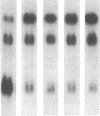
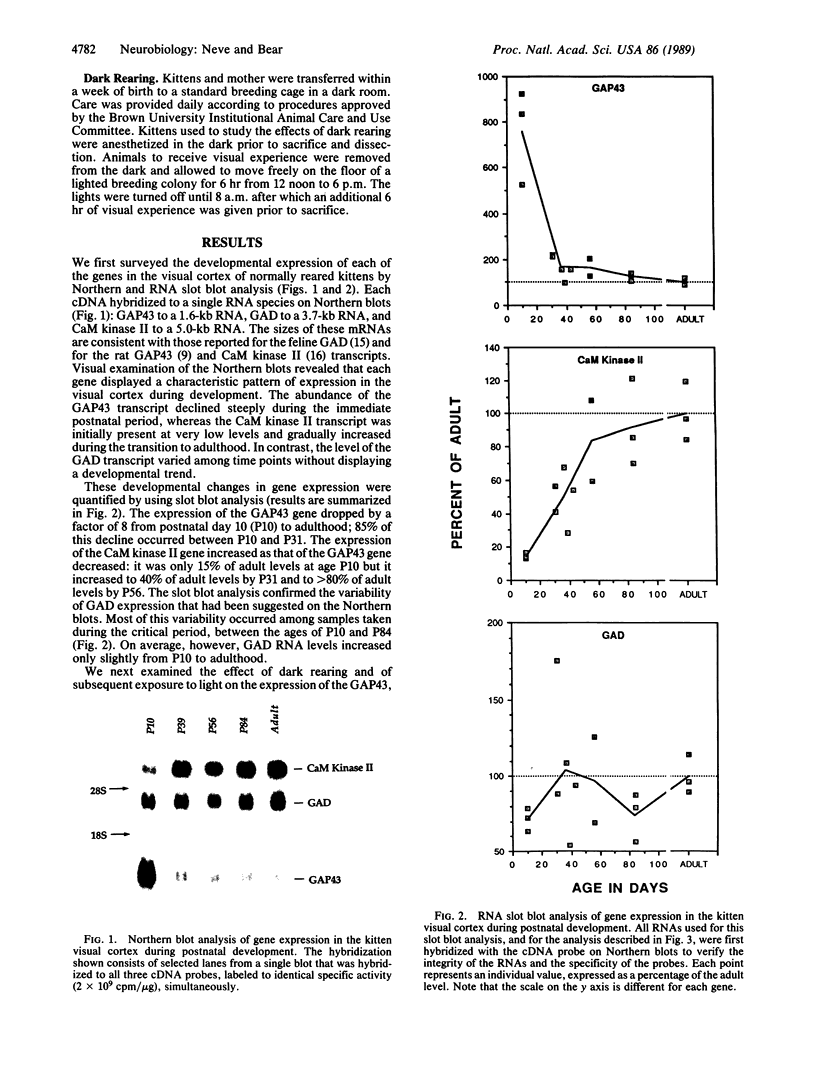
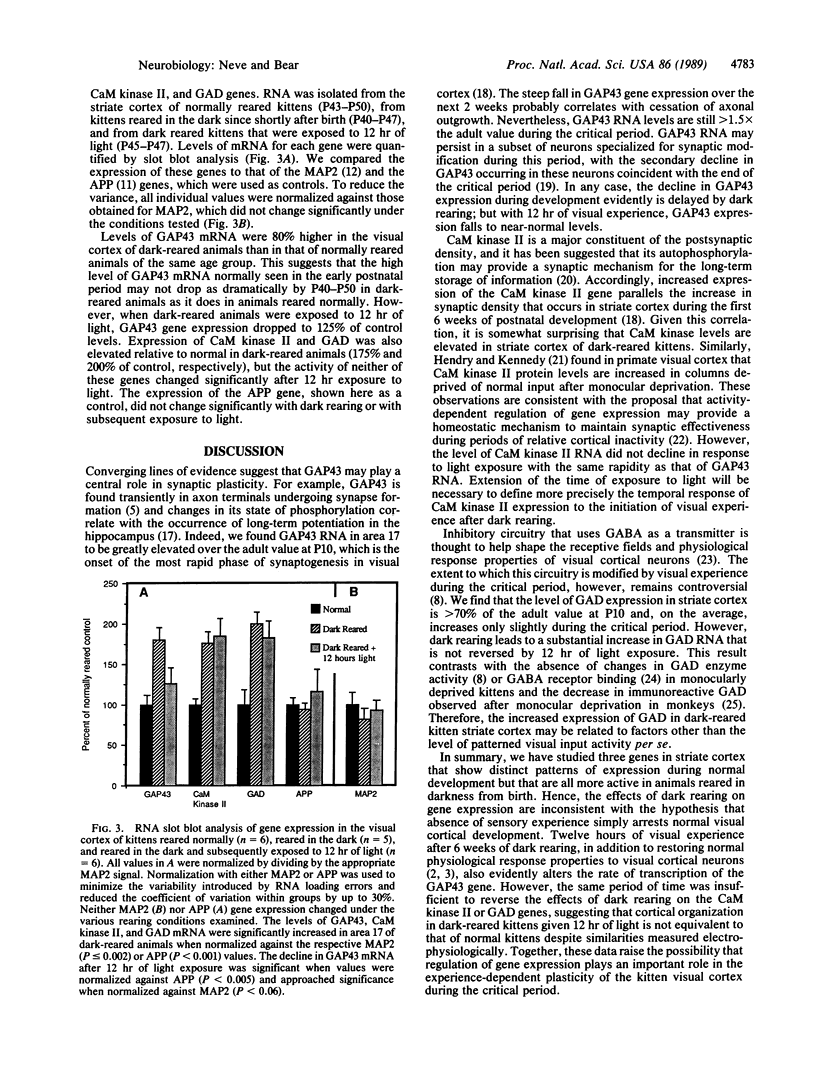
Abstract
We have examined the regulation of expression of the genes for the neuronal growth-associated protein GAP43, the type II calcium/calmodulin-dependent protein kinase, and glutamic acid decarboxylase in the kitten visual cortex during normal postnatal development and after a period of visual deprivation. We find that the mRNA transcripts of these genes display very different patterns of normal development but are all increased in the visual cortex of animals reared in the dark. Upon exposure to light, the transcript of the GAP43 gene drops to near-normal levels within 12 hr.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear M. F., Schmechel D. E., Ebner F. F. Glutamic acid decarboxylase in the striate cortex of normal and monocularly deprived kittens. J Neurosci. 1985 May;5(5):1262–1275. doi: 10.1523/JNEUROSCI.05-05-01262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret P., Gary-Bobo E., Imbert M. Ocular motility and recovery of orientational properties of visual cortical neurones in dark-reared kittens. Nature. 1978 Apr 27;272(5656):816–817. doi: 10.1038/272816a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in the visual system of the cat. J Comp Neurol. 1975 Mar 15;160(2):147–166. doi: 10.1002/cne.901600202. [DOI] [PubMed] [Google Scholar]
- Frégnac Y., Imbert M. Development of neuronal selectivity in primary visual cortex of cat. Physiol Rev. 1984 Jan;64(1):325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- Hanley R. M., Means A. R., Ono T., Kemp B. E., Burgin K. E., Waxham N., Kelly P. T. Functional analysis of a complementary DNA for the 50-kilodalton subunit of calmodulin kinase II. Science. 1987 Jul 17;237(4812):293–297. doi: 10.1126/science.3037704. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Jones E. G. Reduction in number of immunostained GABAergic neurones in deprived-eye dominance columns of monkey area 17. Nature. 1986 Apr 24;320(6064):750–753. doi: 10.1038/320750a0. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Kennedy M. B. Immunoreactivity for a calmodulin-dependent protein kinase is selectively increased in macaque striate cortex after monocular deprivation. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1536–1540. doi: 10.1073/pnas.83.5.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert M., Buisseret P. Receptive field characteristics and plastic properties of visual cortical cells in kittens reared with or without visual experience. Exp Brain Res. 1975;22(1):25–36. doi: 10.1007/BF00235409. [DOI] [PubMed] [Google Scholar]
- Kaufman D. L., McGinnis J. F., Krieger N. R., Tobin A. J. Brain glutamate decarboxylase cloned in lambda gt-11: fusion protein produces gamma-aminobutyric acid. Science. 1986 May 30;232(4754):1138–1140. doi: 10.1126/science.3518061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., McGuinness T. L., Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Feb;81(3):945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Goldring M. A. Feasibility of long-term storage of graded information by the Ca2+/calmodulin-dependent protein kinase molecules of the postsynaptic density. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5320–5324. doi: 10.1073/pnas.85.14.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger D. M., Akers R. F., Nelson R. B., Barnes C. A., McNaughton B. L., Routtenberg A. A selective increase in phosporylation of protein F1, a protein kinase C substrate, directly related to three day growth of long term synaptic enhancement. Brain Res. 1985 Sep 16;343(1):137–143. doi: 10.1016/0006-8993(85)91167-9. [DOI] [PubMed] [Google Scholar]
- Mower G. D., White W. F., Rustad R. [3H]muscimol binding of GABA receptors in the visual cortex of normal and monocularly deprived cats. Brain Res. 1986 Aug 20;380(2):253–260. doi: 10.1016/0006-8993(86)90220-9. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Harris P., Kosik K. S., Kurnit D. M., Donlon T. A. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986 Dec;387(3):271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Perrone-Bizzozero N. I., Finklestein S., Zwiers H., Bird E., Kurnit D. M., Benowitz L. I. The neuronal growth-associated protein GAP-43 (B-50, F1): neuronal specificity, developmental regulation and regional distribution of the human and rat mRNAs. Brain Res. 1987 Jul;388(2):177–183. doi: 10.1016/s0006-8993(87)80012-4. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Selkoe D. J., Kurnit D. M., Kosik K. S. A cDNA for a human microtubule associated protein 2 epitope in the Alzheimer neurofibrillary tangle. Brain Res. 1986 Nov;387(2):193–196. doi: 10.1016/0169-328x(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975 Sep;250(2):305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]