Abstract
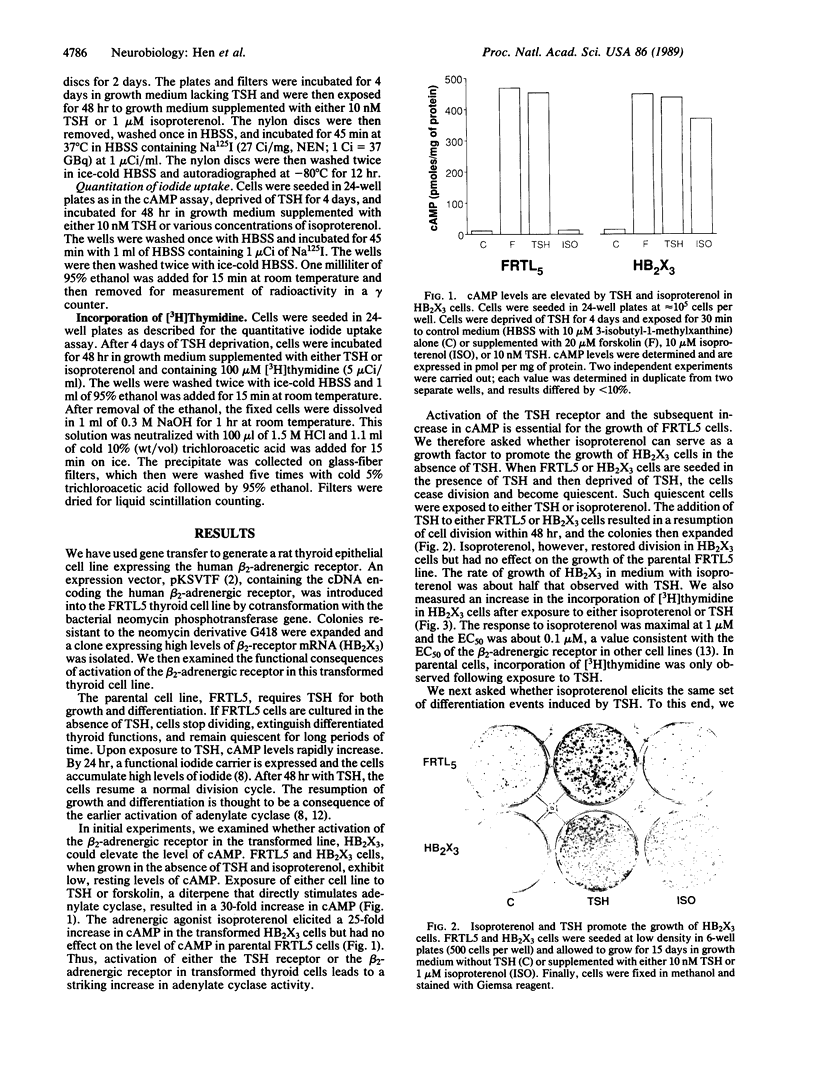
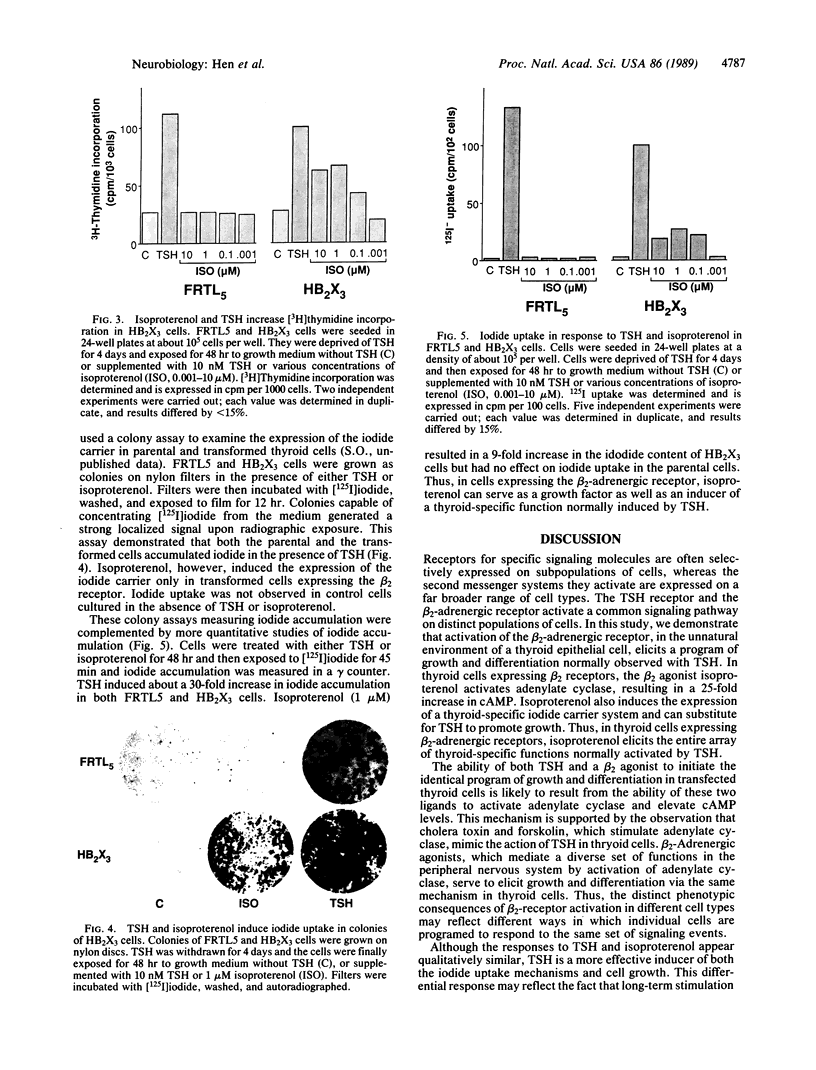
We have introduced the beta 2-adrenergic receptor into the unnatural environment of a thyroid cell to demonstrate that the activation of this receptor initiates diverse cellular programs in different cell types. The thyroid-stimulating hormone (TSH) receptor and the beta 2-adrenergic receptor stimulate a common signaling pathway in distinct populations of cells. In this study, we demonstrate that the activation of the beta 2-adrenergic receptor, transfected into a thyroid epithelial cell, elicits a program of growth and differentiation normally observed with TSH. In thyroid cells expressing beta 2 receptors, the beta 2 agonist isoproterenol activates adenylate cyclase, induces the expression of a thyroid-specific iodide carrier system, and can substitute for TSH to promote growth. Thus, in thyroid cells expressing beta 2-adrenergic receptors, isoproterenol elicits the entire array of thyroid-specific functions normally activated by TSH.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. M., Baetge E. E., Abrass I. B., Palmiter R. D. Isoproterenol response following transfection of the mouse beta 2-adrenergic receptor gene into Y1 cells. EMBO J. 1988 Jan;7(1):133–138. doi: 10.1002/j.1460-2075.1988.tb02792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M., Hnatowich M., Collins S., Kobilka B. K., Deblasi A., Lefkowitz R. J., Caron M. G. Expression of a human cDNA encoding the beta 2-adrenergic receptor in Chinese hamster fibroblasts (CHW): functionality and regulation of the expressed receptors. Mol Pharmacol. 1988 Feb;33(2):133–139. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown E., Kendall D. A., Nahorski S. R. Inositol phospholipid hydrolysis in rat cerebral cortical slices: I. Receptor characterisation. J Neurochem. 1984 May;42(5):1379–1387. doi: 10.1111/j.1471-4159.1984.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Regan J. W., Nakata H., Codina J., Benovic J. L., Gierschik P., Somers R. L., Spiegel A. M., Birnbaumer L., Lefkowitz R. J. Functional reconstitution of the alpha 2-adrenergic receptor with guanine nucleotide regulatory proteins in phospholipid vesicles. J Biol Chem. 1986 Mar 15;261(8):3901–3909. [PubMed] [Google Scholar]
- Cruise J. L., Houck K. A., Michalopoulos G. K. Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha 1 adrenoreceptor by norepinephrine. Science. 1985 Feb 15;227(4688):749–751. doi: 10.1126/science.2982212. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Kobilka T. S., Daniel K., Regan J. W., Caron M. G., Lefkowitz R. J. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988 Jun 3;240(4857):1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- Nemecek G. M., Coughlin S. R., Handley D. A., Moskowitz M. A. Stimulation of aortic smooth muscle cell mitogenesis by serotonin. Proc Natl Acad Sci U S A. 1986 Feb;83(3):674–678. doi: 10.1073/pnas.83.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J., von Euler A. M., Dalsgaard C. J. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985 May 2;315(6014):61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- Seuwen K., Magnaldo I., Pouysségur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988 Sep 15;335(6187):254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- Valente W. A., Vitti P., Kohn L. D., Brandi M. L., Rotella C. M., Toccafondi R., Tramontano D., Aloj S. M., Ambesi-Impiombato F. S. The relationship of growth and adenylate cyclase activity in cultured thyroid cells: separate bioeffects of thyrotropin. Endocrinology. 1983 Jan;112(1):71–79. doi: 10.1210/endo-112-1-71. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Philp N. J., Ambesi-Impiombato F. S., Grollman E. F. Thyrotropin-stimulated iodide transport mediated by adenosine 3',5'-monophosphate and dependent on protein synthesis. Endocrinology. 1984 Apr;114(4):1099–1107. doi: 10.1210/endo-114-4-1099. [DOI] [PubMed] [Google Scholar]
- Wigler M., Perucho M., Kurtz D., Dana S., Pellicer A., Axel R., Silverstein S. Transformation of mammalian cells with an amplifiable dominant-acting gene. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3567–3570. doi: 10.1073/pnas.77.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]







