Abstract
The benefits of drug company–sponsored PAPs remain unclear.
Drug company–sponsored patient assistance programs (PAPs) provide access to brand-name medications at little or no cost and have been advocated as a safety net for inadequately insured patients. Yet little is known about these programs. We surveyed drug company–sponsored PAPs and found much variability in their structures and application processes. Most cover one or two drugs. Only 4 percent disclosed how many patients they had directly helped, and half would not disclose their income eligibility criteria. A better understanding of PAPs might clarify their role in improving access to medications, the adequacy of existing public programs, and their impact on cost-effective medication use.
The ability of Americans to afford prescription medications is a major public health issue. One-third of Americans of all ages and two-thirds of the elderly report difficulty paying for medications.1 More than a quarter of patients have not filled a prescription or have reduced a prescribed dosage because of its high out-of-pocket cost.2 And although many people obtain drug coverage through employer-based or governmental programs, such as Medicaid, sizable numbers of adult Americans have no such coverage whatsoever.3 Even for people with coverage, such as Medicare Part D, patients face cost sharing through tiered copay-ments or coverage gaps, and these out-of-pocket costs could reduce their use of prescribed medications.4 Cost-related medication underuse has important implications for health and paradoxically might increase overall health costs, because care that is potentially preventable by the use of effective medications could cost more than the drugs themselves.5
Patient assistance programs (PAPs) offered by pharmaceutical manufacturers provide eligible patients with access to brand-name medications at little or no cost. These programs have been advocated as a “safety net for millions of needy Americans who are not eligible for comprehensive assistance programs and [are] unable to afford their medications.”6 Further, a majority of nonprofit clinics that serve largely un- and underinsured patients direct scarce resources toward helping their patients obtain medications through PAPs.7 Pharmaceutical Research and Manufacturers of America (PhRMA) estimates that its Partnership for Prescription Assistance (PPA) program, which it launched in 2005 to bring together a variety of private and public programs, has helped 5.5 million Americans.8
There is limited published information describing the benefits offered by drug company–sponsored PAPs.9 A greater understanding of how many programs exist, the benefits they provide, their eligibility criteria, the application process, how patients receive medications, and the number of patients who have been helped by individual programs could help clarify PAPs’ role in providing access to essential medications for patients with inadequate drug coverage. Accordingly, we evaluated the PPA Web site and surveyed programs run by brand-name drug manufacturers.
Study Data And Methods
Electronic review
We searched the PPA Web site (http://www.pparx.org) in July 2007 for programs offering discounted or free medications to patients. We categorized programs into pharmaceutical manufacturer–sponsored programs; government-sponsored programs, such as state Medicaid programs; and third-party programs, such as those offering pharmacy discount cards.
For drug company–sponsored programs, we searched materials available on the PPA Web site to describe their characteristics across the following domains: (1) the number and names of covered medications; (2) the type of benefit, classified as patient assistance (that is, programs that provide medications with or without copayments), copayment or coinsurance assistance (that is, programs that pay the copayments or coinsurance under patients’ existing insurance plans), patient and copayment assistance, rebates, or other; (3) the amount of copayments or coinsurance required, if any; (4) the enrollment criteria regarding whether and under what circumstances patients can have other drug coverage—in particular, Medicare Part D; (5) the financial and clinical eligibility criteria and the documentation required to substantiate self-reported information; (6) the length and readability of the application; (7) the duration of coverage and the process of prescription and program renewal; and (8) the way in which medications are delivered to patients (that is, via their physicians, health care facilities, pharmacies, or mail). We also determined which of the top-selling brand-name medications in the United States in 2006 were covered by drug company–sponsored PAPs.10 Information was recorded using a structured data extraction tool.
Telephone survey
After our electronic review, two coinvestigators (Lee and Agnew-Blais) contacted drug company–sponsored programs by telephone between September and November 2007. If a representative of the program was not reached during the first attempt, we tried to contact the program two more times. We identified ourselves by name, named the institution we were calling from, and stated that we were attempting to obtain more information about the program. We developed a standardized script to gather data across the domains described above to verify information obtained from our electronic review. In addition, we asked how many patients the program had helped. If we received conflicting information between our electronic review and telephone survey, we relied on the latter. Finally, we asked for application forms to be sent to us if they were not available online.
Analysis
Our analysis consisted of only the drug company–sponsored PAPs that were listed on the PPA Web site and that we were also able to contact by phone. We report our results using descriptive statistics with means, medians, and proportions, as appropriate, for quantitative questions. Readability was assessed using the Flesch-Kincaid Grade Level, which is a reliable and valid tool that uses word and sentence length to determine the school-grade reading level of text.11
What We Found
We identified 285 unique programs listed on the PPA Web site, of which 29 percent are not sponsored by pharmaceutical manufacturers. Of 188 drug company–sponsored programs, we contacted 171 (91 percent). The telephone representatives of six programs (3 percent) were unable or refused to provide answers to more than half of our questions. The remaining 165 programs formed the basis of our analysis.
General program characteristics
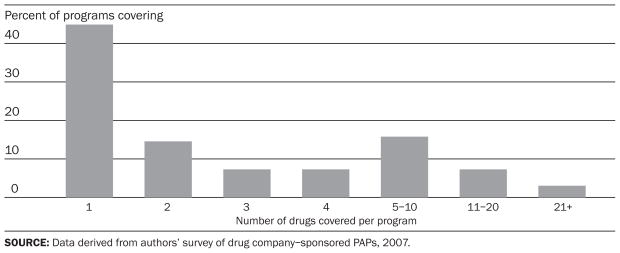
Eighty-two different companies operate the 165 programs we evaluated. Nearly two-thirds of companies have only one program, while others have as many as seven. Collectively, the programs cover 698 medications. The majority of individual programs provide access to only one or two specific drugs (Exhibit 1). Of the top-ten medications in the United States in 2006, all are covered by at least one program, except Zocor, which is now available as a generic (Exhibit 2).12
EXHIBIT 1. Number Of Individual Drugs Covered By Drug Company–Sponsored Patient Assistance Programs (PAPs), 2007.
EXHIBIT 2.
Drug Company–Sponsored Prescription Drug Access Programs For The Ten Top-Selling Medications In The United States In 2006
| Drug | Type of benefit | Income eligibility | Can have other insurance? | Fees or copays required | No. of other drugs covered by program |
|---|---|---|---|---|---|
| Lipitor | PAP | Undisclosed | No | Zero | 62 |
| Pharmacy card | No guidelines | No | Zeroa | 97 | |
| Nexium | PAP | <300% of poverty | No | Zero | 13 |
| PAP | <300% of poverty | Yesb | Zero | 13 | |
| Advair Diskus | PAP | <250% of poverty, spent >$600 on Part D in calendar year | Yesb | Zero | 50 |
| PAP | <250% of poverty | No | $10 | 50 | |
| Aranesp | PAP | <750% of poverty | No | Zero | 5 |
| Prevacid | PAP | <300% of poverty | Yes | Zero | 0 |
| Epogen | PAP | <750% of poverty | No | Zero | 5 |
| Zocor | –c | –c | –c | –c | –c |
| Enbrel | PAP | Undisclosed | Yes | Zero | 0 |
| Seroquel | PAP | <300% of poverty | No | Zero | 13 |
| PAP | <300% of poverty | Yesb | Zero | 13 | |
| Singulair | Pharmacy card | <200% of poverty | Yes | Zerod | 9 |
| PAP | <200% of poverty | Yes | Zero | 11 | |
SOURCES: List of top-selling drugs obtained from IMS Health (see Note 10 in text). Other information derived from authors’ survey of drug company–sponsored pharmacy assistance programs (PAPs), 2007.
NOTES: PAP is patient assistance program. FPL is federal poverty level.
Patients pay 70–85 percent of retail price with discount card.
For Medicare beneficiaries only.
Not applicable; Zocor is available as a generic, so it is not covered by the programs.
Patients pay 80–85 percent of retail price with discount card.
The vast majority (88 percent) are patient assistance programs (that is, they provide medications, with or without copayments, directly to patients who have no or insufficient coverage). Others (2 percent) provide both patient assistance and assistance with copayments. The remaining offer a pharmacy discount card (8 percent), rebates (1 percent), or only copayment assistance (1 percent). As a whole, 8 percent of programs (n = 14) require copayments, ranging from $2 to $150 per prescription (Exhibit 3).
EXHIBIT 3.
Distribution Of Copayments Among Drug Company–Sponsored Patient Assistance Programs (PAPs) That Require Payments, 2007
Only six programs (4 percent) disclosed how many patients they had directly helped; of those that did disclose the information, estimates ranged from “about six a year” to “more than 14,000 patients in 2006 alone.”
Eligibility
The majority of drug company–sponsored programs base eligibility partially on income. Although eighty-seven programs (more than half) would not disclose these income criteria to us, there was a range of criteria among those that did (Exhibit 4). The majority of programs (71 percent) require proof-of-income documents such as tax returns.
EXHIBIT 4.
Income Eligibility Criteria For Drug Company–Sponsored Patient Assistance Programs (PAPs), 2007
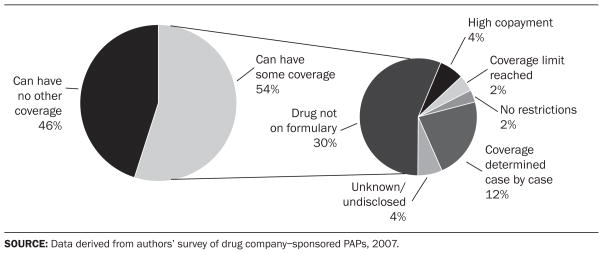
Roughly half of programs allow beneficiaries to have some existing prescription drug coverage (Exhibit 5). Among these, fifty programs (56 percent) cover patients for medications not included on the formulary of their pharmacy benefit plan. Twenty-six programs (29 percent) provide assistance for patients enrolled in Medicare Part D, while an additional fifteen programs (17 percent) consider Part D beneficiaries to be eligible only if they are in the coverage gap known as the “doughnut hole,” in which drug expenses are not covered after the beneficiary spends a specified amount and before coverage resumes again.
EXHIBIT 5.
Distribution Of Drug Company–Sponsored Patient Assistance Programs (PAPs) That Allow Patients To Have Other Coverage, And Circumstances Under Which This Can Occur, 2007
Although 92 percent of programs require patients to submit a prescription as part of the application process, 62 percent do not request any other clinical information. Several programs require additional details, such as the International Classification of Diseases (ICD) code or physician license number for insurance verification (12 percent) or confirmation from a physician that the medications are being used for approved indications (5 percent).
Application process
One program does not require patients to complete an application form at all. Of programs requiring applications, 70 percent make their forms available online. Two additional programs sent us application forms to review when requested. Much variability was seen in the requirements and formats of the applications. Of the 114 application forms available for our review, fifty-four (47 percent) were one or two pages long, fifty-two (46 percent) were three or four pages long, and the remaining eight (7 percent) were five or more pages long. On average, the principal sections of the application forms are written at a tenth-grade reading level (range: grades 6–12). When the disclaimers and disclosures sections that appear in fine print are included, the forms are written at an eleventh-grade reading level (range: grades 7–12).
Administration and fulfillment
Two-thirds of programs provide twelve months of medication coverage; 56 percent provide a three-month supply of medications or less at one time. One-fifth of programs provide automatic refills; the remaining programs require patients to submit refill requests in writing (31 percent) or by telephone (21 percent). In many cases, the medications are delivered to a doctor’s office, care center, or other facility (44 percent); in 28 percent of programs, medications are delivered directly to patients.
Discussion
Our survey identified a large number of PAPs offering discounted or free medications. Drug company–sponsored PAPs collectively cover the most widely prescribed brand-name medications in the United States. In a health care system where many patients either lack prescription drug coverage or have coverage limitations, these programs help some patients obtain important medications.
Limitations of PAPs
Several features of these programs may limit their usefulness. First, the application processes are generally complex, with reading levels greater than those suggested for patients with low health literacy (a problem that is particularly relevant for patients with insufficient insurance coverage).13 Second, instead of supplying patients with medications directly, programs generally give them to patients’ providers, requiring an additional step for patients to obtain them. Third, the majority of programs are focused only on one or two specific drugs, and they vary in the nature of the benefits they provide and the criteria for eligibility. As a result, for patients who require assistance with multiple drugs, there is no standardized application process. Even programs that do cover several drugs are unlikely to be comprehensive enough to meet the needs of patients with multiple chronic diseases. Danielle Chauncey and colleagues found that patients filed application requests for coverage from an average of five distinct programs.14 Although patients frequently rely on clinics that provide care to underserved populations to apply to PAPs on their behalf, completing these applications is burdensome, requiring an average of one hour of personnel time per medication per patient every year. As a result, more than 20 percent of these underfunded clinics do not use manufacturer-sponsored PAPs at all, even though they serve many patients who might benefit from them.15 Accordingly, PAPs’ current structure appears to make accessing them challenging for patients who need them the most.
Lack of transparency
Despite these observations, our analysis highlights the lack of transparency that exists surrounding drug company–sponsored PAPs. Most notably, the number of people who have been helped by these programs and their financial eligibility requirements—and, thus, their role in assisting patients with inadequate coverage—remain unclear. Only six of the programs we surveyed disclosed how many patients to whom they had directly given benefits. More than half of the programs would not tell us their income eligibility criteria. Accordingly, although previous reports have documented the ability of small groups of patients to obtain assistance from pharmaceutical manufacturers, it is unclear what proportion of those who have been helped by PhRMA’s PPA were directly aided by manufacturers and what proportion were referred to existing governmental programs.16
If manufacturer-sponsored PAPs are intended to serve as a “safety net,” but few people can successfully navigate their application processes, then the public’s reliance on them and the resources devoted to them, albeit from private industry, are potentially misplaced. In contrast, if the use of PAPs is in fact highly prevalent, then this highlights the inadequacy of public prescription drug coverage, especially because the financial eligibility criteria of many PAPs overlap with government programs such as Medicaid.
Implications for public drug spending
Drug company–sponsored PAPs may inhibit cost-effective medication use, and their widespread use may have important implications for public drug spending. This potential impact must be better understood. Drug company–sponsored PAPs may steer patients toward and lock them into a particular manufacturer’s product, even when other equally effective and less costly alternatives are available.17 If these patients ultimately acquire better coverage, then they may request unnecessarily expensive medications. In the case of Medicare Part D, patients’ prior use of PAPs that provide subsidies for brand-name products may lead to higher overall individual and public drug spending. This is analogous to the situation in which patients who receive free brand-name drug samples have higher subsequent out-of-pocket drug costs than patients who do not receive such samples.18
Recommendations
Gaining a better understanding of PAPs’ role in the care of patients with inadequate drug coverage should be a clear policy priority. As our results demonstrate, simply asking these programs to report how many patients they have helped may be inadequate. PAPs could be compelled to report this information, perhaps by the Federal Trade Commission, which monitors advertising claims such as those made by the PPA, or the Internal Revenue Service, if drug makers seek tax benefits for providing medications to eligible patients. Of course, even if these strategies were legal and privacy concerns could be adequately addressed, forcing the release of this information may unnecessarily antagonize corporations who legitimately seek to help patients with inadequate coverage.
Alternatively, a prospective system could be created that tracks patients who seek and receive coverage from PAPs and evaluates their subsequent patterns of medication use, including that which is provided by public programs. For example, patients who visit the PPA Web site may be asked to register and provide information about the medications they seek and ultimately receive. Although this may pose an additional barrier to access for an already marginalized group of people, this requirement must be weighed against the importance of information that is necessary for adequate policy evaluation, even for a short period of time.
In summary, our results suggest that numerous drug company–sponsored PAPs exist to provide patients with access to a wide variety of medications but that many details about these programs remain unclear. As a result, the extent to which these programs provide a safety net to patients is poorly understood. Policymakers who seek to improve access to medications and reduce disparities in care would benefit from more information about the specifics of these programs. Given the potential implications of PAPs on medication use for un- and underinsured people and the adequacy and spending levels of public and private prescription drug programs, gaining even more information about PAPs should be a policy priority.
Acknowledgments
This was an unfunded study. William Shrank is supported by a career development award (Grant no. K23HL090505-01) from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
NOTES
- 1.Lester W. The Associated Press/Ipsos Poll: Almost a Third of Americans Say Paying for Drugs Is a Problem in Their Families. [accessed December 21 2007];2004 February 25; http://www.ipsos-na.com/news/pressrelease.cfm?id=2064#.; Tseng CW, et al. Elderly Patients’ Preferences and Experiences with Providers in Managing Their Drug Costs. Journal of the American Geriatrics Society. 2007;5512:1974–1980. doi: 10.1111/j.1532-5415.2007.01445.x. [DOI] [PubMed] [Google Scholar]
- 2.Steinman MA, Sands LP, Covinsky KE. Self-Restriction of Medications Due to Cost in Seniors without Prescription Coverage. Journal of General Internal Medicine. 2001;16(12):793–799. doi: 10.1111/j.1525-1497.2001.10412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhry NK, et al. Should Patients Receive Secondary Prevention Medications for Free after a Myocardial Infarction? An Economic Analysis. Health Affairs. 2007;26(1):186–194. doi: 10.1377/hlthaff.26.1.186. [DOI] [PubMed] [Google Scholar]; Choudhry NK, et al. Cost-Effectiveness of Providing Full Drug Coverage to Increase Medication Adherence in Post–Myocardial Infarction Medicare Beneficiaries. Circulation. 2008;117(10):1261–1268. doi: 10.1161/CIRCULATIONAHA.107.735605. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tamblyn R, et al. Adverse Events Associated with Prescription Drug Cost-Sharing among Poor and Elderly Persons. Journal of the American Medical Association. 2001;285(4):421–429. doi: 10.1001/jama.285.4.421. [DOI] [PubMed] [Google Scholar]
- 4.USA Today/Kaiser Family Foundation/Harvard School of Public Health. [accessed 17 February 2009];Health Care Costs Survey. 2005 August; http://www.kff.org/newsmedia/upload/7371.pdf.
- 5.Cubanski J, Neuman P. Status Report on Medicare Part D Enrollment in 2006: Analysis of Plan-Specific Market Share and Coverage. Health Affairs. 2007;26(1):w1–w12. doi: 10.1377/hlthaff.26.1.w1. (published online 21 November 2006; 10.1377/hlthaff.26.1.w1) [DOI] [PubMed] [Google Scholar]; Goldman DP, Joyce GF, Zheng Y. Prescription Drug Cost Sharing: Associations with Medication and Medical Utilization and Spending and Health. Journal of the American Medical Association. 2007;298(1):61–69. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hsu J, et al. Unintended Consequences of Caps on Medicare Drug Benefits. New England Journal of Medicine. 2006;354(22):2349–2359. doi: 10.1056/NEJMsa054436. [DOI] [PubMed] [Google Scholar]
- 6.Chen JT, Summers KH. Pharmaceutical Manufacturer Prescription Assistance Programs: Are They Worth It? Journal of Managed Care Pharmacy. 2007;13(7):611–613. doi: 10.18553/jmcp.2007.13.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duke KS, Raube K, Lipton HL. Patient-Assistance Programs: Assessment of and Use by Safety-Net Clinics. American Journal of Health-System Pharmacy. 2005;62(7):726–731. doi: 10.1093/ajhp/62.7.726. [DOI] [PubMed] [Google Scholar]; Strum MW, et al. Effects of a Medication Assistance Program on Health Outcomes in Patients with Type 2 Diabetes Mellitus. American Journal of Health-System Pharmacy. 2005;62(10):1048–1052. doi: 10.1093/ajhp/62.10.1048. [DOI] [PubMed] [Google Scholar]
- 8.See the Partnership for Prescription Assistance home page. http://www.pparx.org.
- 9.Chauncey D, et al. Medication Access through Patient Assistance Programs. American Journal of Health-System Pharmacy. 2006;63(13):1254–1259. doi: 10.2146/ajhp050457. [DOI] [PubMed] [Google Scholar]; Chisholm MA, DiPiro JT. Pharmaceutical Manufacturer Assistance Programs. Archives of Internal Medicine. 2002;162(7):780–784. doi: 10.1001/archinte.162.7.780. [DOI] [PubMed] [Google Scholar]
- 10.IMS Health. [accessed 18 February 2009];Top Ten Products by U.S. Sales. 2007 http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Document/Top-Line%20Industry%20Data/2007%20Top%20Products%20by%20Sales.pdf.
- 11.Kincaid JP, et al. Derivation of New Readability Formulas (Automated Readability Index, Fog Count, and Flesch Reading Ease Formula) for Navy Enlisted Personnel. Memphis: Naval Air Station; 1975. [Google Scholar]
- 12.IMS Health. Top Ten Products by U.S. Sales [Google Scholar]
- 13.Davis TC, et al. Low Literacy Impairs Comprehension of Prescription Drug Warning Labels. Journal of General Internal Medicine. 2006;21(8):847–851. doi: 10.1111/j.1525-1497.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen, Summers Pharmaceutical Manufacturer Prescription Assistance Programs. doi: 10.18553/jmcp.2007.13.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chauncey, et al. Medication Access [Google Scholar]
- 15.Duke, et al. Patient-Assistance Programs [Google Scholar]; Sarrafizadeh M, et al. Pharmacist-Facilitated Enrollment in Medication Assistance Programs in a Private Ambulatory Care Clinic. American Journal of Health-System Pharmacy. 2004;61(17):1816–1820. doi: 10.1093/ajhp/61.17.1816. [DOI] [PubMed] [Google Scholar]
- 16.PPA home page. Sarrafizadeh S, et al. Pharmacist-Facilitated Enrollment [Google Scholar]; Harmon GN, et al. Outpatient Medication Assistance Program in a Rural Setting. American Journal of Health-System Pharmacy. 2004;61(6):603–607. doi: 10.1093/ajhp/61.6.603. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services Office of Inspector General. Special Advisory Bulletin on Patient Assistance Programs for Medicare Part D Enrollees. Federal Register. 2005;70(224):70623–70628. [Google Scholar]
- 18.Alexander GC, Zhang J, Basu A. Characteristics of Patients Receiving Pharmaceutical Samples and Association between Sample Receipt and Out-of-Pocket Prescription Costs. Medical Care. 2008;46(4):394–402. doi: 10.1097/MLR.0b013e3181618ee0. [DOI] [PubMed] [Google Scholar]