a manual social class versus 56.0% of non-responders, and 7.7% of responders had a family history of prostate cancer versus 6.4% of non-responders.
The main analysis was carried out on the 1,020 cases (116 with advanced prostate cancer, 317 with high Gleason grade) and 5,044 matched controls who answered at least one of the sun exposure questions. 84% of participants had recorded ethnicity, of whom 5,743 men (99%) self-identified as white. 57.8% of the men with missing ethnicity described themselves as having fair/pale skin colour, which suggests that these men are predominantly white. A sensitivity analysis was carried out, based on 589 cases and 4,551 controls who self-identified as white and had sun exposure data; the results based on these men were very similar to the results based on all 1,020 cases and their controls.
There were no substantial differences between cases and the controls, except that 7.8% of cases had a family history of prostate cancer versus 5.4% controls (p<0.01) and slightly fewer of the cases had a smoking history (64.2% cases, 67.8% controls; p=0.03). The mean age of the cases was 62.5 years versus 62.3 years amongst controls, and 49% of cases were in a manual social class versus 47% of controls.
The median PSA in cases was 5.06 ng/ml (inter-quartile range 3.7-8) and in controls was 1 ng/ml (inter-quartile range 0.6-1.7). Approximately 90% of men had recorded where they mostly lived during different age ranges. Of these, only between 0.85% and 6.37% lived outside the UK (Age 5-12 years: 3.00%; 13-19 years: 3.27%; 20-29 years: 6.37%; 30-39 years: 3.19%; 40-49 years: 2.00%; 50-69 years: 0.85%).
Looking at the associations between the exposure variables, men with medium or olive/brown skin were less likely to burn (2.8%) than men with fair/pale skin (54.3%), as was true also for those with dark hair (27.1%) compared to those with light hair (48.1%). Higher proportions of fair/pale-skinned men than olive/brown-skinned men consistently reported always/mostly protecting their skin from the sun (mid-adulthood: 53.7% vs.38.4%). Similar sun protection patterns were seen among men who burned readily compared to those who burnt rarely/never (mid-adulthood: 59.9% vs. 44.6%). Light haired men were only slightly more likely than dark haired men to protect their skin (mid-adulthood: 51.8% vs. 48.7%). All p-values for the preceding comparisons were ≤ 0.01.
For associations of measures of pigmentation with prostate cancer, we found none for hair colour, a modest trend for men with olive/brown skin (p for trend = 0.19) and similarly for men who burn rarely or never (p for trend = 0.18) to have a higher odds of prostate cancer (Table 1). Men who burnt rarely were slightly more likely to have localised tumours (p = 0.11) but there was no association with tumour grade (Table 2).
Table 1.
Associations of prostate cancer with measures of pigmentation and measures of sun exposure in childhood, adulthood and throughout the life course
| Controls n (%) |
All Cancers n (%) |
Age, sunscreen use and pigmentation* adjusted |
|||
|---|---|---|---|---|---|
| OR | 95% CI | p-trend | |||
| 5,044 | 1,020 | ||||
| Usual reaction of skin when first exposed to sunlight | |||||
| Always/easily burns | 1,910 (38.5) | 358 (35.8) | 1 | ||
| Burns rarely/never | 3,055 (61.5) | 641 (64.2) | 1.11 | (0.95,1.29) | 0.18 |
| Skin colour at age 20 | |||||
| Fair/pale | 2,978 (59.8) | 577 (57.5) | 1 | ||
| Medium | 1,857 (37.3) | 386 (38.5) | 1.03 | (0.89,1.20) | |
| Olive/brown | 142 (2.9) | 41 (4.1) | 1.47 | (1.00,2.17) | 0.19 |
| Natural hair colour at age 20 | |||||
| Light | 2,578 (51.9) | 511 (51.1) | 1 | ||
| Dark | 2,391 (48.1) | 489 (48.9) | 1.00 | (0.87,1.16) | 0.96 |
|
| |||||
| Time spent Outside | |||||
| Lifecourse | |||||
| High | 1,826 (37.3) | 382 (38.7) | 1 | ||
| Medium | 1,880 (38.4) | 355 (35.9) | 0.91 | (0.77,1.08) | |
| Low | 1,196 (24.4) | 251 (25.4) | 0.98 | (0.81,1.19) | 0.72 |
| Childhood (5-19 years) | |||||
| High | 3,301 (68.0) | 656 (67.4) | 1 | ||
| Medium | 423 (8.7) | 91 (9.4) | 1.10 | (0.86,1.42) | |
| Low | 1,128 (23.3) | 226 (23.2) | 0.98 | (0.82,1.17) | 0.93 |
| Adulthood (20-69 years) | |||||
| High | 1,682 (34.5) | 350 (35.6) | 1 | ||
| Medium | 1,170 (24.0) | 221 (22.5) | 0.90 | (0.74,1.09) | |
| Low | 2,030 (41.6) | 412 (41.9) | 0.96 | (0.81,1.15) | 0.71 |
|
| |||||
| Intense Sun Exposure | |||||
| Life course | |||||
| High | 1,285 (27.1) | 265 (27.8) | 1 | ||
| Middle | 2,508 (52.9) | 472 (49.5) | 0.94 | (0.79,1.12) | |
| Low | 948 (20.0) | 217 (22.8) | 1.14 | (0.92,1.42) | 0.32 |
| Childhood (0-19 years) | |||||
| High | 457 (9.5) | 88 (9.1) | 1 | ||
| Middle | 2,149 (44.6) | 450 (46.5) | 1.08 | (0.84,1.40) | |
| Low | 2,215 (45.9) | 430 (44.4) | 1.08 | (0.78,1.32) | 0.72 |
| Adulthood (20-49 years) | |||||
| High | 2,189 (45.3) | 447 (46.3) | 1 | ||
| Middle | 1,865 (38.6) | 347 (35.9) | 0.92 | (0.78,1.08) | |
| Low | 779 (16.1) | 172 (17.8) | 1.12 | (0.90,1.38) | 0.61 |
| Last 2 years** | |||||
| High | 2,426 (49.7) | 473 (48.0) | 1 | ||
| Middle | 1,407 (28.8) | 270 (27.4) | 0.99 | (0.84,1.18) | |
| Low | 1,051 (21.5) | 243 (24.7) | 1.24 | (1.03,1.50) | 0.04 |
|
| |||||
| Number of times severly sunburnt | |||||
| Childhood (0-19 years) | |||||
| none | 2,868 (58.4) | 596 (60.3) | 1 | ||
| 1 | 1,065 (21.7) | 209 (21.1) | 0.98 | (0.82,1.18) | |
| ≥ 2 | 978 (16.5) | 184 (18.6) | 0.97 | (0.79,1.18) | 0.72 |
| Adulthood (20-49 years) | |||||
| none | 2,562 (52.0) | 526 (52.8) | 1 | ||
| 1 | 1,398 (28.4) | 280 (28.1) | 1.01 | (0.85,1.20) | |
| ≥ 2 | 966 (19.6) | 190 (19.1) | 0.98 | (0.81,1.19) | 0.89 |
| Last 2 years** | |||||
| none | 4,660 (94.6) | 940 (94.3) | 1 | ||
| 1 | 211 (4.3) | 46 (4.6) | 1.13 | (0.80,1.59) | |
| ≥ 2 | 56 (1.1) | 11 (1.1) | 0.83 | (0.40,1.72) | 0.92 |
|
| |||||
| Number of sessions on sunbeds per year | |||||
| Childhood (0-19 years) | |||||
| none | 4,839 (97.7) | 973 (97.2) | 1 | ||
| 1 | 23 (0.5) | 4 (0.4) | 0.89 | (0.30,2.65) | |
| ≥ 2 | 89 (1.8) | 24 (2.4) | 1.30 | (0.81,2.08) | 0.32 |
| Adulthood (20-49 years) | |||||
| none | 4,349 (87.8) | 884 (88.5) | 1 | ||
| 1 | 172 (3.5) | 33 (3.3) | 0.95 | (0.63,1.41) | |
| ≥ 2 | 435 (8.8) | 82 (8.2) | 0.95 | (0.73,1.23) | 0.64 |
| Last 2 years** | |||||
| none | 4,773 (96.1) | 965 (96.1) | 1 | ||
| 1 | 33 (0.7) | 7 (0.7) | 0.93 | (0.38,2.26) | |
| ≥ 2 | 163 (3.3) | 32 (3.2) | 0.94 | (0.63,1.41) | 0.74 |
Usual reaction of skin when first exposed to sunlight, natural hair colour, skin colour at age 20. Measures of pigmentation adjusted for age and sunscreen use only.
Prior to the prostate check clinic at ages 50-69
Table 2.
Associations of prostate cancer stage and Gleason grade with the usual reaction of the skin when first exposed to sunlight and measures of sun exposure throughout the life course (odds ratios compare localised versus advanced or low versus high grade cancers)
| Localised | Advanced | Age, sunscreen use and pigmentation* adjusted |
Low Grade (<7) |
High Grade (≥ 7) |
Age, sunscreen use and pigmentation* adjusted |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | OR | 95% CI | p-trend | n (%) | n (%) | OR | 95% CI | p-trend | |
| 899 | 116 | 686 | 317 | |||||||
| Usual reaction of skin when first exposed to sunlight | ||||||||||
| Always/easily burns | 308 (35.0) | 48 (42.5) | 1 | 242 (35.9) | 111 (36.0) | 1 | ||||
| Burns rarely/never | 573 (65.0) | 65 (57.5) | 0.71 | (0.47,1.08) | 0.11 | 432 (64.1) | 197 (64.0) | 0.98 | (0.72,1.31) | 0.87 |
| Time spent Outside (5-69 years) | ||||||||||
| High | 331 (38.0) | 50 (45.1) | 1 | 245 (36.7) | 130 (42.9) | 1 | ||||
| Medium | 311 (35.7) | 42 (37.8) | 0.93 | (0.59,1.47) | 241(36.1) | 107 (35.3) | 0.89 | (0.64,1.23) | ||
| Low | 230 (26.4) | 19 (17.1) | 0.49 | (0.27,0.89) | 0.03 | 182 (27.3) | 66 (21.8) | 0.62 | (0.43,0.91) | 0.02 |
| Intense Sun Exposure (0-last 2 years**) | ||||||||||
| High | 237 (28.1) | 27 (25.5) | 1 | 173 (27.0) | 87 (29.4) | 1 | ||||
| Middle | 419 (49.7) | 52 (49.1) | 0.95 | (0.57,1.58) | 327 (51.0) | 138 (46.6) | 0.81 | (0.58,1.14) | ||
| Low | 187 (22.2) | 27 (25.5) | 1.00 | (0.54,1.83) | 0.98 | 141 (22.0) | 71 (24.0) | 0.87 | (0.54,1.32) | 0.48 |
Usual reaction of skin when first exposed to sunlight, natural hair colour, skin colour at age 20
Prior to the prostate check clinic at ages 50-69
Considering reported levels of sun exposure, overall associations of sun or sunbed exposure in childhood, adulthood and throughout the life course with prostate cancer occurrence were uniformly close to the null, with the minor exception of those men with the lowest levels of intense sun exposure in the 2 years prior to their prostate clinic check, who had an increased risk of prostate cancer (OR = 1.24, p for trend = 0.04) (Table 1).
Amongst men with prostate cancer, those who spent less time outside over the lifecourse appeared to have a reduced risk of both advanced prostate cancer and high Gleason grade (Table 2). There was no evidence of associations between intense sun exposure and stage of cancer or Gleason grade.
Results were not attenuated with adjustment for sunscreen use (age adjusted only) and when additionally adjusted for family history of prostate cancer, smoking, physical activity and occupational social class.
Systematic Literature Review
The electronic MEDLINE search identified 103 papers. Based on a review of the title and abstract, the full text of 42 papers was retrieved for more detailed assessment against our inclusion criteria. Searching the references of these papers retrieved 12 more papers giving a total of 54. Of these, 15 were narrative review papers, six were ecological, five considered racial variation in prostate cancer risk, three related seasonality to prostate cancer, 11 reported only serum vitamin D levels and one was a methodological paper (deriving a summary score for sun exposure). In total, 13 papers reported on either sun exposure and / or skin type, but seven papers duplicated data present in others (15;28-33). The most recent analysis or those papers with the most relevant variables were included, providing six papers comparing sun exposure (34-39) and two comparing skin reaction to sun exposure (35;38) for inclusion in our meta-analyses (Table 3).
Table 3.
Summary of papers included in the meta-analysis
| Paper | Subjects | Methods | Measurement of sun exposure | Confounders adjusted for | Main results* |
|---|---|---|---|---|---|
| Cohort studies | |||||
| Robsahm, 2004 | 39,583 Norwegians, aged 25-94 years, diagnosed with prostate cancer |
Identified from Norway Cancer Registry between 1964 and 1992. 16,457 deaths from prostate cancer. |
Residential sun exposure, based on mean annual UV radiation calculated for region II-VIII relative to region I. |
age at diagnosis, birth cohort, period of diagnosis, stage of disease at diagnosis, occupational sun exposure, childbearing pattern, educational level. |
Relative risk (95% CI) of prostate cancer death, compared with lowest group of UV exposure (residing in region I): Region II = 1.00 (0.90-1.11); III = 1.02 (0.94-1.12); IV = 0.99 (0.90-1.08); V = 1.16 (1.06-1.28); VI = 0.98 (0.90-1.07); VII = 1.10 (1.02-1.21); VIII = 0.98 (0.88-1.07). p-trend = 0.28. |
| John, 2007 | 3528 non-hispanic white men aged 25- 74 years, sampled from US population, followed by enrollment into the NHANES I follow-up study cohort |
Initial baseline interview and dermatologic examination in 1971-1975. 161 cases of prostate cancer (59 fatal cases) with information on sun exposure. Identified from combination of self-report, hospital records & death certificates. |
Self-reported occupational or residential exposure |
age | Relative risk (95% CI) of prostate cancer, compared with the lowest exposure group (both never, rare or occasional) was: Incidence One frequent = 0.80 (0.52-1.24); Both frequent = 1.05 (0.70-1.58). Fatal incidence One frequent = 0.46 (0.21-1.02); Both frequent = 0.70 (0.35-1.40). |
| de Vries, 2007 | 13,541 white male skin cancer patients from southeastern part of the Netherlands |
Identifed from Eindhoven Cancer Registry, diagnosed since 1970 till 2005. 272 prostate cancer patients (61 stage III and IV cases). |
Rates of invasive prostate cancer in skin cancer patients (as a proxy for high sun exposure) compared with rates in general population. |
age, period of diagnosis | Standardized incidence ratio (95% CI) of prostate cancer, compared with general population: Incidence = 0.89 (0.78-0.99); Fatal incidence = 0.73 (0.56-0.94). |
| Case control studies | |||||
| Freedman, 2002 | 97,873 cases were deaths from prostate cancer in 24 states of US. 83,421 controls were deaths, excluding cancer and certain neurological diseases. |
Identified from National Cancer Institute, the National Institute for Occupational Safety and Health and the National Center for Health Statistics database of information recorded on death certificates between 1984 and 1995. |
Residential exposure, based on annual mean daily solar radiation for state reported as residence and birthplace, US Weather Bureau data |
age, sex, race, occupation, physical activity, socioeconomic status |
Odds ratios (95% CI) of prostate cancer mortality, compared to low exposure: Medium = 0.89 (0.86-0.91); High = 0.90 (0.87-0.93). |
| John, 2005 | 450 nonhispanic white cases of advanced prostate cancer, aged 40-79 years, from San Francisco regional cancer registry, during 1997-2000. 455 controls recruited by random digit dialling, matched by age. |
In-person inverviews and structured questionnaire, reflectometer measure. |
Sun exposure index, based on relative difference between constitutive and facultative skin pigmentation as a measure of cumulative lifetime sun exposure |
age, family history, month of pigmentation measurement |
Odds ratios (95% CI) of advanced prostate cancer, compared to lowest quintile (Q1): Q2 = 0.87 (0.58-1.30); Q3 = 0.80 (0.53-1.20); Q4 = 0.95 (0.64-1.42); Q5 = 0.51 (0.33-0.80). P-trend = 0.02. |
| Skin reaction to summer midday sun exposure |
age, family history, month of pigmentation measurement |
Odds ratios (95% CI) of advanced prostate cancer, compared to ‘moderate to severe sunburns’: Mild sunburns = 0.86 (0.65-1.14); No sunburns = 0.72 (0.46-1.11). P-trend = 0.1. |
|||
| Rukin, 2007 | 528 white cases of prostate cancer, mean age 70.2 years, from urology clinics at University Hospital North Staffordshire. 365 controls with benign prostatic hypertophy from same clinics. |
Self-administered questionnaires of lifetime UV exposure |
Average daily sun exposure (hrs per day) since age 20 |
age, skin type | Odds ratios (95% CI) of prostate cancer = 0.78 (0.72-0.85) |
| Skin type | Odds ratios (95% CI) of prostate cancer, compared to Q4 (readily tans): Q3 = 0.74 (0.51-1.07); Q2 = 0.73 (0.49-1.09); Q1 (never tans) = 0.47 (0.26-0.86). P-trend = 0.024. |
||||
Results as reported. For the meta-analysis, results are inverted to make scales comparable where necessary.
Our meta-analysis included the results for time spent outside over the life course from the ProtecT nested case control study for all prostate cancers and, separately, advanced cancers compared to controls. Where necessary, we reversed the scales of the presented results from other studies so that high sun exposure was the baseline category. Our meta-analysis suggested that, on average, men with the lowest exposure to sunlight (compared to men with the highest exposure) had a slightly increased risk of any incident prostate cancer (4 studies, 1,981 cases; random-effects pooled relative risk = 1.13; 95% CI: 0.98 to 1.29; I2 = 66.9%) (Figure 1) as well as of advanced or fatal prostate cancer versus no cancer (6 studies, 115,016 cases; random-effects pooled relative risk = 1.14; 95% CI: 0.98 to 1.33; I2 = 75.7%) (Figure 2). Excluding ProtecT made little difference to the pooled effects estimates which were 1.18 (95% CI: 1.04 to 1.35; I2 = 56.8%) for incident prostate cancer and 1.17 (95% CI: 1.03 to 1.32; I2 = 67.3%) for advanced or fatal prostate cancer. A sensitivity analysis, replacing the ProtecT results for ‘time spent outside’ with those for ‘intense sun exposure over the life course’, made little difference to the pooled effects estimates which were 1.19 (95% CI: 1.07 to 1.31; I2 = 39.6%) for incident prostate cancer and 1.17 (95% CI: 1.04 to 1.31; I2 = 60.3%) for advanced or fatal prostate cancer.
Figure 1.
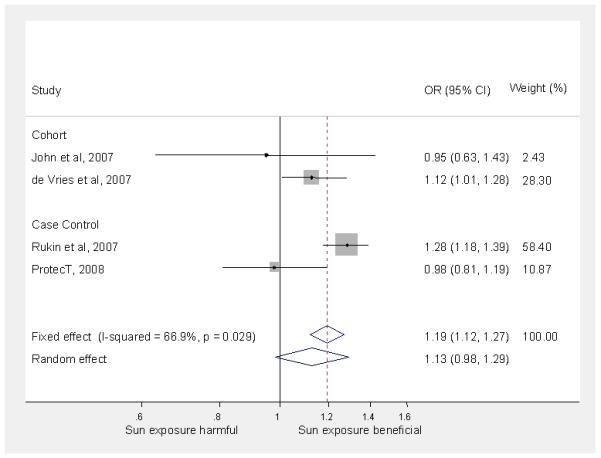
Meta-analysis of association of incident prostate cancer with sun exposure (low vs. high* sun exposure)
Figure 2.
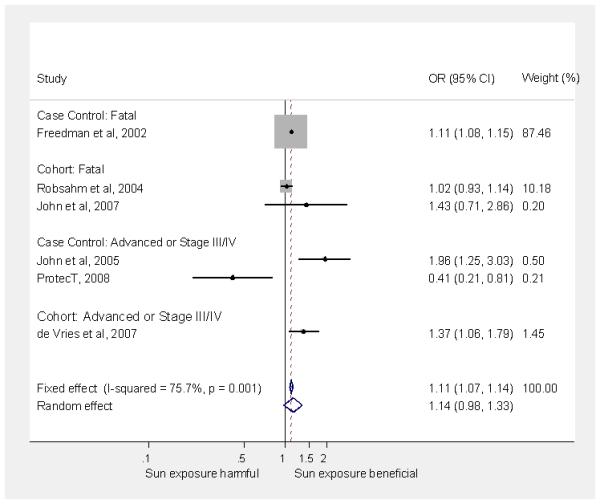
Meta-analysis of association of advanced or fatal prostate cancer (versus no cancer) with sun exposure (low vs. high sun exposure)
Meta-analysis of two studies (ProtecT plus Rukin et al (35) suggests that men who never burn versus those who easily burn have an increased risk of incident prostate cancer (1,117 cases; random-effects pooled relative risk = 1.44; 95% CI: 0.77 to 2.68; I2 = 76.6%). However, in a pooled analysis of two studies (ProtecT plus John et al (38), men who never burn versus those who easily burn had a lower risk of advanced prostate cancer (526 cases; random-effects pooled relative risk = 0.78; 95% CI: 0.57 to 1.05; I2 value =0.0%).
Discussion
Summary of Findings
Our Protect study provides only weak evidence to support the hypothesis that reduced levels of sun exposure could be a risk factor for the development of prostate cancer. Men with olive/brown skin, those who rarely burn and men with the lowest levels of intense sun exposure in the two years prior to attending the prostate check clinic, had an increased risk of all prostate cancers. Olive/brown skin and a tendency not to burn (in other words, a person tans easily), may reflect lower exposure to ultraviolet light of the epidermal layers of the skin where vitamin D production is greatest, hence reducing cutaneous vitamin D synthesis. In parallel with these findings, our meta-analysis of the available literature suggested that men with the lowest exposure to sunlight had an increased risk of both incident and advanced or fatal prostate cancer, although the confidence intervals for the random effects models included the null value, and there was evidence of heterogeneity. Amongst men with prostate cancer in ProtecT, those who burnt rarely or never and those who spent less time outside appeared to have an inverse association with advanced prostate cancer and/or those with a high Gleason grade; and a pooled analysis of two studies (ProtecT and one other) suggested weak evidence that men who burned rarely or never (a marker for reduced cutaneous vitamin D synthesis) had a positive association with prostate cancer overall, albeit an inverse one with advanced prostate cancer.
Sunlight exposure and incidence of prostate cancer
Vitamin D regulates calcium and phosphate levels as well as controlling cellular growth and differentiation (7) (11). The administration of vitamin D analogues slows the growth and inhibits the metastases of prostate tumours in animal models (40;41), and in small phase II trials synthetic vitamin D analogues slowed PSA-defined progression of prostate cancer (42). Some epidemiological studies have suggested a potential protective role of vitamin D against prostate cancer (7-9), although the evidence has been limited and conflicting (4). Since sunlight exposure (and subsequent conversion of vitamin D to circulating 25(OH)D) is the most important source of vitamin D in men, (43-45) these animal and clinical data would be consistent with an inverse association of sunlight and all prostate cancer risk.
Our results for men with prostate cancer, suggesting lower risk of advanced disease amongst men with exposures indicating reduced levels of cutaneous vitamin D synthesis, are more difficult to explain. In contrast with ProtecT, the point estimate from our random effects meta-analysis suggested that studies were, on average, consistent with men with the lowest exposure to sunlight having an increased risk of advanced or fatal prostate cancer. However, the pooled random effects model included the null value and there was moderate heterogeneity. Thus the causal association of sunlight exposure with advanced prostate cancer is far from clear.
ProtecT results amongst men with prostate cancer are in line with some other studies that have shown positive associations of high vitamin D levels with advanced prostate cancer. One study investigating serum levels of the two vitamin D metabolites, 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D found a positive association with both low 25-hydroxyvitamin D (OR = 1.5; 95% CI: 0.8-2.7) and high 25-hydroxyvitamin D levels (OR = 1.7, 95% CI: 1.1-2.4) (46) suggesting that both low and high levels of vitamin D are associated with a higher risk of prostate cancer. Two other studies also found that men with vitamin D levels above the lowest quintile had a higher risk of aggressive prostate cancer (OR comparing highest quintile to lowest = 1.37, 95% CI: 0.92-2.05; p-trend = 0.05) (47) and that men with a deficiency in circulating 25(OH)D had lower risk of total (OR = 0.62, 95% CI: 0.43-0.91) and poorly differentiated (OR = 0.42, 95% CI: 0.23-0.78) prostate cancer than men without deficient levels (48).
There may be a biological rationale explaining why high serum vitamin D may increase the risk of advanced prostate cancer amongst men who have the disease. Raised levels of vitamin D may lead to increased 24-hydroxylase levels, an enzyme that decreases local synthesis of 1,25-dihydroxyvitamin D (49). 24-hydroxylase itself has been found to be cancer-inducing because it inactivates 1,25-dihydroxyvitamin D (50). Certain prostate cancer cell lines have exceptionally high 24-hydroxylase levels (11). A high level of vitamin D may lead to less locally synthesised 1,25-dihydroxyvitamin D, decreasing any beneficial effects, (51;52), whilst increasing the risk of prostate cancer progression due to increased 24-hydroxylation. This mechanism may, therefore, explain our findings that increased sun exposure, a proxy for high vitamin D serum levels, is associated with an increased risk of advanced prostate cancer.
Explaining heterogeneity in the literature
The total number of studies was too small to formally investigate sources of heterogeneity using meta-regression techniques. However, the heterogeneity in results may reflect the challenge of measuring sun exposure and obtaining an accurate description of life-course exposure, whether based on ultraviolet radiation or self-reported exposure. For example, the cohort study by de Vries (36) compares the rate of prostate cancer in skin cancer patients with the rate in the general population. Skin cancer patients may change their sun exposure behaviours or be subject to detection bias as a result of regular medical contact. Rukin (35) recruited cases and controls from a urology clinic, and the controls were men with benign prostatic hypertrophy, which may itself be affected by sun exposure, leading to control selection bias. In both these latter two studies, the men without prostate cancer are likely to be different from the general population. Our results and those of John et al (37), which show a positive relationship of sun exposure and prostate cancer incidence, are population-based and compare prostate cancer patients to non-cancer patients, unlike Rukin (35) and de Vries (36) who find an inverse relationship.
Of the studies included in the meta-analysis for advanced or fatal prostate cancer, our study is the only one based in the UK. Two of these studies, Robsahm (39) and Freedman (34), are essentially ecological with respect to exposure measurement (which is based on solar radiation at region of residence), which may lead to a high rate of misclassification of individual exposures, for example, if older people travel to sunnier climates during winter periods. Exposure based on ultraviolet radiation will be influenced by the amount of time spent outside and the amount of exposed and / or unprotected skin. Levels of sun exposure, based on time spent outside, may differ considerably across different countries. Many studies do not allow for how different patterns of exposure interact to mediate risk. However, vitamin D synthesis due to sun exposure is closely regulated and no more than 15% of the total cutaneous 7-dehydrocholesterol, the substrate for ultraviolet action, is converted to provitamin D3, whether skin is exposed for 30 minutes or 8 hours at the equator (53). Confounding variables such as level of health care and cultural, lifestyle or genetic differences may limit the causal inference that can be made from existing observational studies (54;55). Systematic measurement error in outcome assessment may arise from screening by serum PSA testing, accuracy of diagnosis or recording. There may also be a distortion of the literature by publication bias, where null or unexpected results are not submitted or published as often as results that are ‘statistically significant’ or in line with expectation.
Strength and limitations of our study
We have used recalled sun exposure as a proxy for serum vitamin D levels. Reported measures of sun exposure were found to have varying levels of reproducibility (56), which may have lead to misclassification of sun exposure status. There is potential misclassification of vitamin D status due to a lack of information on other sources of exposure, such as leisure activities, occupational exposure or dietary vitamin D. This misclassification is likely to be non-differential with respect to cancer status. We were able to investigate both behaviours and biological effects (such as propensity to burn), which may be a more accurate reflection of sun exposure than self-reported behaviour alone.
Since the decision to biopsy is based on PSA level, it is possible that some of the controls with PSA<3ng/ml have unidentified prostate cancer (57). However, there was no evidence of an association between PSA level and skin colour, ambience or exposure pattern in the control group (data on request). Therefore any misclassification of cancer status is likely to be non-differential with respect to sun exposure, attenuating effect-estimates. Evidence of associations with advanced prostate cancer must be interpreted carefully as the small number of advanced cases may by chance be unrepresentative. There is the possibility of reverse causality: that men with more advanced cancer may be less able or less inclined to go outdoors. However, this is unlikely as the men were asymptomatic so their behaviour was unlikely to change, and it would only affect variables relating to the time period directly prior to diagnosis.
Approximately 94% of the men lived in the UK all of their lives, and sun tourism has only recently increased (58), so it is possible that there may be little variation due to latitude, but we are looking at variations due to behaviour and skin pigmentation.
Conclusion
Our results from the population-based ProtecT study and our meta-analysis offer little support to the hypothesis that greater sun exposure may protect against the development of prostate cancer. There is similarly weak evidence that, amongst men with prostate cancer, it is possible that higher levels of sun exposure increase the likelihood that those cancers that do develop will be more advanced. The finding that an exposure may reduce prostate cancer development but increase risk of cancer progression has a precedent in the Prostate Cancer Prevention Trial, where the 5-alpha reductase inhibitor finasteride had different effects on incidence and progression. Men in the finasteride arm had fewer prostate cancers overall but an increase in high-grade disease compared to men in the placebo group (59). We believe that the suggestion of a possible adverse effect of higher sunlight exposure on prostate cancer progression warrants further investigation, as this finding has potential implications for both public health and therapeutic attempts to controlling this disease via manipulating vitamin D status.
Supplementary Material
Acknowledgements
This analysis was funded by the World Cancer Research Fund (Grant number 2006/15). The ProtecT (Prostate testing for cancer and Treatment) study is funded by the UK NIHR Health Technology Assessment Programme (projects 96/20/06, 96/20/99). Rebecca Gilbert is supported by a Cancer Research UK Graduate Training Fellowship.
The authors would like to acknowledge the provision of additional epidemiological data by the NHS R&D Directorate supported Prodigal study and the ProMPT (Prostate Mechanisms of Progression and Treatment) collaboration which is supported by the National Cancer Research Institute (NCRI) formed by the Department of Health, the Medical Research Council and Cancer Research UK.
The authors would like to acknowledge the tremendous contribution of all members of the ProtecT study research group, and especially the following who were involved in this research (Prasad Bollina, Sue Bonnington, Debbie Cooper, Michael Davis, Liz Down, Andrew Doble, Alan Doherty, Emma Elliott, David Gillatt, Pippa Herbert, Peter Holding, Joanne Howson, Mandy Jones, Roger Kockelbergh, Howard Kynaston, Teresa Lennon, Norma Lyons, Hilary Moody, Philip Powell, Stephen Prescott, Liz Salter, Pauline Thompson). Department of Health disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Abbreviations
- PSA
Prostate Specific Antigen
- ProtecT
Prostate Testing for Cancer and Treatment study
Footnotes
Statements:
Our results provide some support for the hypothesis that greater sun exposure may protect against the development of prostate cancer. However amongst men with prostate cancer, our data indicate the possibility that higher levels of sun exposure may increase progression to a more advanced stage or grade.
Our findings have potential implications for both public health and therapeutic attempts at controlling this disease via manipulation of vitamin D status.
Conflicts of Interest: none
Reference List
- 1.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001 Sep;37(Supplement 8):4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK Commonly diagnosed cancers worldwide. Mar 19, 2008. http://info cancerresearchuk org/cancerstats/geographic/world/commoncancers/
- 3.Gronberg H. Prostate cancer epidemiology. The Lancet. 2003 Mar 8;361(9360):859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund / American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR; Washington DC: 2007. [Google Scholar]
- 5.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992 Dec 15;70(12):2861–9. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG, Hanchette CL. UV, latitude, and spatial trends in prostate cancer mortality: all sunlight is not the same (United States) Cancer Causes Control. 2006 Oct;17(8):1091–101. doi: 10.1007/s10552-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States) Cancer Causes Control. 2005 Mar;16(2):83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG. Vitamin D and the epidemiology of prostate cancer. Seminars in Dialysis. 2005 Jul;18(4):276–89. doi: 10.1111/j.1525-139X.2005.18403.x. [DOI] [PubMed] [Google Scholar]
- 9.Polek TC, Weigel NL. Vitamin D and prostate cancer. J Androl. 2002 Jan;23(1):9–17. doi: 10.1002/j.1939-4640.2002.tb02596.x. [DOI] [PubMed] [Google Scholar]
- 10.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006 Feb;96(2):252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lou YR, Qiao S, Talonpoika R, Syvala H, Tuohimaa P. The role of Vitamin D3 metabolism in prostate cancer. The Journal of Steroid Biochemistry and Molecular Biology. 2004 Nov;92(4):317–25. doi: 10.1016/j.jsbmb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Kricker A, Armstrong B. Does sunlight have a beneficial influence on certain cancers? Prog Biophys Mol Biol. 2006 Sep;92(1):132–9. doi: 10.1016/j.pbiomolbio.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 13.van der Rhee HJ, de Vries E, Coebergh JWW. Does sunlight prevent cancer? A systematic review. Eur J Cancer. 2006 Sep;42(14):2222–32. doi: 10.1016/j.ejca.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Shlomo Y, Evans S, Ibrahim F, Patel B, Anson K, Chinegwundoh F, Corbishley C, Dorling D, Thomas B, Gillatt D, Kirby R, Muir G, et al. The Risk of Prostate Cancer amongst Black Men in the United Kingdom: The PROCESS Cohort Study. Eur Urol. 2008 Jan;53(1):99–105. doi: 10.1016/j.eururo.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Luscombe CJ, French ME, Liu S, Saxby MF, Jones PW, Fryer AA, Strange RC. Outcome in prostate cancer associations with skin type and polymorphism in pigmentation-related genes. Carcinogenesis. 2001 Sep 1;22(9):1343–7. doi: 10.1093/carcin/22.9.1343. [DOI] [PubMed] [Google Scholar]
- 16.Grant WB. Geographic variation of prostate cancer mortality rates in the United States: Implications for prostate cancer risk related to vitamin D.[comment] Int J Cancer. 2004 Apr 23;111(3):470–1. doi: 10.1002/ijc.20220. [DOI] [PubMed] [Google Scholar]
- 17.Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, Jewell D, Powell P, Gillatt D, Dedman D, Mills N, Smith M, et al. Prostate Testing for Cancer and Treatment (ProtecT) feasibility study. Health Technology Assessment (Winchester, England) 2003;7(14):1–88. doi: 10.3310/hta7140. [DOI] [PubMed] [Google Scholar]
- 18.Ohori M, Wheeler TM, Scardino PT. The New American Joint Committee on Cancer and International Union Against Cancer TNM classification of prostate cancer. Clinicopathologic correlations. Cancer. 1994 Jul 1;74(1):104–14. doi: 10.1002/1097-0142(19940701)74:1<104::aid-cncr2820740119>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Harvey I, Frankel S, Marks R, Shalom D, Nolan-Farrell M. Non-melanoma skin cancer and solar keratoses II analytical results of the South Wales Skin Cancer Study. Br J Cancer. 1996 Oct;74(8):1308–12. doi: 10.1038/bjc.1996.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey I, Frankel S, Marks R, Shalom D, Nolan-Farrell M. Non-melanoma skin cancer and solar keratoses. I. Methods and descriptive results of the South Wales Skin Cancer Study. Br J Cancer. 1996 Oct;74(8):1302–7. doi: 10.1038/bjc.1996.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J, Colditz GA, Hunter DJ. Risk factors for skin cancers: a nested case-control study within the Nurses' Health Study. Int J Epidemiol. 2006 Dec;35(6):1514–21. doi: 10.1093/ije/dyl197. [DOI] [PubMed] [Google Scholar]
- 22.Whiteman D, Green A. Wherein lies the truth? Assessment of agreement between parent proxy and child respondents. Int J Epidemiol. 1997 Aug;26(4):855–9. doi: 10.1093/ije/26.4.855. [DOI] [PubMed] [Google Scholar]
- 23.Whiteman DC, Valery P, McWhirter W, Green AC. Risk factors for childhood melanoma in Queensland, Australia. Int J Cancer. 1997 Jan 6;70(1):26–31. doi: 10.1002/(sici)1097-0215(19970106)70:1<26::aid-ijc4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Green A, Siskind V, Bain C, Alexander J. Sunburn and malignant melanoma. Br J Cancer. 1985 Mar;51(3):393–7. doi: 10.1038/bjc.1985.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green A, Bain C, McLennan R, Siskind V. Risk factors for cutaneous melanoma in Queensland. Recent Results Cancer Res. 1986;102:76–97. doi: 10.1007/978-3-642-82641-2_6. [DOI] [PubMed] [Google Scholar]
- 26.Whiteman DC, Stickley M, Watt P, Hughes MC, Davis MB, Green AC. Anatomic Site, Sun Exposure, and Risk of Cutaneous Melanoma. J Clin Oncol. 2006 Jul 1;24(19):3172–7. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luscombe CJ, Fryer AA, French ME, Liu S, Saxby MF, Jones PW, Strange RC. Exposure to ultraviolet radiation: association with susceptibility and age at presentation with prostate cancer. Lancet. 2001 Aug 25;358(9282):641–2. doi: 10.1016/S0140-6736(01)05788-9. [DOI] [PubMed] [Google Scholar]
- 29.Bodiwala D, Luscombe CJ, French ME, Liu S, Saxby MF, Jones PW, Fryer AA, Strange RC. Associations between prostate cancer susceptibility and parameters of exposure to ultraviolet radiation. Cancer Lett. 2003 Oct 28;200(2):141–8. doi: 10.1016/s0304-3835(03)00416-6. [DOI] [PubMed] [Google Scholar]
- 30.Bodiwala D, Luscombe CJ, French ME, Liu S, Saxby MF, Jones PW, Ramachandran S, Fryer AA, Strange RC. Susceptibility to prostate cancer: studies on interactions between UVR exposure and skin type. Carcinogenesis. 2003 Apr;24(4):711–7. doi: 10.1093/carcin/bgg021. [DOI] [PubMed] [Google Scholar]
- 31.Bodiwala D, Luscombe CJ, Liu S, Saxby M, French M, Jones PW, Fryer AA, Strange RC. Prostate cancer risk and exposure to ultraviolet radiation: further support for the protective effect of sunlight. Cancer Lett. 2003 Mar 31;192(2):145–9. doi: 10.1016/s0304-3835(02)00710-3. [DOI] [PubMed] [Google Scholar]
- 32.Rukin N, Blagojevic M, Luscombe CJ, Liu S, Saxby MF, Ramachandran S, Fryer AA, Jones PW, Strange RC. Associations between timing of exposure to ultraviolet radiation, T-stage and survival in prostate cancer. Cancer Detect Prev. 2007;31(6):443–9. doi: 10.1016/j.cdp.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 33.John EM, Dreon DM, Koo J, Schwartz GG. Residential sunlight exposure is associated with a decreased risk of prostate cancer. The Journal of Steroid Biochemistry and Molecular Biology. 2004 May;89-90:549–52. doi: 10.1016/j.jsbmb.2004.03.067. [DOI] [PubMed] [Google Scholar]
- 34.Freedman DM, Dosemeci M, McGlynn K. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: a composite death certificate based case-control study. Occup Environ Med. 2002 Apr 1;59(4):257–62. doi: 10.1136/oem.59.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rukin NJ, Zeegers MP, Ramachandran S, Luscombe CJ, Liu S, Saxby M, Lear J, Strange RC. A comparison of sunlight exposure in men with prostate cancer and basal cell carcinoma. Br J Cancer. 2007 Feb 12;96(3):523–8. doi: 10.1038/sj.bjc.6603576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Vries E, Soerjomataram I, Houterman S, Louwman MWJ, Coebergh JW. Decreased Risk of Prostate Cancer after Skin Cancer Diagnosis: A Protective Role of Ultraviolet Radiation? Am J Epidemiol. 2007 Apr 15;165(8):966–72. doi: 10.1093/aje/kwk084. [DOI] [PubMed] [Google Scholar]
- 37.John EM, Koo J, Schwartz GG. Sun Exposure and Prostate Cancer Risk: Evidence for a Protective Effect of Early-Life Exposure. Cancer Epidemiol Biomarkers Prev. 2007 Jun 1;16(6):1283–6. doi: 10.1158/1055-9965.EPI-06-1053. [DOI] [PubMed] [Google Scholar]
- 38.John EM, Schwartz GG, Koo J, Van Den Berg D, Ingles SA. Sun Exposure, Vitamin D Receptor Gene Polymorphisms, and Risk of Advanced Prostate Cancer. Cancer Res. 2005 Jun 15;65(12):5470–9. doi: 10.1158/0008-5472.CAN-04-3134. [DOI] [PubMed] [Google Scholar]
- 39.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway) Cancer Causes Control. 2004 Mar;15(2):149–58. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 40.Willis MS, Wians FH. The role of nutrition in preventing prostate cancer: a review of the proposed mechanism of action of various dietary substances. Clin Chim Acta. 2003 Apr;330(1-2):57–83. doi: 10.1016/s0009-8981(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 41.Lokeshwar BL, Schwartz GG, Selzer MG, Burnstein KL, Zhuang SH, Block NL, Binderup L. Inhibition of Prostate Cancer Metastasis in Vivo: A Comparison of 1,25-Dihydroxyvitamin D (Calcitriol) and EB1089. Cancer Epidemiol Biomarkers Prev. 1999 Mar 1;8(3):241–8. [PubMed] [Google Scholar]
- 42.Stewart LV, Weigel NL. Vitamin D and Prostate Cancer. Exp Biol Med. 2004 Apr 1;229(4):277–84. doi: 10.1177/153537020422900401. [DOI] [PubMed] [Google Scholar]
- 43.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–8. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Platz EA, Rimm EB, Willett WC, Kantoff PW, Giovannucci E. Racial Variation in Prostate Cancer Incidence and in Hormonal System Markers Among Male Health Professionals. J Natl Cancer Inst. 2000 Dec 20;92(24):2009–17. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- 45.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999 May 1;69(5):842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 46.Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, Stattin P, Harvei S, Hakulinen T, Luostarinen T, Dillner J, Lehtinen M, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004 Jan 1;(1):8. doi: 10.1002/ijc.11375. 2004. [DOI] [PubMed] [Google Scholar]
- 47.Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, Horst RL, Hollis BW, Huang WY, Shikany JM, Hayes RB, For the Prostate LCaOCSTPT Serum Vitamin D Concentration and Prostate Cancer Risk: A Nested Case-Control Study. J Natl Cancer Inst. 2008 Jun 4;100(11):796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikhak B, Hunter DJ, Spiegelman D, Platz EA, Hollis BW, Giovannucci E. Vitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, and prostate cancer risk. Prostate. 2007 Jun 15;67(9):911–23. doi: 10.1002/pros.20570. [DOI] [PubMed] [Google Scholar]
- 49.Miller GJ, Stapleton GE, Hedlund TE, Moffat KA. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1alpha,25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res. 1995 Sep;1(9):997–1003. [PubMed] [Google Scholar]
- 50.Albertson DG, Ylstra B, Segraves R, Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW, Pinkel D. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat Genet. 2000 Jun;25(2):144–6. doi: 10.1038/75985. [DOI] [PubMed] [Google Scholar]
- 51.Peehl DM, Feldman D. The role of vitamin D and retinoids in controlling prostate cancer progression. Endocrine-Related Cancer. 2003 Jun;10(2):131–40. doi: 10.1677/erc.0.0100131. [DOI] [PubMed] [Google Scholar]
- 52.Ma JF, Nonn L, Campbell MJ, Hewison M, Feldman D, Peehl DM. Mechanisms of decreased Vitamin D 1 alpha-hydroxylase activity in prostate cancer cells. Mol Cell Endocrinol. 2004;221:1–2. 67–74. doi: 10.1016/j.mce.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Holick MF. McCollum Award Lecture, 1994: vitamin D--new horizons for the 21st century. Am J Clin Nutr. 1994 Oct 1;60(4):619–30. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- 54.Wolpowitz D, Gilchrest BA. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol. 2006 Feb;54(2):301–17. doi: 10.1016/j.jaad.2005.11.1057. [DOI] [PubMed] [Google Scholar]
- 55.Waltz P, Chodick G. International comparisons of prostate cancer mortality rates with dietary practices and sunlight levels. Urologic Oncology: Seminars and Original Investigations. 2007;25(1):85. doi: 10.1016/j.urolonc.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 56.English DR, Armstrong BK, Kricker A. Reproducibility of reported measurements of sun exposure in a case-control study. Cancer Epidemiol Biomarkers Prev. 1998 Oct 1;7(10):857–63. [PubMed] [Google Scholar]
- 57.Thompson IM, Ankerst DP, Chi C, Lucia MS, Goodman PJ, Crowley JJ, Parnes HL, Coltman CA., Jr. Operating Characteristics of Prostate-Specific Antigen in Men With an Initial PSA Level of 3.0 ng/mL or Lower. JAMA. 2005 Jul 6;294(1):66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]
- 58.European Travel Commission European Tourism Insights 2007. European Travel Commission. 2008 May; [Google Scholar]
- 59.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, et al. The Influence of Finasteride on the Development of Prostate Cancer. N Engl J Med. 2003 Jul 17;349(3):215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


