Abstract
A GPI anchor bearing unsaturated fatty acid lipid chains (1) was synthesized by a highly convergent strategy employing the para-methoxybenzyl group for permanent hydroxyl protection. The final global deprotection was achieved by an efficient three-step, one-pot procedure to give an 81% isolated yield of the target structure.
Glycosylphosphatidylinositols (GPIs) are a class of complex glycolipids that are ubiquitously expressed by eukaryotic cells and are operative in many biological processes, most notably the anchorage of extracellular molecules, such as surface proteins and glycoproteins, to the cell membrane.1 Over 30 GPI anchors have been identified since the T. brucei variant surface glycoprotein GPI anchor was first characterized by Ferguson and co-workers in 1988.2 All GPIs identified so far contain the following conserved core: H2NEt-(P)-6-Manα(1→2)Manα(1→6)Manα(1→4)GlcNH2α (1→6)myo-inositol-1-(P)-glycerolipid. Structural diversity among GPI anchors arises from additional phosphoethanolamine and carbohydrate moieties linked to the various locations of the core, as well as modifications in the lipid chains, which may contain unsaturated bonds that are crucial to bioactivity.3
In order to probe the effects of such structural modifications and better understand the scope and mechanism of GPI anchoring, it is necessary to have access to pure and structurally well-defined samples of GPI anchors, GPI analogues, and functionalized GPIs. Advances in chemical synthesis have enabled the preparation of GPI anchors by several research groups,4 including ours,5 but limitations associated with current synthetic strategies prevent the inclusion of some important functional groups. For example, most reported GPI syntheses have employed the benzyl group for permanent hydroxyl protection, which precludes the incorporation of alkene, alkyne, azide, thiol, thioether, and other functionalities that are intolerant to Pd-catalyzed hydrogenolysis. To address this issue, Nikolaev and co-workers4i utilized the benzoyl group for permanent protection to achieve the first synthesis of GPI anchors containing unsaturated lipid chains. However, the usage of acyl groups for hydroxyl protection complicates the presence of other esters and/or peptides/glycopeptides in the target molecule due to sensitivity to deprotection with sodium methoxide. For instance, the reported deacylation step in Nikolaev’s synthesis gave ≤ 40% yield, likely a result of degradation of the ester-linked lipid chains.4i Ideally, such a precarious and low-yielding step should be avoided, particularly when dealing with valuable and complex late-stage intermediates. Therefore, the efficient synthesis of GPI anchors containing unsaturated lipids and other useful functional groups has essentially remained an unsolved problem.
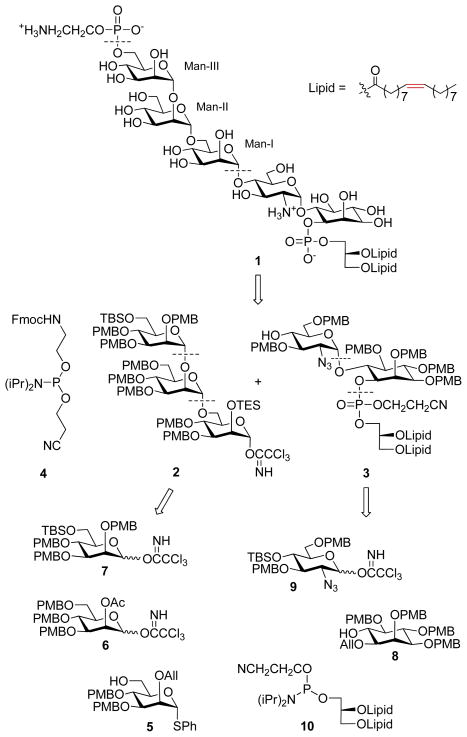
In this work, we sought to develop a new strategy for the synthesis of functionalized GPIs using the para-methoxybenzyl (PMB) group for permanent hydroxyl protection. PMB ethers can be cleaved under mild acidic or oxidative conditions, which we anticipated would provide the flexibility necessary for the incorporation of a wide range of functional groups in target molecules. As a demonstration of the value and feasibility of this methodology in the preparation of functionalized GPIs, as well as other glycoconjugates, we set out to synthesize a GPI anchor 1, which bears unsaturated fatty acid lipid chains. Scheme 1 depicts the retrosynthesis of target molecule 1. A convergent strategy was designed for the assembly of the target GPI from trimannose 2, pseudodisaccharide 3, and phosphorylating reagent 4. Fragment 3 was stitched together with mannose building blocks 5–7, while 4 was synthesized from inositol derivative 8, glucosaminyl donor 9, and phosphoglycerolipid precursor 10. For the glycosylations, we mainly utilized the Schmidt trichloroacetimidate method,6 which can be achieved under relatively mild conditions.
Scheme 1.
Retrosynthesis of GPI anchor 1
Mannose building blocks 5–7 were prepared from common mannose derivative 11 (Scheme 2). Thioglycoside alcohol 5 was obtained from known diol 127 via a three-step sequence including stannylene acetal-directed regioselective 3-O-methoxybenzylation, 2-O-allylation, and regioselective para-methoxybenzylidene ring opening using DIBAL-H. Compound 6 was derived from 11 via orthoester 15,8 which after deacetylation-methoxybenzylation was treated with acetic acid to open the orthoester ring, providing a hemiacetal that was then converted to the corresponding imidate. The synthesis of 7 started with BF3-promoted glycosylation of 11 with allyl alcohol. Subsequent deacetylation and differentiation of the 6-O-position by reaction with para-methoxytrityl (PMTrt) chloride afforded triol 16. Methoxybenzylation of the 2-, 3-, and 4-O-positions was followed by swapping the PMTrt group for a tert-butyldimethylsilyl (TBS) group to produce 17. Finally, Pd-catalyzed deallylation revealed the anomeric hydroxyl group, which was transformed into imidate 7.
Scheme 2.
Synthesis of mannose building blocks 5–7a
a Conditions: (a) Bu2SnO, toluene, reflux; PMBCl, CsF, DMF. (b) NaH, AllBr, DMF, 68% (two steps). (c) DIBAL-H, CH2Cl2, 82%. (d) NaOMe, MeOH. (e) NaH, PMBCl, DMF, 76% (two steps). (f) AcOH-H2O (1:1), 70%. (g) Cl3CCN, DBU, CH2Cl2, 77%. (h) AllOH, BF3·OEt2, MS 4 Å, CH2Cl2, 66% (two steps from D-mannose). (i) NaOMe, MeOH. (j) PMTrtCl, pyridine, 66% (two steps). (k) NaH, PMBCl, DMF, 73%. (l) AcOH, H2O, CH2Cl2, 94%. (m) TBSCl, pyridine, 88%. (n) PdCl2, AcOH, NaOAc, CH2Cl2, H2O, 82%. (o) Cl3CCN, DBU, CH2Cl2, 73%.
With monosaccharides 5, 6, and 7 in hand, the construction of fragment 2 commenced (Scheme 3). The glycosylation of 5 by imidate 6 proceeded smoothly using catalytic TMSOTf in CH2Cl2 at 0 °C. The reaction generated only the α-product 18, which was deacetylated with K2CO3/MeOH to give 19 in 66% yield over two steps. Reaction of 19 with imidate 7 under the same conditions gave trimannose 20 in a 5:1 α/β ratio, a figure that was improved to α-only (76% yield) upon switching the solvent from CH2Cl2 to Et2O. Compound 20 was designed as a versatile key intermediate for the synthesis of various GPIs containing a substituent at the Man-I 2-O-position. For the synthesis of GPI anchor 1, however, the allyl group of 20 was replaced with a TES group to insure later compatibility with the lipidic unsaturated bonds. Thus, 20 was subjected to Cha’s deallylation protocol,9 which proved to be the most effective after attempting several methods. The resulting alcohol then underwent protection by TESOTf to give 21, which was converted to imidate 2 in two steps, including NIS/AgOTf-promoted hydrolysis of the thioacetal and then treatment of the resulting hemiacetal with Cl3CCN and DBU.
Scheme 3.
Synthesis of trimannose 2a
a Conditions: (a) TMSOTf (cat.), MS 4 Å, CH2Cl2, 0 °C. (b) K2CO3, MeOH, 66% (two steps). (c) 7, TMSOTf (cat.), MS 4 Å, Et2O, 0 °C, 76%. (d) Ti(OiPr)4, cyclopentylmagnesium chloride, THF, Et2O, 82%. (e) TESOTf, 2,6-lutidine, CH2Cl2, 90%. (f) NIS, AgOTf, TTBP, wet CH2Cl2, 74%. (g) Cl3CCN, DBU, CH2Cl2, 88%.
En route to optically pure inositol 8 (Scheme 4), racemate (±)-22 was generated from myo-inositol using a reported procedure.10 Here, we protected both the 1-O- and 6-O-positions as allyl ethers to give (±)-23, which underwent acid-catalyzed methanolysis of the ketals followed by methoxybenzylation, resulting in (±)-25. Deallylation using Cha’s method9 was again effective, providing diol (±)-26 in 85% yield. Positions 1 and 6 were differentiated at this stage using stannylene acetal-directed selective allylation to protect the 1-O-position (72%), and the free 6-O-position of (±)-8 was then used as an anchoring point for chiral resolution. After esterification with (1S)-(−)-camphanic chloride, the diastereomeric products were separated by preparative HPLC to afford optically pure (−)-27, which was saponified to give inositol alcohol 8 in 44% yield over two steps, including the enantiomeric resolution (maximum yield 50%).11
Scheme 4.
Synthesis of inositol 8a
a Conditions: (a) cyclohexanone dimethyl ketal, TsOH, DMF, 38%. (b) NaH, AllBr, DMF, 81%. (c) AcCl, CH2Cl2, MeOH, 98%. (d) NaH, PMBCl, DMF, 73%. (e) Ti(OiPr)4, cyclohexylmagnesium chloride, THF, Et2O, 85%. (f) Bu2SnO, toluene, reflux; AllBr, CsF, DMF, 72%. (g) (1S)-(−)-camphanic chloride, DMAP, Et3N, CH2Cl2, 46%. (h) 1M NaOH, MeOH, THF, 95%.
Preparation of glucosaminyl donor 9 (Scheme 5) began with the transformation of known sugar 28,12 which contained a non-participating azido group at the 2-position, to allyl glycoside 29. Deacetylation with NaOMe formed a triol that was reacted with para-anisaldehyde dimethyl acetal to afford 30. A PMB group was installed at the 3-O-position, and then regioselective opening of the para-methoxybenzylidene ring using HCl and NaBH3CN provided 32. After usage of TBSOTf to protect the 4-O-position, the anomeric allyl ether was removed by a protocol involving Ir-catalyzed isomerization to the corresponding vinyl ether and subsequent hydrolysis using Hg(II).13 The resulting hemiacetal 33 was readied for glycosylation by conversion to imidate 9.
Scheme 5.
Synthesis of glucosamine 9a
a Conditions: (a) AllOH, SnCl4, MS 4 Å, CH2Cl2, 81%. (b) NaOMe, MeOH. (c) anisaldehyde dimethyl acetal, CSA, DMF, 77% (two steps). (d) NaH, PMBCl, DMF, 97%. (e) NaBH3CN, dry HCl in Et2O, MS 4 Å, THF, 71%. (f) TBSOTf, 2,6-lutidine, CH2Cl2, 85%. (g) [Ir(COD)(PMePh2)2]PF6, H2, THF; then HgCl2, HgO, acetone, H2O, 85%. (h) Cl3CCN, DBU, CH2Cl2, 83%.
The glycosylation of inositol derivative 8 with glucosaminyl imidate 9 was achieved with catalytic TMSOTf in CH2Cl2 at 0 °C to form pseudodisaccharide 34 (Scheme 6). While the conversion rate was very good (84%), stereoselectivity slightly favored the undesired β anomer (α/β 1.0:1.6). However, when Et2O was used as the solvent, a moderately improved stereoselectivity was obtained (α/β 1.2:1.0). After the anomeric mixture was separated by preparative HPLC (separation by silica gel column following the next step was also possible), the Ir/Hg deallylation protocol was utilized to expose the inositol 1-O-position, giving 35 in 96% yield. Next, the unsaturated phospholipid was installed by reaction with freshly prepared phosphoramidite 10 under the influence of tetrazole. The intermediate phosphite was selectively oxidized in situ to a phosphate using tert-butyl hydroperoxide at −40 °C. Exposure of the product to Et3N·3HF for 5 days removed the presumably hindered 4-O-TBS group, which gave compound 3 as a 1:1 diastereomeric mixture, originating from the stereogenic phosphorus atom, in 56% yield over two steps. The resulting mixture was separated by preparative HPLC to facilitate the characterization of 3 and subsequent complex intermediates.
Scheme 6.
Synthesis of lipidated pseudodisaccharide 3a
a Conditions: (a) TMSOTf (cat.), MS 4 Å, Et2O, 44%. (b) [Ir(COD)(PMePh2)2]PF6, H2, THF; then HgCl2, HgO, acetone, H2O, 96%. (c) 10, tetrazole, CH3CN, CH2Cl2; then t-BuOOH, −40 °C. (d) Et3N·3HF, THF, CH3CN, 56% (two steps).
The key step in the final stage of the synthesis was to couple the trimannose and peusdodisaccharide fragments (Scheme 7). Trimannose imidate 2 reacted smoothly with 3 in the presence of catalytic TMSOTf to afford the desired α-pseudopentasaccharide in 64% yield. Overnight treatment of the product with Et3N·3HF removed the TBS and TES groups to provide diol 36, of which the anomeric JCH coupling constants confirmed α stereochemistry for all mannose units.14 Installation of the phosphoethanolamine group by treatment with phosphoramidite 4 for a short period (1 h) was selective for the Man-III 6-O-position to give 37.15 To obtain the target GPI 1, a three-step, one-pot deprotection protocol was developed to efficiently remove all of the protecting groups from 37 in 4 h: (i) Zn-mediated reduction of the azide; (ii) removal of the base-labile Fmoc and cyanoethoxy groups with DBU; (iii) hydrolysis of all PMB ethers with 10% TFA. The target GPI anchor 1 was finally obtained in 81% yield after purification with a Sephadex LH-20 column and was characterized with 1H NMR spectroscopy and MALDI-TOF MS.
Scheme 7.
Assembly of GPI anchor 1a
a Conditions: (a) TMSOTf (cat.), MS 4 Å, CH2Cl2, 64%. (b) Et3N·3HF, THF, CH3CN, 70%. (c) 4, tetrazole, CH3CN, CH2Cl2; then t-BuOOH, −40 °C, 50% for 37 (61% BRSM). (d) Zn, AcOH, CH2Cl2, 2 h; DBU, CH2Cl2 1 h; CH2Cl2-TFA (9:1), 1 h, 81% (three steps).
In summary, a GPI anchor containing unsaturated lipid chains was efficiently synthesized using the PMB group for hydroxyl protection. This represents a potentially generally useful strategy for the design and synthesis of uniquely functionalized GPIs, as well as other complex oligosaccharides, which may not be easily accessible by means of traditional protection methods, such as using benzyl, acetyl, or benzoyl groups. We are currently using this synthetic strategy to prepare various biofunctionalized GPI anchors aimed at probing cell surface GPIomics.
Supplementary Material
Acknowledgments
This work was funded by NIH/NIGMS (R01GM090270). We thank Dr. B. Shay and Dr. L. Hryhorczuk for MS measurements, and Dr. B. Ksebati for help with some NMR spectroscopy experiments.
Footnotes
Supporting Information Available: Experimental procedures and spectroscopic data. These materials are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Ferguson MAJ, Williams AF. Annu Rev Biochem. 1988;57:121–138. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]; (b) Englund PT. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]; (c) Paulick MG, Bertozzi CR. Biochemistry. 2008;47:6991–7000. doi: 10.1021/bi8006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson MAJ, Homans SW, Dwek RA, Rademacher TW. Science. 1988;239:753–759. doi: 10.1126/science.3340856. [DOI] [PubMed] [Google Scholar]
- 3.Almeida IC, Camargo MM, Procópio DO, Silva LS, Mehlert A, Travassos LR, Gazzinelli RT, Ferguson MAJ. EMBO J. 2000;19:1476–1485. doi: 10.1093/emboj/19.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Murakata C, Ogawa T. Carbohydr Res. 1992;235:95–114. doi: 10.1016/0008-6215(92)80081-b. [DOI] [PubMed] [Google Scholar]; (b) Mayer TG, Kratzer B, Schmidt RR. Angew Chem Int Ed. 1994;33:2177–2181. [Google Scholar]; (c) Campbell AS, Fraser-Reid B. J Am Chem Soc. 1995;117:10387–10388. [Google Scholar]; (d) Baeschlin DK, Chaperon AR, Charbonneau V, Green LG, Ley SV, Lücking U, Walther E. Angew Chem Int Ed. 1998;37:3423–3428. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3423::AID-ANIE3423>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]; (e) Ruda K, Lindberg J, Garegg PJ, Oscarson S, Konradsson P. J Am Chem Soc. 2000;122:11067–11072. [Google Scholar]; (f) Martín-Lomas M, Khiar N, García S, Koessler JL, Nieto PM, Rademacher TW. Chem Eur J. 2000;6:3608–3621. doi: 10.1002/1521-3765(20001002)6:19<3608::aid-chem3608>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]; (g) Hewitt MC, Snyder DA, Seeberger PH. J Am Chem Soc. 2002;124:13434–13436. doi: 10.1021/ja027538k. [DOI] [PubMed] [Google Scholar]; (h) Ali A, Gowda DC, Vishwakarma RA. Chem Commun. 2005:519–521. doi: 10.1039/b414119a. [DOI] [PubMed] [Google Scholar]; (i) Yashunsky DV, Borodkin VS, Ferguson MAJ, Nikolaev AV. Angew Chem Int Ed. 2006;45:468–474. doi: 10.1002/anie.200502779. [DOI] [PubMed] [Google Scholar]
- 5.(a) Xue J, Guo Z. J Am Chem Soc. 2003;125:16334–16339. doi: 10.1021/ja0382157. [DOI] [PubMed] [Google Scholar]; (b) Wu X, Guo Z. Org Lett. 2007;9:4311–4313. doi: 10.1021/ol701870m. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt RR, Michel J. Angew Chem Int Ed. 1980;19:731–732. [Google Scholar]
- 7.Crich D, Banerjee A. J Am Chem Soc. 2006;128:8078–8086. doi: 10.1021/ja061594u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garegg PJ, Maron L. Acta Chem Scand, Ser B. 1979;B33:39–41. [Google Scholar]
- 9.Lee J, Cha JK. Tetrahedron Lett. 1996;37:3663–3666. [Google Scholar]
- 10.Garegg PJ, Iverson T, Johansson R, Lindberg B. Carbohydr Res. 1984;130:322–326. [Google Scholar]
- 11.The correct enantiomer was identified by comparison of optical rotation with an authentic sample (see supporting information).
- 12.Alper PB, Hung SC, Wong CH. Tetrahedron Lett. 1996;37:6029–6032. [Google Scholar]
- 13.Oltvoort JJ, Van Boeckel CAA, De Koning JH, Van Boom JH. Synthesis. 1981:305–308. [Google Scholar]
- 14.See supporting information.
- 15.Regiochemistry of 37 was confirmed by 1H/COSY NMR spectroscopy: The Man-I 2-H signal did not shift downfield, as would be expected if phosphorylation occurred at this site.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.