Abstract
Polycationic systems based on poly(hexamethylene biguanide) (PHMBG), branched polyethyleneimine (PEI) and poly(N-vinylguanidine) (PVG) have been evaluated as heterogeneous catalysts for the transesterification of sunflower oil by methanol. Insoluble networks are synthesized via crosslinking of PHMBG by either 4,4′-methylenebis(N,N-diglycidylaniline) or polyisocyanate prepolymer, PEI with sebacoyl chloride, and PVG with 1,4-butanediol diglycidyl ether. PHMBG and its crosslinked networks appeared to be remarkably efficient catalysts, enabling 80–100% triglyceride conversion within 0.5 h at 70°C. PEI-based networks catalyzed triglyceride transesterification with rates 8- to 12-fold slower than their PHMBG-based counterparts. The PVG-based networks, which were devoid of hydrophobic moieties, appeared to be inefficient catalysts due to limited accessibility of the basic guanidine groups to reactants. The PHMBG networks were shown to be recyclable by a simple centrifugal filtration. After 15 cycles of recovery and reuse, only 10–15% decline in performance was observed.
Keywords: poly(hexamethylene biguanide), poly(ethylene imine), cross-linking, heterogeneous catalysis, methanolysis, biodiesel
Introduction
Development of catalysts for the synthesis of biodiesel (fatty acid methyl esters) directly from renewable feedstocks such as vegetable oils by their transesterification with short-chain alcohols is an emerging sector of the modern chemical industry.1 Selection of an appropriate catalyst is of fundamental importance for the design of a sustainable transesterification process. Alcohol-soluble alkaline catalysts such as sodium and potassium hydroxides or methoxides are most commonly used at present in biodiesel production, mainly because of their low cost and their efficiency in promoting the transesterification at relatively low temperatures. In the homogeneous catalysis, the immiscible glycerol phase, which accumulates during the course of the reaction, solubilizes homogeneous alkaline catalysts and, hence, removes them from the reaction medium. The recovery of the homogeneous catalyst after the reaction is a significant problem, because aqueous quenching resulting in the formation of a stable emulsion and saponification make separation of methyl esters (biodiesel) and glycerol difficult. Yet, the recovery of the co-product, glycerol generated during the biodiesel production, and its sale into the commercial market enables reduction in the production costs.2 Also, a large amount of wastewater can be produced in the separation and cleaning of the catalyst and products.3 Therefore, heterogeneous catalysts present advantages over homogeneous catalysts in that the former are reusable and easy to separate from the reaction products, do not form soaps, produce more pure biodiesel, simplify glycerol purification and are more tolerant to water in the feedstock. The present work aimed at finding an efficient catalyst for heterogeneous catalysis of the methanolysis of vegetable oils. A variety of both basic and acidic heterogeneous catalysts are known, including metal oxides, hydroxides and salts supported on high-surface area materials, zeolites, and ion-exchange resins. 4,5 The use of solid organic bases in biodiesel catalysis is much less widespread, despite their proven catalytic efficiency in a variety of other organic reactions where organic bases act as proton sponges. 6–8 Organic bases acting as heterogeneous catalysts for the transesterification of vegetable oils, including guanidines anchored on polymers or encapsulated in zeolite cages, have been reported.9 A number of supported guanidines have shown high turnover frequencies on repeated use.10 Encapsulated bases were not very efficient owing to diffusional limitations on the transfer rates of bulky triglycerides. Salts of amino acids that are insoluble in methanol, glycerol, and fatty acid esters were investigated as catalysts for the methanolysis of triglycerides.1 Salts of 2-amino-5-guanidinovaleric acid, carnitine, betaine, and taurine, and especially those containing quaternary ammonium or guanidine groups, displayed significant catalytic activity.
Herein, we designed polymeric, organic base catalysts specifically for heterogeneous biodiesel synthesis based on commercially available and non-toxic biguanide, amine, or guanidine functionalities; these polymers are readily converted into insoluble species by onestep cross-linking reactions. The resulting polymer network beads are recovered easily from mixtures of alcohol and triglycerides using straightforward laboratory techniques. The polymers used in this study are poly(hexamethylene biguanide), which is commonly applied as an antiseptic,11 branched poly(ethylene imine), produced for industrial and medicinal uses on a large scale,12,13 and poly(N-vinylguanidine) obtained by modification of a commercially available polyvinylamine. Cross-linked poly(hexamethylene biguanide), in particular, exhibited high catalytic efficiency and was readily recovered by centrifugation, as is shown in the work described in this paper.
Experimental
Chemicals
Branched PEI (nominal average molecular weight, 25 kDa) was obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO), dialyzed against DI water (12–14 kDa MW cutoff), and its solution pH was adjusted to 7.5. The PEI is highly branched with a molar ratio of primary to secondary to tertiary amino groups of 1:2:1. After dialysis and removal of lower molecular weight fractions, the Mw was 38 kDa and Mw/Mn=1.55. Poly(hexamethylene biguanide) (PHMBG) was a generous gift from Arch UK Biocides, Ltd. (Manchester, UK) supplied as a 20 wt% aqueous solution (Cosmocil CQ) with a reported Mw of 2674 and a polydispersity of 1.89. Ganex® V-220 (copolymers of vinylpyrrolidone and long-chain α-olefins) was obtained from International Specialty Products Corp. (Wilmington, DE). Sunflower seed oil from Helianthus annuus, anhydrous methanol (water content below 0.005%), sodium methoxide (97%), 1,5,7-triazabicyclo[4.4.0]dec-5-ene (98%, TBD), sorbitan monooleate (Span 80), polysorbate (Tween 20), toluene 2,4-diisocyanate (TDI), chlorobenzene (98%), 4,4′-methylenebis(N,N-diglycidylaniline), 1,4-butanediol diglycidyl ether (>95%), and molecular sieves were all from Sigma-Aldrich Chemical Co. Lupamin® 9095 (20–22% aqueous solution of polymer representing 90% hydrolyzed poly(N-formamide), average molecular weight 340 kDa), was kindly supplied by BTC, a specialty chemical distribution group of BASF, Germany. Prior to further modifications, Lupamin 9095 was completely hydrolyzed into polyvinylamine by 37% hydrochloric acid at 80 °C followed by polymer purification by precipitation into 2M NaOH and dialysis (molecular weight cutoff, 12–14 kDa) and drying. All other chemicals and solvents were obtained from commercial sources and were of highest purity available. The acid value (the number of mg of potassium hydroxide required to neutralize the free acids in 1.0 g of the oil) and the saponification value (SAP, the number of milligrams of potassium hydroxide or sodium hydroxide required to saponify 1g of oil) of the sunflower oil were measured by a commercial laboratory to be 0.29 and 190.8 mg KOH/g, respectively. Prior to use, both methanol and sunflower oil were stored over alumosilicate molecular sieves (4Å). The mean molecular weight of the oil was taken as 3x(MW of KOH)/(SAP in g/g) = 3×56.1/0.001×190.8=882.1 Da, which corresponded well with the reported molecular weight of sunflower seed oil of 876.6. Da.14
Syntheses
PHMBG-based networks were synthesized either by cross-linking PHMBG with 4,4′-methylenebis(N,N-diglycidylaniline) through reaction of the four glycidyl groups of the latter with the amino-imine moieties of PHMBG, or by cross-linking with polymeric isocyanate. Prior to the use, the freshly synthesized networks were kept under dry conditions in a desiccator. To ensure the polymer solubility in organic solvent, the PHMBG was fully converted into its protonated form as follows. PHMBG (35 g, 28.3 mmol, 141 meq of secondary amino groups) was dissolved in 200 mL of deionized water and the resulting solution was mixed with 200 mL of aqueous 40 wt% sodium hydroxide, forming a precipitate. The precipitate was filtered off and suspended in 400 mL of anhydrous ethanol, the suspension was filtered off and the filtrate was dried under vacuum.
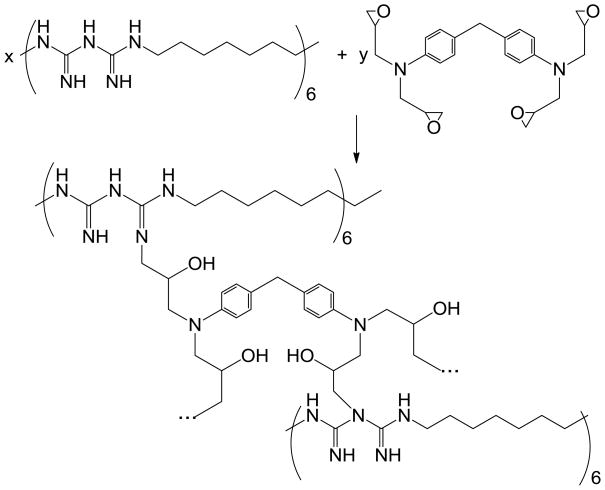
Method 1 (Scheme 1)
Scheme 1.
Cross-linking of poly(hexamethylene biguanide) (PHMBG) by 4,4′-methylenebis(N,N-diglycidylaniline).
The dry polymer (30 g) was dissolved in 400 mL of anhydrous ethanol at 90°C and a solution of 5 g (11.8 mmol, 47.3 meq of glycidyl groups) of 4,4′-methylenebis(N,N-diglycidylaniline) in 70 mL dry acetonitrile was added to the PHMBG solution dropwise. The resulting mixture was kept in a sealed reactor at 80°C for 16 h, with periodic shaking. Then the reaction vessel was allowed to equilibrate at room temperature and disassembled, and the rubbery product was recovered, washed by ethanol and dried under vacuum at 40°C for 24 h. 1H NMR 15 (CD3OD, 400 MHz): δ, 7.1 (2H, benzyl), 6.7 (2H, benzyl α to –N-C(C)), 3.6 (1H, -OH), 2.7–3.0 (2H, methylene α to –N-C(C))), 2.0 (1H, amine), 1.3 (2H, methylene), 0.9 (3H, methyl). Elemental analysis, found: C, 63.1; H, 9.13; N, 22.4. The synthesized resin (designation, XL-PHMBG M1; effective secondary amine/glycidyl ratio, ca.4) was dispersible and swellable, but insoluble in methanol. The content of accessible biguanide groups in the resin (3.5 meq/g) and pKa in water (11.0) were measured at 20°C by colloidal potentiometric titration using a 736 GP Titrino potentiometric titration system (Metrohm Ltd, Herisau, Switzerland), as described previously. 16
The dispersions were obtained by cryo-grinding the dry resin in liquid nitrogen, followed by dispersing the ground resin particles in methanol by sonication. The mean diameter of the dispersed particles (184±45 μm) was measured using a Malvern Mastersizer 2000 light scattering instrument (Malvern Instruments Ltd., UK). Uncrosslinked PHMBG polymer treated by sodium hydroxide as described above was completely soluble in methanol, forming clear solutions at concentrations up to 5–8 wt%.
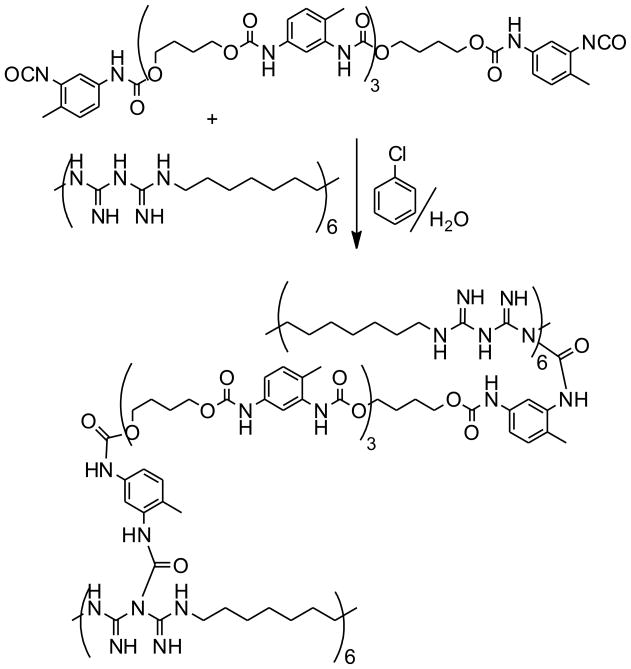
Method 2 (Scheme 2)
Scheme 2.
Cross-linking of PHMBG by polyurethane prepolymer via interfacial polymerization.
Method 2 involved synthesis of a polymeric crosslinker.16 In brief, toluene 2,4-diisocyanate (TDI, 21.7 g, 125 mmol) was dissolved into cyclohexanone (150 mL) in a 250 mL round-bottom flask. The mixture was placed in an 80 °C oil bath and agitated using a magnetic stirrer. 1,4-Butanediol (3.7 g, 41 mmol) was added dropwise. The flask was purged with nitrogen and allowed to react for 24 h. Solvents and unreacted component admixtures were removed at 100 °C under 15 Torr for 8 h. The number-average molecular weight of the synthesized cross-linker was measured to be 1.3 kDa (Pd, 1.4) using size-exclusion chromatography in THF, with calibration curve developed using poly(propylene glycol) molecular weight standards.
For PHMBG cross-linking, the polyurethane prepolymer (3 g) was dissolved in chlorobenzene (4 g) at 70 °C and the resulting solution was added to a solution of PHMBG (4 g) and Ganex® V-220 (0.5 g) in 50 mL deionized water. The mixture was emulsified by vortexing and the reaction flask was kept at 70°C for 3 h under high-speed continuous stirring using a mechanical impeller. Then the stirring was discontinued and the mixture was equilibrated at room temperature. The resulting solids were vacuum-filtered and washed with acetone, methanol, and excess deionized water. The mean diameter of particles dispersed in methanol was measured to be 127±14 μm as described above. 1H NMR of the resin (CD3OD, 400 MHz): δ, 9.2 (1H, NH α to benzyl), 7.3, 7.2 (2H, benzyl), 3.9 (2H, CH2 α to – O-C(O)N), 2.9 (2H, CH2 α to –N-C(N)), 2.1 (1H, -NH), 1.5–1.65 (2H, methylene β to –N-C(N)), 1.3 (2H, methylene). The content of accessible biguanide groups in the resin and pKa in water were measured to be 3.1 meq/g and 11.2, respectively. The resin obtained via Method 2 was designated XL-PHMBG M2.
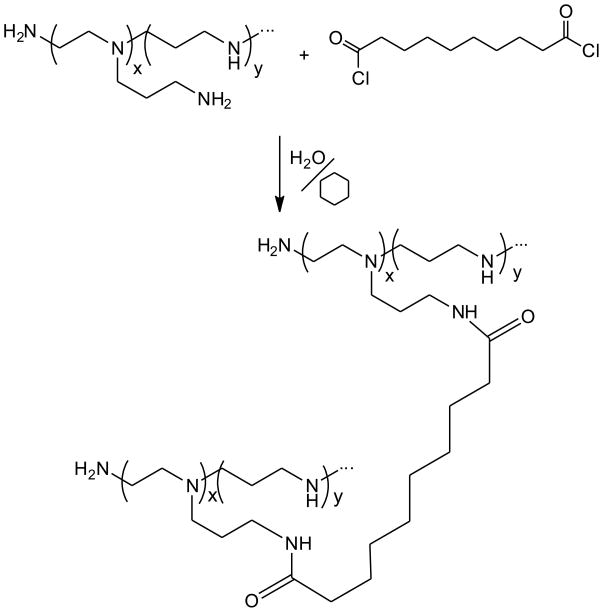
Cross-linking of poly(ethylene imine) (PEI) with sebacoyl chloride (Scheme 3)
Scheme 3.
Cross-linking of branched poly(ethylene imine) with sebacoyl chloride via interfacial reaction.
PEI-based networks were prepared via interfacial polycondensation reaction between branched PEI and sebacoyl chloride. A 2% v/v solution of Span 80 in cyclohexane (50 mL) was mixed with 15 mL of 10 wt% aqueous solution of PEI (pH adjusted to 8.0). The mixture was emulsified by vortexing and 0.96 g (4 mmol) sebacoyl chloride in 2 mL of cyclohexane were added. The reaction was allowed to proceed for 3 min with intermittent vortexing and then the emulsion was diluted with 50 mL of cyclohexane. The solid reaction product in the form of beads settled within 1 h and was then mechanically separated from the liquid and rinsed three times with 50 mL of cyclohexane. Transfer of the beads into the aqueous phase was achieved by dispersing the PEI product in 50 mL of aqueous solution of Tween 20 (30% v/v) and gradually adding 250 mL of deionized water. The beads were then recovered on a Buchner filter and rinsed several times with deionized water to remove traces of impurities. Following lyophilization from 20 wt% aqueous suspension, the cross-linked beads were suspended at 1 wt% in methanol, where the average particle size was measured to be 242±26 μm as described above. The produced beads swelled, but did not dissolve in methanol at 70°C within 72 h. The content of titratable amine groups in the resin and pKa in water were measured to be 12.3 meq/g and 9.8, respectively. The cross-linked PEI resin was designated XL-PEI.
The molar ratios of the PHMBG or PEI to their corresponding crosslinkers reported above correspond to the minimum crosslinker fractions resulting in methanol-insoluble network species. A two-fold decrease in the molar crosslinker:polymer ratio set in the network synthesis resulted in a significant fraction of network dissolved in methanol at 70°C. PVG species was observed to be insoluble in methanol even without crosslinker.
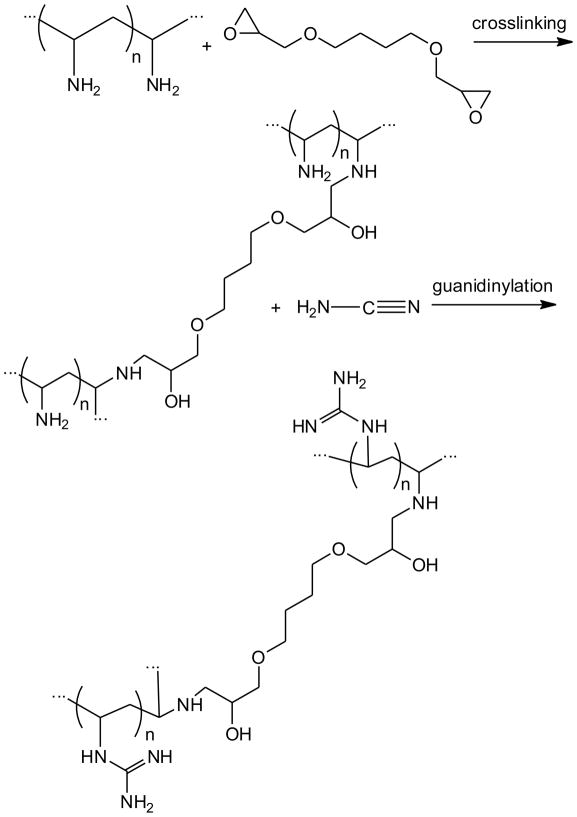
Cross-linking and guanidinylation of polyvinylamine (Scheme 4)
Scheme 4.
Cross-linking and guanidinylation of polyvinylamine.
PVAm prepared by 100% hydrolysis of Lupamin® 9095 (6 g, 140 mmol) was dissolved in 50 mL deionized water and 10 mL 37% HCl, and 200 mL of water were added under stirring. Cross-linker, 1,4-butanediol diglycidyl ether (1.42 g, 7 mmol) was added and the resulting solution was kept at 50°C under vigorous stirring for 24 h resulting in a suspension of cross-linked gel particles. The suspension was then subjected to guanidinylation by an aqueous solution of cyanamide following the previously described procedure.18 The resulting gel particles were precipitated in excess acetone, washed by acetone, dried, redissolved in deionized water, dialyzed against water (MWCO 1 kDa) and lyophilized. FTIR and NMR characterization of the resulting polymer has been described in detail elsewhere.18 The pKa of the gel in water was measured by titration to be 13.1, in good agreement with previously published data. The PVG-based gel particles were insoluble in methanol where the average particle size was measured to be 460±63 μm as described above. The cross-linked poly(N-vinylguanidine) resin was designated XL-PVG.
Network swelling
Swelling of the networks in methanol was estimated by placing a weighed amount of dry network (weight, Wd) in 20-fold excess solvent, equilibrating the suspension at room temperature for 5 days with shaking at 200 rpm, separating the network by centrifugation (3,000 g, 5 min), swift network removal and immediate weighing (swollen weight, Ws). The swelling degree was expressed as S,%=100×(Ws−Wd)/Wd. The S estimates for XL-PHMBG M1, XL-PHMBG M2, XL-PEI, and XL-PVG were 670, 750, 900, and 160 wt%, respectively. The experiments were conducted in triplicate. Standard deviations for S values in methanol were approximately 25 to 30%. Rapid methanol evaporation and the solvent entrapment in the interstices between the network particles were major sources of imprecision. Swelling of the networks in sunflower oil, measured analogously to the one in methanol, was estimated to be in the order of 100 to 200 wt%, with the majority of the weight increase due to the apparent entrapment of the viscous oil between the particles as well as adsorption on the polymer surface.
Catalyst activation and recovery
For activation, a solid polymer sample was suspended in a 3 M sodium methoxide solution in methanol and the resulting suspension was shaken at room temperature for 15 min. The suspension was then filtered under vacuum using Whatman 934-AH glass microfiber filters and the solid was washed multiple times with excess THF and methanol. The activated catalyst was kept in a desiccator under anhydrous conditions. The methanol wash-outs were mixed with deionized water (1:1 v/v) and the pH of the resulting solutions was measured. The end-point of the washing was determined to be at pH 7.0, indicative of the complete removal of sodium methoxide. Catalyst was recovered after a 2 h reaction by centrifugation at 4,000 g for 5 min using Amicon® Ultra-4 Centrifugal Filter Units (Millipore Co., Billerica, MA) followed by catalyst rinse with 3 M sodium methoxide solution in methanol, anhydrous methanol and vacuum-drying (~120 μ Torr). Recovered catalyst was kept in a desiccator under anhydrous conditions prior to reuse. With cross-linked PHMBG networks, weight-based recovery yields in a single recovery step were approximately 97%.
Transesterification reaction measurements
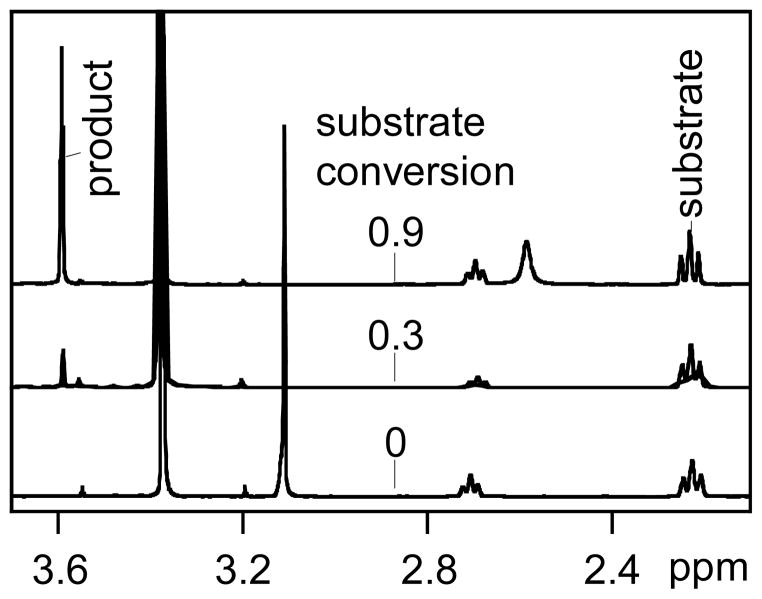
Sunflower oil (4.2 mL, 5.2 mmol) was mixed with 3.0 mL (74 mmol) of methanol. At time t=0, a measured amount of catalyst was added to glass reaction vials, the mixture was briefly vortexed, and the reaction commenced under vigorous shaking at a given temperature, using a VWR Nutating mixer placed in an oven. Reaction samples (70 μL) were withdrawn intermittently by syringe, dissolved in deuterated chloroform (630 μL), and analyzed by 1H NMR. The NMR measurements were performed at 25±0.5 °C using a Bruker Avance-400 spectrometer. 1H resonance frequency was 400.13 MHz and proton decoupling was applied throughout. The conversion of the oil to biodiesel at a given time t was calculated as: 19 Ft = 2 I(3.6)/3I(2.2), where I(3.6) and (I(2.2) are integrations of the product (methyl ester, singlet at 3.60 ppm) and the substrate (methylene located α to the carbonyl group in the oil, triplet at 2.23 ppm), respectively. Typical NMR results at varying degrees of conversion are depicted in Fig. 1.
Fig. 1.
Typical 1H NMR spectra of the sunflower oil-methanol mixtures at various degrees of transesterification attained with XL-PHMBG M2 as a catalyst. Signals corresponding to methyl ester and methylene located α to the carbonyl group in oil are designated “product” and “substrate”, respectively.
Results and discussion
Base-catalyzed, heterogeneous transesterification of vegetable oils by methanol (also termed transmethylation or methanolysis 20,21) (Scheme 5) is well-studied and has been established to be of a rather complex nature due to the large contributions of multistep chemical reactions as well as mass transfer factors, in the overall kinetics and yield of the fatty acid methyl esters (FAME). These esters are valuable as fuels as they are combustible in diesel engines without modifications.22 The methanolysis reaction occurs primarily between methoxide ions and glycerides adsorbed on the catalyst surface.
Scheme 5.
Overall stoichiometry of the sunflower oil methanolysis.
Of note, methanol and vegetable oil are immiscible liquids requiring proper agitation for the reaction(s) to proceed. As methanolysis produces methanol-soluble FAME, the mass transfer limitations and hence, the rate of the reaction change with increasing conversion.23 Side reactions such as saponification of glycerides and methyl esters and neutralization of free fatty acids by catalyst also can occur. Herein, we concentrated on comparative evaluation of the basic polymer networks as transesterification catalysts, and thus the reaction conditions (product/substrate ratio, vigorous mixing) were set to highlight the catalyst performance. It has been shown previously that with alkaline catalysis, a 6:1 molar ratio of alcohol to vegetable oil results in high conversions (93–98%) of vegetable oil to ester, whereas below the 6:1 ratio conversion decreases. 24,25 In our experiments, a 14.2-fold molar excess of methanol over oil was utilized throughout, which enabled readily quantifiable kinetics with directly comparable reaction rates. Spontaneous methanolysis in the absence of any catalysts has been detected at 70°C, but was observed to be minor (below 7% FAME production after 2 h). Our catalysts were both uncross-linked polymers and their cross-linked networks, all of which initially formed heterogeneous suspensions in the oil/methanol emulsions. While the cross-linked networks maintained their particulate structure and were recoverable from the reaction mixture by a simple filtration, the uncross-linked polymers eventually dissolved and were impossible to recover without solvent evaporation or precipitation, which require large inputs of either energy or solvent, or both. To compare the catalyst performance in our system, we have chosen to quantify the conversion rate in the initial period of the TG methanolysis, where the TG conversion appeared to follow pseudo-first order reaction kinetics, similarly to some other heterogeneous base-catalyzed methanolysis processes: 23
| (1) |
where r is the reaction rate, C is the triglyceride concentration at time t and kobs is the observed rate constant, which takes into account both the mass transfer and the chemical Ct reaction rate. Since TG concentration is related to the conversion degree, , eqn (1) can be transformed into , which upon integration yields:
| (2) |
The observed rate constant, kobs, was thus found from the initial slope of the ln(1−Ft) vs. time plots, which have been observed to be linear (R2>0.98 in all cases).
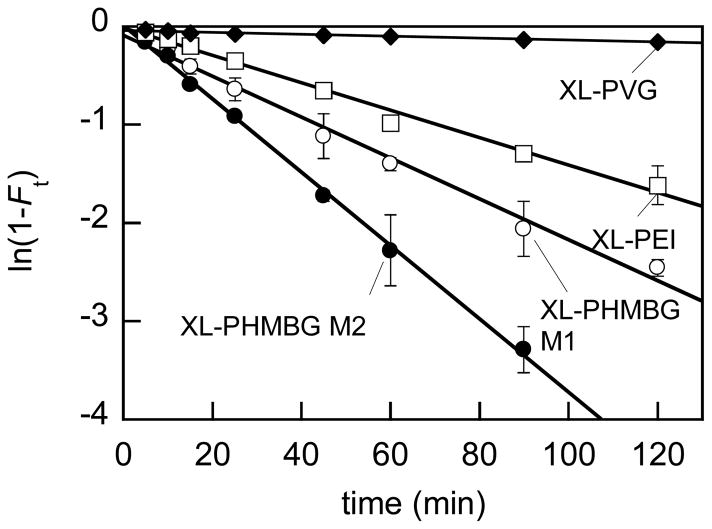
The results of the network testing at 70°C are illustrated in Fig. 2 and comparative results of all measurements are collected in Table 1.
Fig. 2.
Kinetics of sunflower oil methanolysis catalyzed by polymeric networks at 70°C. Initial effective oil and methanol concentrations were 0.73 and 10.3 M, respectively; effective catalyst concentration in all experiments was 14 mg/mL, which corresponds to ca. 42, 180, and 65 mM in the case of XL-PHMBG M1 and M2, XL-PEI, and XL-PVG, respectively. Experiments were conducted in triplicate and standard deviations are shown. Ft denotes the conversion degree (eqn (2)).
Table 1.
Kinetic parameters for the methanolysis of sunflower seed oil catalyzed by organic bases at 70°C.
| Catalysta | Half-life (t1/2, min)b | Second-order rate constant (k″, M−1min−1)c |
|---|---|---|
| TBD | 3±1 | 2.3 |
| PHMBG | 11±3 | 1.4 |
| XL-PHMBG M1 | 28±5 | 0.60 |
| XL-PHMBG M2 | 18±0.4 | 0.94 |
| PEI | 41±6 | 0.093 |
| XL-PEI | 47±5 | 0.083 |
| PVG | 154±13 | 0.067 |
| XL-PVG | 214±14 | 0.050 |
The catalysts are abbreviated as follows: TBD: triazabicyclodecene; PHMBG: poly(hexamethylene biguanide); XL-PHMBG M1: cross-linked poly(hexamethylene biguanide) (Method 1); XL-PHMBG M2: cross-linked poly(hexamethylene biguanide) (Method 2); PEI: poly(ethylene imine); XL-PEI: cross-linked poly(ethylene imine); PVG: poly(N-vinylguanidine); XL-PVG-cross-linked poly(N-vinylguanidine).
Half-life was calculated from t1/2=ln(2)/kobs, where kobs is the experimentally observed reaction rate constant.
Apparent second-order rate constants were calculated as a mean of three measurements from k″=ro/CsCcat, where ro=kobsCs is the initial reaction rate, Cs and Ccat are initial effective concentrations of the substrate (oil) and catalyst, respectively. The Ccat values were calculated as effective molar concentrations of the catalytic groups.
As is seen in Fig. 2 and Table 1, PHMBG-based networks are efficient catalysts, with PHMBG being the most active among polymeric species studied.
Based on the k″ values, PHMBG activity is comparable to that of triazabicyclodecene (TBD), one of the strongest organic, low molecular weight bases known that has been shown to be active in oil transesterification. 22 Substrate conversion of 100% was observed with both TBD and PHMBG within 0.5 h at 70°C and at effective catalyst concentrations of 100 and 42 mM, respectively. Methanolysis in the presence of cross-linked PHMBG networks was 1.5–2.4-fold slower than that with uncross-linked PHMBG, which can be attributed to the differences in the particle size between cross-linked and uncross-linked species. With PHMBG, a clear methanolic solution was attained after 2-hour kinetics at 70°C, whereas the particle size of the cross-linked species did not change significantly under identical conditions, and the suspensions remained heterogeneous. Of importance for practical applications of the PHMBG-based gels, they enabled greater than 90% conversion of the triglycerides within 1 h. Interestingly, the content of the accessible biguanide groups in the cross-linked XL-PHMBG gels measured by potentiometric titration in water was essentially equal to that of the PHMBG solution. That is, the consumption of the amino/imino moieties of the biguanide groups due to the cross-linking (Schemes 1 and 2) did not significantly diminish the capacity of the resulting gels for protonation in water. The pKa of the XL-PHMBG M1 and M2 measured in water was equal to that of PHMBG polymer. However, in this case the size of the catalyst particles evidently affected the reaction rate due to the effect of intraparticle diffusion resistance 23 (compare complete dissolution of PHMBG vs 184±45 μm and 127±14 μm particles of XL-PHMBG M1 and XL-PHMBG M2, respectively, in methanol). Such resistance in the case of relatively bulky and hydrophobic reactants such as triglycerides is significant. 3 Similar considerations apply to the case of uncross-linked PEI (liquid fully dispersible in methanol/oil emulsion at 70°C) and its XL-PEI gel counterpart, which retained its particulate structure under the reaction conditions (Table 1). The overall slower catalysis rate with the PEI-based catalysts than with the PHMBG-based species can be explained by the lower basicity of the primary and secondary amino and imino groups relative to that of biguanide groups. On the other hand, poly(N-vinylguanidine) (PVG) and its cross-linked counterpart, XL-PVG, appeared to be inefficient catalysts (Fig. 2 and Table 1), despite their higher basicity in water compared to that of the PHMBG- and PEI-based species. This interesting observation indicates the effect of the low accessibility of the catalytic guanidine groups of PVG and XL-PVG by the triglyceride and methanol reactants. The PVG-based species were insoluble in methanol or oil due to the lack of hydrophobic moieties. The swelling degree of XL-PVG in methanol was 4.2 to 4.7-fold lower than that of the XL-PHMBG and 5.6-fold lower than XL-PEI networks (see Experimental). Analogously, the accessibility of the catalytic groups played an important role in the catalysis of transesterification of vegetable oils by basic gel-type ion-exchange resins of cross-linked polystyrene quaternized by either trimethylamine or dimethylethanolamine.3
It is interesting to note that although Gelbard et al., who were first to report on the efficient catalysis of the transesterification of triglycerides from vegetable oils by soluble and immobilized guanidines and biguanides, 10,22,26,27 mentioned PHMBG as an available polymer applied as a biocide, they did not test it as a catalyst. Instead, they adopted a synthetic route toward catalysts where low molecular weight catalytic species such as pentamethylguanidine and the like were immobilized on cross-linked, non-catalytic polystyrene gel beads. The guanidines immobilized but not covalently attached onto poly(styrene/divinylbenzene) gels demonstrated only slightly lower activity than their homogeneous analogs and reached the same high conversions after prolonged reaction time. 10 The immobilized guanidines slowly leached from the gel supports and participated in substitution reactions during the recycling experiments to form inactive guanidinium compounds. Covalent grafting of N-heptaalkyl biguanides on non-porous chloromethylpolystyrene gel beads afforded recyclable heterogeneous catalysts with yields above 90% obtained in less than 15 min at 70°C in methanolysis of vegetable oils. 27 This is approximately 4.5-fold greater reaction rate than that observed in our work with XL-PHMBG M2 networks. However, in order to achieve equal effective concentrations of catalytic biguanide groups as in our PHMBG-based networks, at least a 10-fold higher volume fraction of the polystyrene beads surface-modified with biguanide would be required, which can affect both stirring and the product recovery.
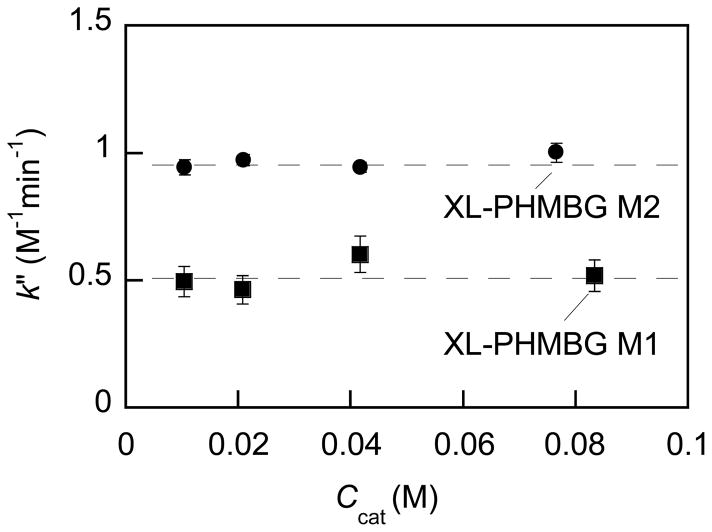
In order to test a degree of accessibility of the catalytic groups in our PHMBG networks, we varied the effective concentration of the catalyst and the results are shown in Fig. 3.
Fig. 3.
Apparent second-order rate constant (k″) versus effective concentration of biguanide groups of PHMBG networks. Apparent second-order rate constant was calculated as a mean of three measurements as described in Table 1. The Ccat values were calculated as effective molar concentrations of the biguanide groups.
As is seen in Fig. 3, within the concentration range studied, the apparent second-order rate constant is independent of the biguanide group concentration, which at Cs=const indicates that the catalytic rate constant is proportional to the Ccat, i.e., increase in the mass of catalyst yielded proportional increase in the biguanide group concentration. That is, in these experiments the same fraction of the biguanide groups was accessible for the reactants, which proves reproducibility of our measurements and can be advantageous in the process scaleup.
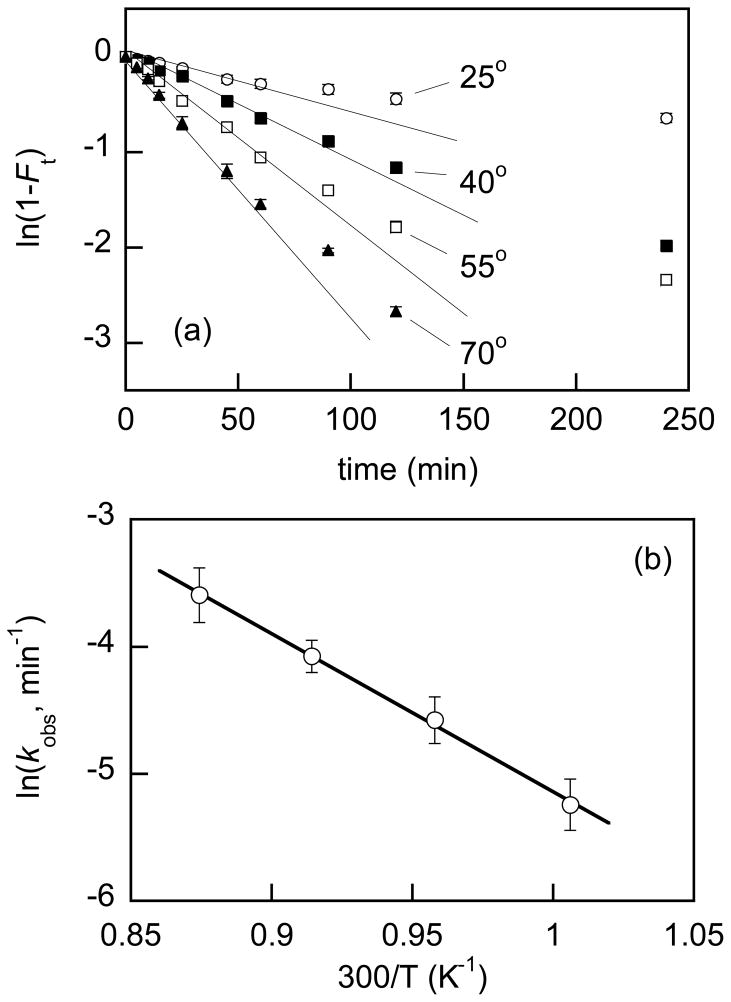
Methanolysis in the presence of PHMBG-based networks was studied in the temperature range from 25 to 70°C and representative kinetic data and corresponding Arrhenius plot are shown in Fig. 4. The appreciable efficiency of the XL-PHMBG M1 network is illustrated by the approximately 50% triglyceride conversion after 2 h even at room temperature. The Arrhenius model applied to the temperature dependence of the kobs values,28 yielded activation energies (Ea) of 31–35 kJ/K mol over the temperature range studied with both PHMBG-based networks. These values are well within the typical Ea ranges observed in heterogeneously catalyzed methanolysis of vegetable oils with other basic catalysts.28,29
Fig. 4.
Temperature dependence of sunflower oil methanolysis catalyzed by XL-PHMBG M1 networks in the temperature range 25–70°C (a) and corresponding Arrhenius plot (b). Initial effective oil and methanol concentrations are 0.73 and 10.3 M, respectively; effective catalyst concentration ca. 42 mM. Experiments were conducted in triplicate and standard deviations are shown. Solid lines represent initial slopes.
In addition to the catalytic efficiency of the PHMBG-based networks shown here, we addressed the question of their recovery and reuse. It was observed that under anhydrous conditions, both XL-PHMBG M1 and XL-PHMBG M2 networks were recovered efficiently and could be reused for at least 15 cycles without significant loss of catalytic activity. The XL-PHMBG M1 and XL-PHMBG M2 networks that demonstrated k″ values of 0.59±0.08 and 0.94±0.024 M−1min−1, respectively, in the first cycle of use, also showed k″ values of 0.54±0.10 and 0.80±0.14 M−1min−1, respectively, in the 15-th cycle, with only 10–15% decline in performance and approximately 75% of recovery on a weight basis. The gradual losses of the catalyst in each step (which were less than 3 to 4 wt%) are attributable to the minor losses after centrifugal filtration (see Experimental). In general, our PHMBG-based networks demonstrated much higher levels of regeneration than observed with cationic, commercially available macroporous ion-exchange resins.3
Conclusions
The present work was undertaken on the simple premise that efficient polymeric catalysts for the transesterification of vegetable oil by methanol can be developed based on commercially available polybases that are cross-linked to render them insoluble in oil/methanol mixtures so that they can be readily recovered by filtration. Three polycationic systems were studied, composed of poly(hexamethylene biguanide) (PHMBG, pKa in water ~11), branched polyethyleneimine (PEI, pKa, 9.7), and poly(N-vinylguanidine) (PVG, pKa ~13). While PHMBG and PEI are commercially available, PVG was obtained via guanidinylation of polyvinylamine by cyanamide. The networks were synthesized by cross-linking of PHMBG by either 4,4′-methylenebis(N,N-diglycidylaniline) or polyisocyanate prepolymer, PEI with sebacoyl chloride, and PVG with 1,4-butanediol diglycidyl ether. Comparison of the methanolysis rates in anhydrous conditions revealed that uncross-linked PHMBG was a remarkably efficient catalyst, enabling 100% triglyceride conversion within 0.5 h at 70°C. The PHMBG-based networks also demonstrated 100% conversion, but the kinetics were 1.5- to 2.4-fold lower than those with uncross-linked PHMBG (which is soluble in methanol) due to the less efficient heterogeneous catalysis by the cross-linked networks. The PEI-based networks catalyzed the methanolysis, but with rates 8- to 12-fold lower than their PHMBG-based counterparts. The PVG-based networks devoid of hydrophobic moieties appeared to be inefficient catalysts, probably due to the lack of swelling in the oil/methanol mixtures and, hence, insufficient availability of the basic guanidine groups. The PHMBG networks, which demonstrated the best catalytic rates, were also shown to be recyclable by a simple centrifugal filtration. After 15 cycles of recovery and reuse, only 10–15% decline in performance and approximately 75% of recovery on a weight basis was observed with these networks.
Acknowledgments
The authors are grateful to National Center for Research Resources (Puerto Rico) for their support of this work under grants P20 RR016470, S06 GM-08216, and GM-08102.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sivasamy A, Cheah KY, Fornasiero P, Kemausuor F, Zinoviev S, Miertus S. ChemSusChem. 2009;2:278–300. doi: 10.1002/cssc.200800253. [DOI] [PubMed] [Google Scholar]
- 2.Haas MJ, McAloon AJ, Yee WC, Foglia TA. Bioresource Technol. 2006;97:671–678. doi: 10.1016/j.biortech.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Kim M, Salley SO, Ng KYS. Energy & Fuels. 2008;22:3594–3599. [Google Scholar]
- 4.Hara M. ChemSusChem. 2009;2:129–135. doi: 10.1002/cssc.200800222. [DOI] [PubMed] [Google Scholar]
- 5.Jérôme F, Pouilloux Y, Barrault J. ChemSusChem. 2008;1:586–613. doi: 10.1002/cssc.200800069. [DOI] [PubMed] [Google Scholar]
- 6.Raab V, Harms K, Sundermeyer J, Kovacević B, Maksić ZB. J Org Chem. 2003;68:8790–8797. doi: 10.1021/jo034906+. [DOI] [PubMed] [Google Scholar]
- 7.Raab V, Kipke J, Gschwind RM, Sundermeyer J. Chemistry. 2002;8:1682–1693. doi: 10.1002/1521-3765(20020402)8:7<1682::aid-chem1682>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Maksic ZB, Kovacevic B. J Org Chem. 2000;65:3303–3309. doi: 10.1021/jo991592a. [DOI] [PubMed] [Google Scholar]
- 9.Schuchardt U, Sercheli R, Vargas RM. J Braz Chem Soc. 1998;9:199–210. [Google Scholar]
- 10.Schuchardt U, Vargas RM, Gelbard G. J Molec Catalysis A: Chem. 1996;109:37–44. [Google Scholar]
- 11.Bromberg L, Hatton TA. Anal Chem. 2009;81:5637–5645. doi: 10.1021/ac9003437. [DOI] [PubMed] [Google Scholar]
- 12.Vicennati P, Giuliano A, Ortaggi G, Masotti A. Curr Med Chem. 2008;15:2826–2839. doi: 10.2174/092986708786242778. [DOI] [PubMed] [Google Scholar]
- 13.Hughes MA, Wood J, Rosenberg E. Ind Eng Chem Res. 2008;47:6765–6774. [Google Scholar]
- 14.Guana G, Sakuraia N, Kusakabe K. Chem Eng J. 2009;146:302–306. [Google Scholar]
- 15.Bianco A, Furrer J, Limal D, Guichard G, Elbayed K, Raya J, Piotto M, Briand JP. J Comb Chem. 2000;2:681–690. doi: 10.1021/cc0000489. [DOI] [PubMed] [Google Scholar]
- 16.Hattori T, Nakata Y, Kato R. Anal Sci. 2003;19:1525–1528. doi: 10.2116/analsci.19.1525. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Keller MW, Moore JS, White SR, Sottos NR. Macromolecules. 2008;41:9650–9655. [Google Scholar]
- 18.Bromberg L, Hatton TA. Polymer. 2007;48:7490–7498. [Google Scholar]
- 19.Gelbard G, Bres O, Vargas RM, Vielfaure F, Schuchardt UF. J Am Oil Chem Soc. 1995;72:1239–1241. [Google Scholar]
- 20.Vicente G, Martinez M, Aracil J, Esteban A. Ind Eng Chem Res. 2005;44:5447–5454. [Google Scholar]
- 21.Boocock DGB, Konar SK, Mao V, Lee C, Buligan S. J Am Oil Chem Soc. 1998;75:1167–1172. [Google Scholar]
- 22.Schuchardt U, Vargas RM, Gelbard G. J Molec Catalysis A: Chem. 1995;99:65–70. [Google Scholar]
- 23.Veljković VB, Stamenković OS, Todorović ZB, Lazić ML, Skala DU. Fuel. 2009;88:1554–1562. [Google Scholar]
- 24.Freedman B, Pryde EH, Mounts TL. J Amer Oil Chem Soc. 1984;61:1638–1643. [Google Scholar]
- 25.Freedman B, Butterfield RO, Pryde EH. J Amer Oil Chem Soc. 1986;63:1375–1380. [Google Scholar]
- 26.Gelbard G, Vielfaure-Joly F. Tetrahedron Lett. 1998;39:2743–2746. [Google Scholar]
- 27.Gelbard G, Vielfaure-Joly F. React Funct Polym. 2001;48:65–74. [Google Scholar]
- 28.Noureddini H, Zhu D. J Am Oil Chem Soc. 1997;74:1457–1463. [Google Scholar]
- 29.Liu X, Piao X, Wang Y, Zhu S. Energy & Fuels. 2008;22:1313–1317. [Google Scholar]