Abstract
Background
Medication errors represent a major public health concern, and inadequate prescription drug labels have been identified as a root cause of errors. A new prescription medication labeling system was implemented by Target pharmacies in May 2005 and aimed to improve health outcomes.
Objectives
To evaluate whether the new Target label influenced patient health services utilization.
Subjects
Derived from 2 large health plans.
Research Design and Measures
Using administrative claims, we identified patients with 1 of 9 chronic diseases who filled prescriptions at Target pharmacies and a matched sample who filled prescriptions at other community pharmacies. We stratified our cohort into new and prevalent medication users and evaluated the impact of the Target label on outpatient, emergency department and inpatient health services use. We used linear regression and segmented linear regression to evaluate the new-user and prevalent-user analyses, respectively.
Results
Our sample included 23,745 Target pharmacy users and 162,369 matched non-Target pharmacy users. In the new-user analysis, we found no significant change in rates of both outpatient (event rate ratio: 0.53; 95% CI: 0.15–1.86) and inpatient and emergency department (Event rate ratio: 0.88; 95% CI: 0.62–1.24) health services utilization in Target users after implementation when compared with non-Target users. Similarly, in the prevalent user analysis, we found no change in the level or slope of outpatient or emergency/inpatient services in Target users after implementation of the new label when compared with non-Target users.
Conclusions
We found no statistically significant change in health services use attributable to the implementation of the new prescription drug label at Target pharmacies. These findings highlight the challenge of influencing health outcomes with interventions to improve health literacy.
Keywords: labels, prescription medication, health literacy, health outcomes
According to the Institute of Medicine (IOM) 2006 report, Preventing Medication Errors, more than 500,000 adverse drug events occur in the outpatient setting annually in the United States,1 and poor understanding of drug labeling was cited as a root cause.1 Recent studies indicate that prescription drug labels are highly variable and not patient centered;2,3 nearly half of adults may misunderstand the common dosage instructions and warnings on them.4–6 Patients frequently do not communicate with their physicians or pharmacists about appropriate administration of prescription drugs or their risks, thus relying on labels for such information.7,8 Improved prescription drug labels may reduce the frequency of prescription drug errors and improve health outcomes.9
In May 2005, Target Corporation pharmacies implemented a newly designed prescription drug label aimed at improving readability and understanding, consistent with published evidence regarding optimal format.10 The new design applies increased surface area, larger font for administration directions and warnings, and more white space. The labels incorporate a built-in pouch to store medication information leaflets, facilitating access to detailed medication information throughout the course of therapy. The labels are also color coded within families to minimize errors caused by taking medication prescribed for other family members.
Communication of prescription label information has been described as a necessary means of information acquisition and appropriate medication-taking behavior in several health behavior theories.11 Numerous studies have evaluated the relationship between prescription label format and readability and understanding, but there is little information linking label improvements and patient health outcomes.10 Evidence to suggest that improving labels, a simple and low-cost intervention, can improve health even modestly would have important clinical and public health implications. We sought to assess the relationship between implementation of the new prescription drug label at Target pharmacies with health outcomes, as measured by health services use.
METHODS
The new prescription drug label was implemented on May 1, 2005 at all Target Pharmacies. Using pharmacy claims data, we identified cohorts of subjects with common chronic diseases who filled prescriptions at Target and non-Target community pharmacies, and we compared rates of health services use in both groups.
Data Sources
Our sample was derived from beneficiaries commercially insured by Blue Cross Blue Shield of Minnesota (3,141,907 adult members, including those age >65, with eligibility from July 1, 2003 to May 1, 2006) or Horizon Blue Cross Blue Shield of New Jersey (1,847,187 members under 65 with eligibility from July 1, 2003 to May 1, 2006).
Sample
We included patients 18 years and over who were continuously enrolled during the study period, November 2003 to May 2006. From this population, we identified subjects who filled a prescription for a medication used to treat 1 of 9 chronic conditions (atrial fibrillation, diabetes, hyperlipidemia, congestive heart failure, osteoporosis, hypertension, depression, coronary artery disease, and asthma) and had an emergency department, inpatient, or outpatient visit with a diagnosis for that condition during the study period. The medications used for these conditions and the related ICD-9 diagnosis codes are listed elsewhere.12 The first prescription filled in a class was defined as the index prescription. Patients who filled their first prescription at Target pharmacies were considered Target users regardless of where they filled subsequent prescriptions. For each Target pharmacy user, 10 subjects who filled all prescriptions at non-Target pharmacies (non-Target users) were matched on patient gender, age within decade, medication acquired, and calendar quarter of fill date.
Cohort Identification
New User Analysis
From the study sample, we identified “new users” of medications in any of the study classes as patients who filled an eligible prescription during the pre-label change period (May 1, 2004 to November 1, 2004) or the post-label change period (May 1, 2005–November 1, 2005) and had not filled a prescription in the same class in the preceding 6 months. We restricted our sample to patients who had an inpatient, outpatient, or emergency department diagnosis for a chronic disease associated with the drug initiated in the 365 days preceding the index prescription. For the new user analysis, we excluded patients over the age of 65 because prescription coverage changed for many who enrolled in a Medicare Part D plan in January 2006.
Prevalent User Analysis
Target-users who filled a medication between January 1, 2004 and January 31, 2005 and had filled a prescription for a medication in the same class in the prior 6 months were identified as prevalent users. Non-Target users were matched on index prescription filled and calendar quarter of filling, gender, and age within decade. We restricted our sample to subjects who had an inpatient, outpatient, or emergency department diagnosis for a chronic condition associated with the prescription at any point during the study period.
Follow-Up
For new users, follow-up began on the date of the index prescription and ended on the date of the label change (May 1, 2005) for the pre-label change cohort or May 1, 2006 for the post-label change cohort to ensure equal follow-up time for both cohorts (ie, a minimum of 6 months). For the prevalent user analysis, subjects were followed through May 1, 2006. Prevalent users were censored upon turning 65 due to incomplete data related to Medicare Part D enrollment.
Outcomes
Condition-specific outcomes were ascertained during the follow-up period for each patient. The outcomes for each condition and the ICD-9 diagnosis and procedure codes used to identify these outcomes are listed elsewhere.12 We identified inpatient, emergency department, and outpatient diagnoses as separate outcomes. Because of small number of treat-and-release emergency department visits, emergency department, and inpatient outcomes were pooled.
Analysis
New Users
We used a Poisson model to compare outcome rates in Target and non-Target patients. We calculated condition-specific event counts for each subject during the follow-up period. Log person-days of follow-up was used as an offset. We adjusted for baseline covariates measured before the index date including age (linear and quadratic terms), sex, indicators for the presence of 9 chronic conditions, and the number of distinct prescription medications filled during the index month. The interaction of the time variable (before vs. after intervention) and the exposure variable (Target vs. non-Target pharmacies) was the parameter of interest.
We conducted sensitivity analyses to adjust for the “dose” of exposure among users of Target pharmacies in the new-user analysis; we compared patients who filled (1) all their prescriptions at Target (Target consistent) versus (2) those who filled the first prescription at Target and at least 1 subsequent prescription at other pharmacy (Target occasional), with patients who filled prescriptions only at non-Target pharmacies.
Prevalent User Analysis
Monthly outcome rates were calculated for Target and non-Target patients by condition as the number of events during the month divided by the number of subjects contributing to the cohort that month. Subjects with each condition contributed to the denominator for that condition beginning the month of their index date and ending at the first of May 1, 2006, or turning 65. Although patients may have filled prescriptions for multiple conditions, each patient was included only once in this analysis, classified on the basis of the medication they filled first.
Monthly outcome rates were plotted for each condition and setting (inpatient/emergency department and outpatient) for the Target pharmacy and non-Target users from January 1, 2004 to May 1, 2006. We used segmented linear regression analysis to establish the baseline relationship between event rates in Target and non-Target pharmacy users and to identify sudden deviations from this relationship after the introduction of the Target label.13 We used proc autoreg in SAS version 9. A Durbin-Watson test was used to detect serial autocorrelation of error terms; where needed, autocorrelation parameters were included in the model.
RESULTS
We identified 23,745 Target pharmacy users and 162,369 matched non-Target pharmacy users (Table 1). A total of 4807 new-users and 122,346 prevalent users were analyzed.
TABLE 1.
Sample Characteristics
| Characteristics | New Users |
Prevalent Users |
||
|---|---|---|---|---|
| Target (n = 679) | Non-Target (n = 4182) | Target (n = 16,421) | Non-Target (n = 105,925) | |
| N (%) | N (%) | N (%) | N (%) | |
| Female | 395 (58.2) | 2263 (54.8) | 10,156 (61.9) | 62,390 (59.8) |
| Mean age | 44.0 (11.7) | 45.9 (11.1) | 57.9 (15.7) | 59.4 (14.9) |
| Diagnosis of | ||||
| Atrial fibrillation | 16 (2.4) | 70 (1.7) | 1353 (8.2) | 9445 (8.9) |
| Asthma | 5 (0.8) | 30 (0.7) | 3135 (19.1) | 21,159 (20.0) |
| CAD | 29 (4.3) | 207 (5.0) | 2811 (17.1) | 20,521 (19.4) |
| Congestive heart failure | 2 (0.3) | 21 (0.5) | 1160 (7.1) | 8938 (8.4) |
| Depression | 293 (43.2) | 1617 (39.1) | 4952 (30.2) | 27,788 (26.2) |
| Diabetes | 54 (8.0) | 280 (6.8) | 3193 (19.4) | 23,609 (22.3) |
| Hypertension | 169 (24.9) | 1154 (27.9) | 11,093 (67.6) | 77,524 (73.2) |
| Hyperlipidemia | 110 (16.2) | 723 (17.5) | 6365 (38.8) | 40,866 (38.6) |
| Osteoporosis | 24 (3.5) | 206 (5.0) | 1976 (12.0) | 12,351 (11.7) |
| Medication use | ||||
| Calcium channel blockers | 12 (1.8) | 91 (2.2) | 739 (4.5) | 5210 (4.9) |
| Warfarin | 7 (1.0) | 32 (0.8) | 314 (1.9) | 2031 (1.9) |
| Digoxin | 0 (0) | 0 (0) | 110 (0.7) | 795 (0.8) |
| Diabetes | 45 (6.6) | 214 (5.2) | 709 (4.3) | 5085 (4.8) |
| Inhaled steroids | 5 (0.7) | 28 (0.7) | 493 (3.0) | 3079 (2.9) |
| Bisphosphonates | 24 (3.5) | 206 (5.0) | 806 (4.9) | 4931 (4.7) |
| Antiplatelet | 0 (0) | 0 (0) | 102 (0.6) | 791 (0.8) |
| Leukotriene modifiers | 0 (0) | 2 (0.1) | 145 (0.9) | 909 (0.9) |
| Antidepressant | 293 (43.2) | 1617 (39.1) | 3429 (20.9) | 18,352 (17.3) |
| Beta blocker | 90 (13.3) | 574 (13.9) | 2432 (14.8) | 16,562 (15.6) |
| ACE/ARB | 88 (13.0) | 607 (14.7) | 2326 (14.2) | 15,954 (15.1) |
| Statin | 115 (16.9) | 761 (18.4) | 1722 (10.5) | 11,534 (10.9) |
| Healthcare utilization | ||||
| Mean no. unique medications (SD) | 2.4 (2.1) | 2.3 (1.7) | 5.1 (4.7) | 5.2 (4.9) |
New User Analysis
We found no significant difference in overall rates of outpatient or inpatient and emergency department health services utilization in Target users and nonusers during the pre-label change period (Table 2). Similarly, we found no significant difference in rates of outpatient or inpatient and emergency health services utilization before and after implementation in non-Target pharmacy users. In our parameter of interest, the interaction of the use of Target pharmacies and the post-implementation period, we found statistically insignificant trends of reduced rates of both outpatient (event rate ratio: 0.53; 95% CI: 0.15–1.86) and inpatient and emergency (event rate ratio: 0.88; 95% CI: 0.62–1.24) health services utilization in Target users after implementation when compared with non-Target users.
TABLE 2.
Effect of Target Label on Event Rates Among New Users
| Parameter | Inpatient Event Rate Ratio* | Outpatient Event Rate Ratio* |
|---|---|---|
| Age | 1.01 (0.99, 1.03) | 0.99 (0.99, 1) |
| Age2 | 1 (1, 1) | |
| Female sex | 0.6 (0.39, 0.92) | 0.8 (0.7, 0.92) |
| Time of initiation in non-Target users (post-May 1, 2005 vs. pre) | 0.88 (0.59, 1.32) | 0.98 (0.85, 1.12) |
| Target user | 0.9 (0.44, 1.84) | 1.17 (0.94, 1.46) |
| Target × time interaction | 0.53 (0.15, 1.86) | 0.88 (0.62, 1.24) |
| Depression | 2.16 (1, 4.68) | 30.05 (16.74, 53.95) |
| Asthma | 0.24 (0, 40.82) | 7.36 (2.22, 24.35) |
| Hyperlipidemia | 0.9 (0.33, 2.48) | 0.16 (0.03, 0.81) |
| Osteoarthritis | 0.89 (0.14, 5.6) | 5.82 (2.64, 12.84) |
| Diabetes | 2.58 (1.24, 5.37) | 32.73 (18.57, 57.69) |
| Congestive heart failure | 2.01 (0.87, 4.62) | 7.84 (3, 20.48) |
| Coronary artery disease | 4.27 (2.45, 7.45) | 2.35 (1.28, 4.34) |
| Atrial fibrillation | 0.61 (0.19, 1.96) | 1.93 (0.68, 5.46) |
| Hypertension | 1.96 (1.12, 3.43) | 0.92 (0.6, 1.41) |
| No. distinct drugs used in month of first fill | ||
| 5 or more | 8.19 (4.56, 14.71) | 1.65 (1.34, 2.02) |
| 4 | 4.09 (2.04, 8.22) | 1.31 (1.03, 1.67) |
| 3 | 2.61 (1.32, 5.17) | 1.35 (1.11, 1.63) |
| 2 | 1.43 (0.72, 2.85) | 1.03 (0.87, 1.22) |
With 95% confidence intervals.
Of the 679 new users who filled index prescriptions at Target, 198 (29.2%) filled all their prescriptions at Target, whereas the remaining 481 used at least 1 other pharmacy. The Target label change did not have a significant effect on outpatient visit rates in either group relative to non-Target users (RR Target consistent = 1.46, 0.70–3.06; RR Target occasional = 0.80, 0.54–1.18), nor did it significantly affect relative rates of inpatient or ED visits (RR Target consistent = 0.39, 0.01–12.27; RR Target occasional = 0.66, 0.16–2.77).
Prevalent User Analysis
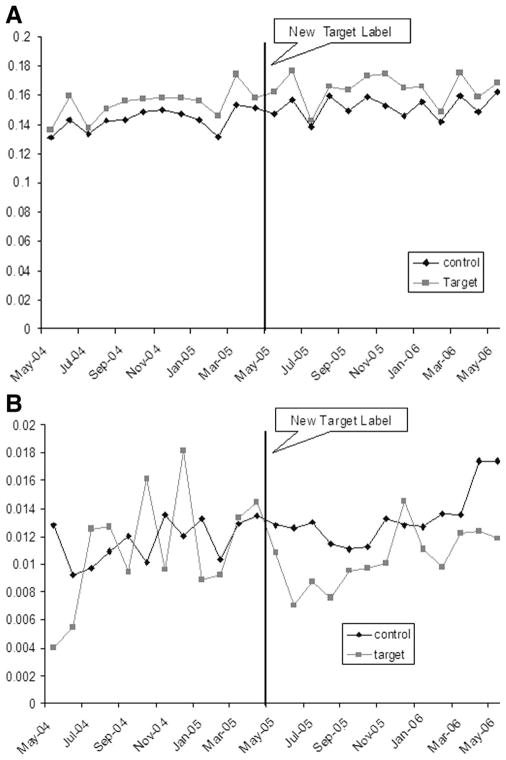
Monthly rates of outpatient and emergency department/inpatient visits, aggregating health service use in all study diseases, are depicted in Figure 1. The baseline monthly outpatient visit rates and ED and hospitalization rates were not statistically different in Target and non-Target pharmacy users (Table 3). We found no change in the level or slope of outpatient or emergency/inpatient services in Target users after implementation of the new label (Table 3).
FIGURE 1.
A, outpatient event rates by cohort. B, inpatient/ER event rates by cohort.
TABLE 3.
Effect of Label Change on Outcomes in Prevalent Users
| Parameter | Outpatient Events/100 Subjects |
Inpatient Events/100 Subjects |
||
|---|---|---|---|---|
| Estimate (95% CI) | P | Estimate (95% CI) | P | |
| Baseline rate in non-Target users | −13.78 (13.16, 14.4) | <0.001 | 1.06 (0.81, 1.31) | <0.001 |
| Pre May 05 slope in non-Target users | 0.1 (0, 0.2) | 0.043 | 0.02 (−0.02, 0.06) | 0.320 |
| Baseline rate difference between Target and non-Target users | 0.85 (−0.01, 1.72) | 0.053 | −0.26 (−0.62, 0.1) | 0.153 |
| Pre May 05 slope difference between Target and non-Target users | 0.05 (−0.09, 0.19) | 0.467 | 0.04 (−0.02, 0.09) | 0.186 |
| Intercept effect of label change | 0.04 (−1.25, 1.34) | 0.946 | −0.39 (−0.91, 0.14) | 0.143 |
| Slope change post May05 in non-Target users | −0.07 (−0.2, 0.05) | 0.243 | 0.02 (−0.04, 0.07) | 0.545 |
| May 05 intercept change in non-Target users | 0.2 (−0.72, 1.12) | 0.661 | −0.22 (−0.59, 0.15) | 0.241 |
| Slope effect of label change | −0.07 (−0.25, 0.11) | 0.462 | −0.04 (−0.12, 0.04) | 0.293 |
DISCUSSION
Little is known about the relationship between labeling improvements and health outcomes. We assessed a natural experiment, the implementation of an improved prescription drug label at Target pharmacies, and its’ effect on health services use. In an analysis of both new and prevalent users of chronic medications, we found no significant reduction in outpatient, emergency department, or inpatient visits associated with the implementation of the new label.
It is unlikely that changes to a prescription label would significantly impact outpatient visits or chronic disease outcomes. The literature describing the extent and associations between health literacy and health outcomes has repeatedly shown strong, gradient relationships with health knowledge, but associations with disease outcomes have not been as clear.14–17 Most health literacy interventions have had only limited success at affecting patient behavior and improving health outcomes, despite demonstrated knowledge gains.18
It is more conceivable that rates of inpatient and emergency room visits could be reduced with a more comprehendible drug label. In our study, no significant relationship was identified. Nonetheless, one may argue that improving labels is valuable regardless of whether or not we can demonstrate changes in outcomes when improved readability and comprehension is demonstrated.10,19 Negative results of this study should not be interpreted as a rationale to abort the use of the new Target label or to discontinue developing improved prescription drug information.
The nonsignificant results may be due to insufficient sample size rather than lack of effect. We would expect that improved prescription drug labels would have the greatest effect on use of new users of medications when delivered with initial prescriptions. However, even evaluating a large national pharmacy in 2 large insurer populations provided us with a relatively small sample size of new users of chronic medications. We did not have the sample size to assess rates of specific adverse events, forcing us to aggregate health services use. The heterogeneity of the outcomes that we combined led to wide confidence intervals and made it challenging to detect signals in the data.
Our results are only generalizable to the population that we studied. Our findings may not apply to patients who are uninsured, beneficiaries of Medicaid, or the elderly, who may be expected to experience the greatest benefit from the intervention.20 It is also possible that sicker or older patients selected Target pharmacies after learning about the new label designs, but our matching should have limited such selection bias.
Regardless of these findings, it is widely believed that providing patients with prescription drug labels that are easier to read and understand is of value. Nonetheless, such simple interventions are unlikely to substantially affect health outcomes. Reliance on prescription drug labels to communicate messages about safe and appropriate medication use will not be sufficient, and continued efforts to improve provider-patient communication about medication use and better systems to support appropriate administration will play an integral role in improving care.
Acknowledgments
Supported by a Pioneer Award from the Robert Wood Johnson Foundation. Also, supported by a career development award from the National Heart, Lung, and Blood Institute (HL-090505) (to W.H.S.) and by a career development award from the National Institute of Health (AG-027400) (to M.A.B.).
Footnotes
This project was approved by the Partners Healthcare Institutional Review Board.
References
- 1.Aspden P, Wolcott J, Bootman L, et al., editors. Institute of Medicine. Preventing Medication Errors. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 2.Shrank WH, Agnew-Blais J, Choudhry NK, et al. The variability and quality of medication container labels. Arch Intern Med. 2007;167:1760–1765. doi: 10.1001/archinte.167.16.1760. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Standardizing Medication Labels: Confusing Patients Less: Workshop Summary. Washington, DC: National Academies Press; 2008. [Google Scholar]
- 4.Wolf MS, Shrank WH, Choudhry NK, et al. Variability in pharmacy interpretations of physician prescriptions. Med Care. 2009;47:370–373. doi: 10.1097/MLR.0b013e31818af91a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf MS, Davis TC, Shrank W, et al. A critical review of FDA-approved Medication Guides. Patient Educ Couns. 2006;62:316–322. doi: 10.1016/j.pec.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Davis TC, Wolf MS, Bass PF, et al. Literacy and misunderstanding of prescription drug labels. Ann Intern Med. 2006;145:887–894. doi: 10.7326/0003-4819-145-12-200612190-00144. [DOI] [PubMed] [Google Scholar]
- 7.Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166:1855–1862. doi: 10.1001/archinte.166.17.1855. [DOI] [PubMed] [Google Scholar]
- 8.Svarstad BL, Bultman DC, Mount JK. Patient counseling provided in community pharmacies: effects of state regulation, pharmacist age, and busyness. J Am Pharm Assoc (Wash) 2004;44:22–29. doi: 10.1331/154434504322713192. [DOI] [PubMed] [Google Scholar]
- 9.Shrank WH, Avorn J. Educating patients about their medications: the potential and limitations of written drug information. Health Aff (Millwood) 2007;26:731–740. doi: 10.1377/hlthaff.26.3.731. [DOI] [PubMed] [Google Scholar]
- 10.Shrank W, Avorn J, Rolon C, et al. Effect of content and format of prescription drug labels on readability, understanding, and medication use: a systematic review. Ann Pharmacother. 2007;41:783–801. doi: 10.1345/aph.1H582. [DOI] [PubMed] [Google Scholar]
- 11.Wogalter MS, Sojourner RJ. Research on pharmaceutical labeling: an information processing approach. In: Park DC, Morrell RW, Shifren K, editors. Processing of Medical Information in Aging Patients Cognitive and Human Factors Perspectives. Mahwah, MJ: Lawrence Erlbaum Associates Publishers; 1999. pp. 291–310. [Google Scholar]
- 12.Shrank WH, Gleason PP, Canning C, et al. Can improved prescription medication labeling influence adherence to chronic medications? An evaluation of the target pharmacy label. J Gen Intern Med. 2009;24:570–578. doi: 10.1007/s11606-009-0924-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 14.Dewalt DA, Berkman ND, Sheridan S, et al. Literacy and health outcomes: a systematic review of the literature. J Gen Intern Med. 2004;19:1228–1239. doi: 10.1111/j.1525-1497.2004.40153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arozullah AM, Lee SY, Khan T, et al. The roles of low literacy and social support in predicting the preventability of hospital admission. J Gen Intern Med. 2006;21:140–145. doi: 10.1111/j.1525-1497.2005.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker DW, Wolf MS, Feinglass J, et al. Health literacy and mortality among elderly persons. Arch Intern Med. 2007;167:1503–1509. doi: 10.1001/archinte.167.14.1503. [DOI] [PubMed] [Google Scholar]
- 17.Morris NS, MacLean CD, Littenberg B. Literacy and health outcomes: a cross-sectional study in 1002 adults with diabetes. BMC Fam Pract. 2006;7:49. doi: 10.1186/1471-2296-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pignone M, DeWalt DA, Sheridan S, et al. Interventions to improve health outcomes for patients with low literacy. a systematic review. J Gen Intern Med. 2005;20:185–192. doi: 10.1111/j.1525-1497.2005.40208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis TC, Federman A, Bass PF, et al. Improving patient understanding of prescription container label instructions. J Gen Intern Med. 2009;24:57–62. doi: 10.1007/s11606-008-0833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis TC, Wolf MS, Bass PF, III, et al. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med. 2006;21:847–851. doi: 10.1111/j.1525-1497.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]