Abstract
Purpose
P-glycoprotein limits tissue penetration of many antiretroviral drugs. We characterized effects of the P-glycoprotein substrate cyclosporin A on T cell P-glycoprotein activity in HIV-infected AIDS Clinical Trials Group study A5138 participants.
Methods
We studied P-glycoprotein activity on CD4 and CD8 T cells in 16 participants randomized to receive oral cyclosporin A (n=9) or not (n=7) during initiation antiretroviral therapy (ART) that did not include protease or non-nucleoside reverse transcriptase inhibitors.
Results
CD4 T cell P-glycoprotein activity decreased by a median of 8 percentage points with cyclosporin A/ART (difference between cyclosporin A/ART versus ART only P=0.001). Plasma trough cyclosporin A concentrations correlated with change in P-glycoprotein activity in several T cell subsets.
Conclusions
Oral cyclosporin A can inhibit peripheral blood CD4 T cell P-glycoprotein activity. Targeted P-glycoprotein inhibition might enhance delivery of ART to T cells.
Keywords: HIV/AIDS, Antiretroviral therapy, P-glycoprotein, Cyclosporin A, T lymphocytes
Introduction
P-glycoprotein, the efflux transporter encoded by ABCB1, limits drug entry into tissues and cells [1, 2]. Inhibition of P-glycoprotein may enhance penetration of substrate drugs [3, 4]. There is interest in the role of P-glycoprotein during human immunodeficiency virus (HIV) therapy [5] because protease inhibitors and other antiretrovirals are P-glycoprotein substrates [6–9] and P-glycoprotein expression on CD4 T lymphocytes could decrease antiviral effects [10–12]. Protease inhibitors can inhibit P-glycoprotein activity [13–15] but increased cellular P-glycoprotein expression can be induced ex vivo by protease inhibitors or non-nucleoside reverse transcriptase inhibitors (NNRTIs) [16–19]. Limited data also suggest that P-glycoprotein overexpression in cell lines may influence HIV-associated effects, conferring relative resistance to infection [20, 21] and apoptosis [22, 23]. An ABCB1 polymorphism that affects P-glycoprotein expression [24] may predict more favorable virologic responses to antiretroviral therapy (ART) [25–27] and/or decreased drug toxicity [28, 29], although data are conflicting.
Targeted P-glycoprotein inhibition has been pursued as a strategy to restore susceptibility of multidrug resistant cancer cells to chemotherapy [30–33]. The immunosuppressant drug, cyclosporin A, inhibits P-glycoprotein activity [34, 35]. This may explain in part the increased oral bioavailability of some chemotherapeutic agents with concomitant cyclosporin administration [36]. Oral cyclosporin A increased plasma levels (trough and area-under-the-curve) of the HIV protease inhibitor (PI) nelfinavir in a single study of seven HIV-infected subjects [37].
Immune activation during HIV infection predicts disease progression [38, 39], suggesting that immunosuppressive therapy might paradoxically be beneficial during ART. AIDS Clinical Trials Group (ACTG) study A5138 tested the hypothesis that concomitant cyclosporin A would enhance immune reconstitution during ART initiation. Although no sustained effects were seen [40], A5138 provided an opportunity to assess the impact of cyclosporin A on P-glycoprotein activity during ART initiation. The ART regimens during the first 14 days of A5138 included neither PIs nor NNRTIs which could affect P-glycoprotein activity. We hypothesized that cyclosporin A would inhibit peripheral blood T cell P-glycoprotein activity. The objective of this study was to determine the effects of cyclosporin A on T cell P-glycoprotein efflux activity ex vivo using specimens from a clinical trial who were receiving PI and NNRTI-sparing ART in the presence and absence of cyclosporin A.
Methods
ACTG protocol A5138
Primary A5138 results have been reported [40]. Briefly, ART-naïve HIV-infected individuals initiated twice-daily co-formulated abacavir/zidovudine/lamivudine. Participants were randomized to also receive either cyclosporin A (Neoral; Sandoz) 4mg/kg twice daily, or no cyclosporin A during the first 14 days of ART. On day 15 all participants added efavirenz 600 mg once daily. Trough cyclosporin A concentrations were obtained at days 3, 7, 10, and 14. The primary study was registered as trial NCT00031070 (http://www.clinicaltrials.gov). The primary study protocol and all substudies were approved by local Institutional Review Boards at each clinical trial site. All participants in this substudy provided written informed consent under the A5138 protocol version that included P-glycoprotein assays.
Dye efflux assay
Acid-citrate-dextrose anticoagulated whole blood was shipped to Vanderbilt University at ambient temperature, and assayed within 24 hours of phlebotomy. Three specimens not processed within 24 hours were not analyzed. The activity of P-glycoprotein in peripheral blood T cells was determined by measuring the cellular efflux of the fluorescent P-glycoprotein substrate 3,3′-diethyloxacarbocyanine iodide [DiOCB2(3)] as described elsewhere [13, 41]. The cellular efflux of DiOC2(3), which is a specific P-glycoprotein substrate [42], has been demonstrated to be directly related to functional P-glycoprotein in these cells [43, 44]. Briefly, CD4+ and CD8+ T cells were loaded with DiOC2(3) by incubating 1 mL of ACD anti-coagulated whole blood with 1 mL of 100 nM DiOC2(3) in PBS for 15 minutes at 37°C. Cells were collected by centrifugation, washed with ice-cold PBS and resuspended in 600 μL of RPMI 1640 plus 50 mM HEPES (pH 7.4). Verapamil was added to one-half of each sample to a final concentration of 19 μM resulting in greater than 95% inhibition of the cellular efflux of DiOC2(3) by P-glycoprotein. Then 100 μL aliquots of each sample, plus or minus verapamil, were added in duplicate to each well of a 96 deep-well microplate. For each microplate, a sample from one of six healthy adults with previously determined P-gp activity was included as an assay control. The microplate was then incubated at 37°C for 60 minutes. After incubation, T cells were labeled with fluorochrome-conjugated monoclonal antibodies (CD62L-PE, CD45RA-APC, and CD4-PerCP-Cy5.5 or CD8-PerCP-Cy5.5) for 30 minutes on ice. Erythrocytes were lysed with Optilyse B essentially as described by the manufacturer (Immunotech, Marseille, France), leukocytes were pelleted by centrifugation, washed with ice-cold PBS, and fixed with 2% paraformaldehyde in PBS prior to analysis by flow cytometry.
Flow cytometry analysis
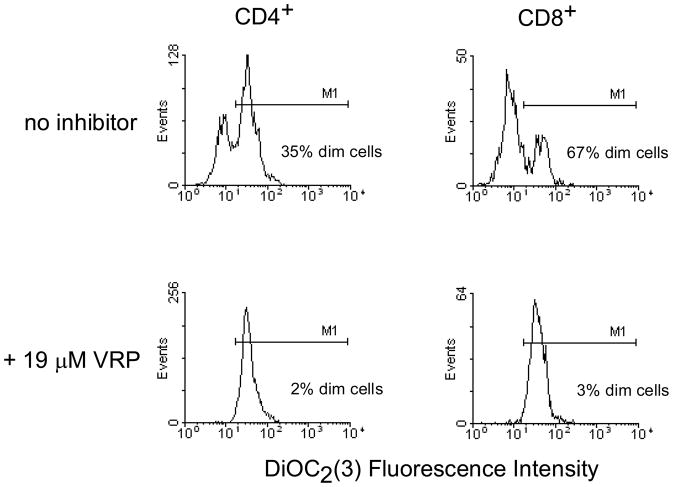
T cell DiOC2(3) content was quantified using a FACSCalibur flow cytometer fitted with a Multiwell AutoSampler (Becton Dickinson, San Jose, CA). For each sample, data from at least 10,000 electronically gated lymphocytes were collected. Fluorescence of DiOC2(3) was detected following excitation at 488 nm, through a 530 nm band-pass filter. Data were analyzed with WinMDI software version 2.8 (J. Trotter, The Scripps Institute). Naive T cells were defined by surface staining for both CD45RA and CD62L (L-selectin). The percentage of the total and naïve CD4+ or CD8+ T cells that were dim (dye-efflux positive) was determined by gating on parallel control samples with near complete P-glycoprotein inhibition (>95%) by verapamil as illustrated in Figure 1. For each subject net dye efflux (P-glycoprotein activity) was reported as the percentage dim cells after subtracting background dim cell percentage in verapamil control incubations.
Figure 1.
Flow cytometry histograms illustrating 3,3′-diethyloxacarbocyanine iodide [DiOC2(3)] efflux by total CD4+ and CD8+ T cells and near complete inhibition of dye efflux by verapamil (VRP). M1 delineates the cells within the population that are dye efflux negative (bright).
Plasma cyclosporin A concentrations
Plasma for cyclosporin A trough assay was obtained immediately prior to the morning dose, and concentrations determined by standard monoclonal antibody or fluorescent polarization radioimmunoassay at commercial laboratories. Results are presented as nanograms/mL of plasma.
Statistical analysis
Distributions of baseline, week 2 and week 4 laboratory values are summarized with median values and interquartile ranges (IQR). Exact Wilcoxon rank-sum and sign tests compared distribution of P-glycoprotein efflux changes from baseline between and within arms, respectively, and Spearman’s correlation assessed the association between efflux changes and cyclosporin A trough levels. The median-unbiased point estimate of the shift parameter between the two distributions and exact 95% confidence interval was estimated from the Hodges-Lehmann procedure. For example, a positive shift means efflux values from the cyclosporin A/ART arm are shifted lower than those in the ART alone arm. Subjects who did not complete two weeks of cyclosporin A (in the cyclosporin A arm) and four weeks of ART were excluded from these analyses; results presented are for the as-treated patient study population based on ART and cyclosporin A administration. Separate inference for changes from baseline to day 14 and day 28 was performed, and no adjustments were made for multiple comparisons across time.
Results
Of 42 A5138 participants, our analysis comprises 16 individuals. The other 26 were excluded because they either enrolled to A5138 before the P-glycoprotein study was implemented (n=16) or were not in the as-treated study population (n=10). Baseline characteristics (age, race/ethnicity, sex, CD4 T cells, and plasma HIV-1 RNA) did not differ by group (9 randomized to cyclosporin A/ART; 7 to ART alone; Table), and were similar to the other 26 participants (data not shown). Of the 16 participants, 14 had CD4 T cell P-glycoprotein assay data at day 14 (6 in the cyclosporin A/ART arm; 8 in the ART alone arm), and 13 had data at day 28 (5 and 8, respectively). Median baseline P-glycoprotein activities, expressed as the percentage of dye efflux positive (dim) cells, within T cell subsets were: 23% in total CD4 T cells, 28% in naïve CD4 T cells, 47% in total CD8 T cells, and 76% in naive CD8 T cells.
Table.
Baseline demographics and HIV disease parameters in total group and by treatment arm
| Total (N = 16) | ART alone (n = 7) | ART + cyclosporin A (n = 9) | |
|---|---|---|---|
| Age, median (range) | 40 (21–55) | 32 (21–49) | 43 (23–55) |
| Race/ethnicity, n (%) | |||
| Black non-Hispanic | 4 (25) | 1 (14) | 3 (33) |
| White non-Hispanic | 11 (69) | 5 (71) | 6 (67) |
| Hispanic | 1 (6) | 1 (14) | 0 (0) |
| Male sex, n (%) | 15 (94) | 7 (100) | 8 (89) |
| CD4 T cells/mm3, median (interquartile range) | 326 (261–397) | 276 (263–404) | 340 (229–392) |
| HIV-1 RNA (log10 copies/mL plasma), median (interquartile range) | 4.9 (4.3–5.5) | 5.1 (4.3–5.5) | 4.9 (4.4–5.0) |
ART = antiretroviral therapy
P>0.05 for all between-arm comparisons, by Fisher’s exact or Kruskal-Wallis tests.
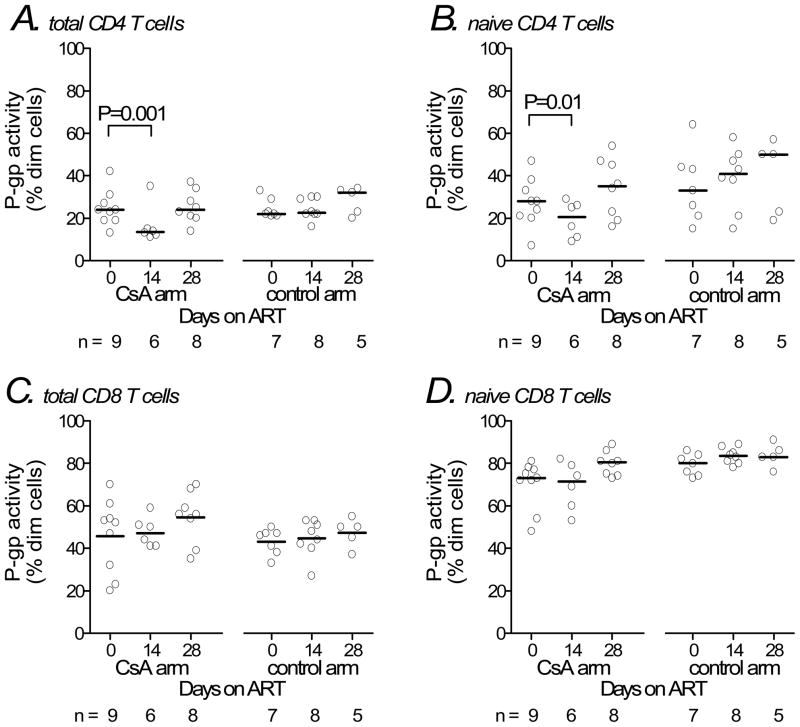
Median baseline P-glycoprotein activity in total CD4 T cells was 24% in the cyclosporin A/ART arm and 22% in the ART alone arm (Figure 2A). Median changes from baseline to week 2 were an 8 percentage point decrease in the cyclosporin A/ART arm (relative decrease of 42% in proportion of dim cells), and no change in the ART alone arm (95% CI for shift in distributions= 5–12 percentage points; P=0.001). At 28 days (two weeks after discontinuing cyclosporin A and initiating efavirenz), median changes from baseline in CD4 T cell P-glycoprotein activity no longer differed between arms. In naïve CD4 T cells, median baseline P-glycoprotein activity was 28% in the cyclosporin A/ART arm and 33% in the ART alone arm. The median changes from baseline to week 2 were a 9 percentage point decrease in the cyclosporin A/ART arm, and no change in the ART alone arm (Figure 2B; 95% CI for shift in distributions= 3–22 percentage points; P=0.01). At 28 days the median change from baseline in naïve CD4 T cell P-glycoprotein activity no longer differed between arms. In total and naïve CD8 T cells there were no significant changes in P-glycoprotein activity from baseline to day 14 (Figures 2C and D).
Figure 2.
P-glycoprotein activity in T cell subsets among study participants. Samples were analyzed at baseline (receiving neither ART nor cyclosporin A), study day 14 (all receiving zidovudine/lamivudine/abacavir, with or without cyclosporin A), and study day 28 (all receiving zidovudine/lamivudine/abacavir/efavirenz without cyclosporin A). Markers represent individual study participants. Horizontal lines represent median values. Sample sizes for groups at each time-point are shown at the bottom of each panel. Panels represent total CD4 T cells (A), naïve CD4 T cells (B), total CD8 T cells (C), and naïve CD8 T cells (D). P-values shown are for the Hodges-Lehmann shift parameter and correspond to the change in distribution of P-glycoprotein activities within the cyclosporin A arm during the first 14 days of therapy. ART = antiretroviral therapy; CsA = cyclosporin A; P-gp = P-glycoprotein.
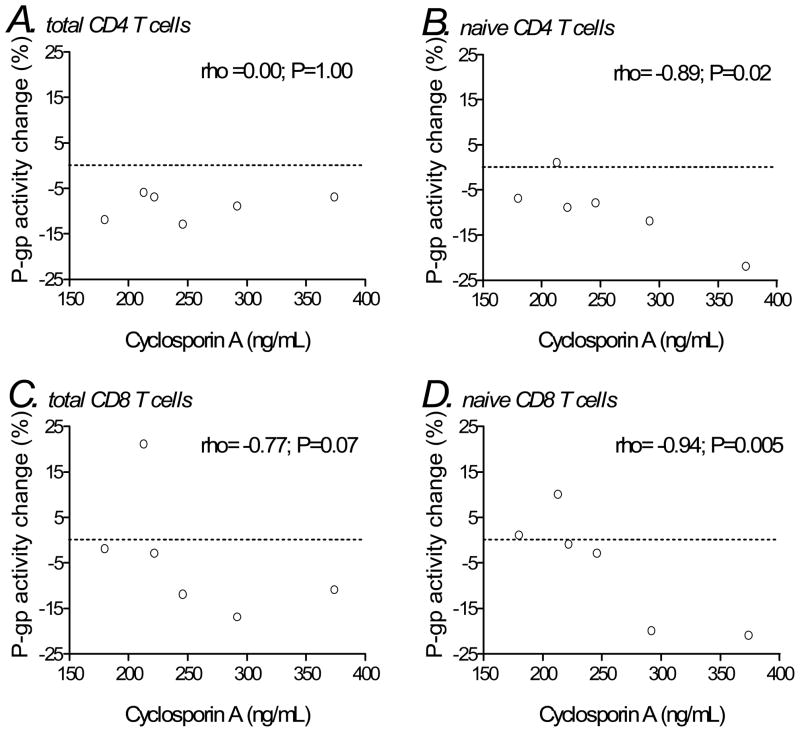
We next assessed correlations between day 14 trough plasma cyclosporin A concentrations and change in P-glycoprotein activity from baseline to day 14. In total CD4 T cells we found no significant correlation (Spearman’s rho= 0.00; P=1.00; Figure 3A), whereas in naïve CD4 T cells there was an inverse correlation (−0.89; P=0.02; Figure 3B). In CD8 T cells there was a trend toward an inverse correlation (−0.77; P=0.07), which in naive CD8 T cells reached statistical significance (−0.94; P=0.005; Figure 3C and D).
Figure 3.
Associations between P-glycoprotein activity (change from baseline to week 14) and plasma trough cyclosporin A concentrations at day 14 in T cell subsets among individuals in the cyclosporin A arm. Panels represent total CD4 T cells (A), naïve CD4 T cells (B), total CD8 T cells (C), and naïve CD8 T cells (D). Correlation coefficients (rho) and P-values shown are for Spearman correlations. The horizontal dotted lines represent no change in P-glycoprotein activity from baseline.
ART = antiretroviral therapy; CsA = cyclosporin A; P-gp = P-glycoprotein.
Discussion
This study demonstrates that oral cyclosporin A inhibits peripheral blood T cell P-glycoprotein activity in HIV-infected adults, and quantifies the magnitude of this inhibition. An inhibitory effect was demonstrable on total CD4 T cells, less so on naïve CD4 T cells, and not on CD8 T cells (Figure 2). This difference among cell types may reflect the lower baseline P-glycoprotein activity on total CD4 T cells [41], which potentially allows detection of inhibition by cyclosporin A even at relatively low drug concentrations. This would be consistent with the lack of observed correlation between cyclosporin A concentrations and P-glycoprotein inhibition in total CD4 T cells (Figure 3A). In contrast, a correlation was seen in naïve CD4 and CD8 T cell subsets (Figure 3B–D), suggesting that the greater absolute P-glycoprotein activity in these cell populations may have made a concentration-dependent inhibition more demonstrable (i.e. only the highest cyclosporin A concentrations provided an inhibitory effect). It was notable that in the total CD4 T cell population (Figure 3A), all subjects had a >5% decrease in P-glycoprotein activity, regardless of trough cyclosporin A concentration, without an apparent dose response. In contrast, in the naïve CD8 T cell subsets (with greatest baseline P-glycoprotein activity) >5% decreases in P-glycoprotein activity were only seen in two subjects with the highest cyclosporin A trough concentrations (Figure 3D). We did not perform cyclosporin A dose-response experiments using our assay; previous studies have demonstrated full inhibition of P-glycoprotein function in lymphocytes when exposed to much higher concentrations of cyclosporin A than the trough levels observed in these study subjects [43].
Importantly, during the first 14 days the ART regimen did not include protease inhibitors or NNRTIs, medications reported to inhibit and/or induce P-glycoprotein activity, and which could therefore confound analyses focused on cyclosporin A. After day 14, all A5138 participants received efavirenz, which can induce P-glycoprotein expression under some conditions. Exposing a colon adenocarcinoma cell line to high concentrations of efavirenz for three days caused a two-fold increase in P-glycoprotein expression but somewhat decreased P-glycoprotein activity [18]. In contrast, administering oral efavirenz to rats for six days did not increase intestinal P-glycoprotein activity [45]. In the present study, 14 days of efavirenz (days 14 to 28) did not markedly alter peripheral blood T cell P-glycoprotein activity in the ART alone arm. We cannot exclude modest induction of P-glycoprotein by efavirenz following discontinuation of cyclosporine A. Recent data in cell culture [46, 47] and murine [48] models have suggested that abacavir may also serve as a P-glycoprotein substrate. Zidovudine and lamivudine did not affect P-glycoprotein [47]. Although we cannot exclude an inhibitory effect of abacavir in this study, all subjects received the same dose of abacavir for the same length of time, and only as-treated data were included in analyses, so any differences between groups would be most likely due to cyclosporin A exposure.
Our study has several limitations, and these results should be considered preliminary. Since specimen collection for this study did not begin until after A5138 began enrolling, not all A5138 participants were eligible for inclusion in this analysis. Demographics, CD4 and HIV-1 RNA levels at baseline did not differ between A5138 subjects included and not included in this analysis (data not shown). This study required overnight shipment of specimens to the P-glycoprotein assay laboratory. Specimens assayed within 24 hours at ambient temperature have not shown substantial changes in efflux activity compared to fresh specimens (authors’ unpublished data), but we cannot exclude additional effects resulting from overnight shipping. Certainly, the in vivo pharmacodynamics of these relationships are complicated, and cannot be fully characterized by P-glycoprotein activity and cyclosporin A concentrations alone. Factors we did not assess in this study such as other cellular drug transporters and hepatic metabolism likely influence these relationships. Although PI- and NNRTI-sparing regimens are not currently recommended ART regimens in clinical practice, this study design provided a unique opportunity to investigate the effects of short-term cyclosporin A in this context, and the insights provided are relevant. Nonetheless, our data cannot be used to determine effects of cyclosporin A or other P-glycoprotein inhibitors used for longer periods of time or with ART regimens containing ritonavir or other PI. In addition, the absolute changes in P-glycoprotein efflux activity are small, and the clinical relevance of such changes is unknown. Finally, because of the small sample size in this study, there was not adequate statistical power to detect modest or moderate differences between all groups.
Optimizing pharmacokinetic profiles of long-term ART remains a priority. Adjunctive pharmacologic manipulation is well established in HIV therapeutics, primarily involving targeted inhibition of CYP3A isoforms to enhance plasma exposure for protease inhibitors [49]. While cyclosporin A may not be well suited to this purpose because of its immunosuppressive properties, studies should continue to evaluate targeted inhibition of P-glycoprotein and other drug transporters, and associated pharmacokinetic and clinical consequences.
Acknowledgments
The authors thank the patients who participated in the study and the following investigators who contributed to A5138: Tania George (Bristol-Myers Squibb, Plainsboro, NJ); Kathy Burgner, Jane Baum, and Steve Arrington (Case Western Reserve University, Cleveland, OH); Laurie Myers and Janeen Duffy (Frontier Science and Technology Research Foundation, Buffalo, NY); Dr. Jerry M. Tolson (GlaxoSmithKline, Research Triangle, NC); Dr. W. Keith Henry (Hennipen County Medical Center, Minneapolis, MN); Dr. Elizabeth Adams and Elaine Ferguson (National Institutes of Health, Division of AIDS, Bethesda, MD); Dr. Harold A. Kessler (Rush-Presbyterian–St. Luke’s Medical Center, Chicago, IL); Nasreen Jahed and Dr. Tine De Marez (Social and Scientific Systems, Silver Spring, MD); Monique Givens (University of Colorado, Denver).
Footnotes
These data were previously presented in part at the 13th Conference on Retroviruses and Opportunistic Infections, February 2006, Denver, CO (abstract 564).
Financial interest disclosures: A5138 was supported by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases (AI68636). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. Additional grant support included a Vanderbilt Clinical Research Scholars award (K12 RR17697) and K23 AT02508 (T.H.); AI38855 (L.S., M.P., H.W.); AI25879 and AI36219 (M.M.L.); AI69423 and AI50410 (C.P.); AI069439, AI54999, and MH071205 (D.W.H.). Pharmaceutical support for A5138 was provided by GlaxoSmithKline and Bristol-Myers Squibb. T.H. has received research funding from Merck & Co., Inc. D.W.H. has received research grants from Bavarian Nordic, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck, Tanox, and Tibotec, and is on Scientific Advisory Boards for Glaxo Smith Kline and Tibotec. The other authors have no potential conflicts to declare.
References
- 1.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borst P, Schinkel AH, Smit JJ, Wagenaar E, Van Deemter L, Smith AJ, Eijdems EW, Baas F, Zaman GJ. Classical and novel forms of multidrug resistance and the physiological functions of P-glycoproteins in mammals. Pharmacol Ther. 1993;60:289–299. doi: 10.1016/0163-7258(93)90011-2. [DOI] [PubMed] [Google Scholar]
- 3.Fromm MF, Kim RB, Stein CM, Wilkinson GR, Roden DM. Inhibition of P-glycoprotein-mediated drug transport: A unifying mechanism to explain the interaction between digoxin and quinidine. Circulation. 1999;99:552–557. doi: 10.1161/01.cir.99.4.552. [DOI] [PubMed] [Google Scholar]
- 4.Mayer U, Wagenaar E, Dorobek B, Beijnen JH, Borst P, Schinkel AH. Full blockade of intestinal P-glycoprotein and extensive inhibition of blood-brain barrier P-glycoprotein by oral treatment of mice with PSC833. J Clin Invest. 1997;100:2430–2436. doi: 10.1172/JCI119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75:13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim AE, Dintaman JM, Waddell DS, Silverman JA. Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J Pharmacol Exp Ther. 1998;286:1439–1445. [PubMed] [Google Scholar]
- 8.Srinivas RV, Middlemas D, Flynn P, Fridland A. Human immunodeficiency virus protease inhibitors serve as substrates for multidrug transporter proteins MDR1 and MRP1 but retain antiviral efficacy in cell lines expressing these transporters. Antimicrob Agents Chemother. 1998;42:3157–3162. doi: 10.1128/aac.42.12.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CG, Gottesman MM. HIV-1 protease inhibitors and the MDR1 multidrug transporter. J Clin Invest. 1998;101:287–288. doi: 10.1172/JCI2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones K, Bray PG, Khoo SH, Davey RA, Meaden ER, Ward SA, Back DJ. P-Glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance? AIDS. 2001;15:1353–1358. doi: 10.1097/00002030-200107270-00004. [DOI] [PubMed] [Google Scholar]
- 11.Jones K, Hoggard PG, Sales SD, Khoo S, Davey R, Back DJ. Differences in the intracellular accumulation of HIV protease inhibitors in vitro and the effect of active transport. AIDS. 2001;15:675–681. doi: 10.1097/00002030-200104130-00002. [DOI] [PubMed] [Google Scholar]
- 12.Turriziani O, Di Marco P, Antonelli G, Dianzani F. May the drug transporter P glycoprotein affect the antiviral activity of human immunodeficiency virus type 1 proteinase inhibitors? Antimicrob Agents Chemother. 2000;44:473–474. doi: 10.1128/aac.44.2.473-474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donahue JP, Dowdy D, Ratnam KK, Hulgan T, Price J, Unutmaz D, Nicotera J, Raffanti S, Becker M, Haas DW. Effects of nelfinavir and its M8 metabolite on lymphocyte P-glycoprotein activity during antiretroviral therapy. Clin Pharmacol Ther. 2003;73:78–86. doi: 10.1067/mcp.2003.11. [DOI] [PubMed] [Google Scholar]
- 14.Drewe J, Gutmann H, Fricker G, Torok M, Beglinger C, Huwyler J. HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem Pharmacol. 1999;57:1147–1152. doi: 10.1016/s0006-2952(99)00026-x. [DOI] [PubMed] [Google Scholar]
- 15.Washington CB, Duran GE, Man MC, Sikic BI, Blaschke TF. Interaction of anti-HIV protease inhibitors with the multidrug transporter P-glycoprotein (P-gp) in human cultured cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:203–209. doi: 10.1097/00042560-199811010-00001. [DOI] [PubMed] [Google Scholar]
- 16.Chandler B, Almond L, Ford J, Owen A, Hoggard P, Khoo S, Back D. The effects of protease inhibitors and nonnucleoside reverse transcriptase inhibitors on p-glycoprotein expression in peripheral blood mononuclear cells in vitro. J Acquir Immune Defic Syndr. 2003;33:551–556. doi: 10.1097/00126334-200308150-00001. [DOI] [PubMed] [Google Scholar]
- 17.Perloff MD, Von Moltke LL, Marchand JE, Greenblatt DJ. Ritonavir induces P-glycoprotein expression, multidrug resistance-associated protein (MRP1) expression, and drug transporter-mediated activity in a human intestinal cell line. J Pharm Sci. 2001;90:1829–1837. doi: 10.1002/jps.1133. [DOI] [PubMed] [Google Scholar]
- 18.Stormer E, von Moltke LL, Perloff MD, Greenblatt DJ. Differential modulation of P-glycoprotein expression and activity by non-nucleoside HIV-1 reverse transcriptase inhibitors in cell culture. Pharm Res. 2002;19:1038–1045. doi: 10.1023/a:1016430825740. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Wring SA, Woolley JL, Brouwer KR, Serabjit-Singh C, Polli JW. Induction of P-glycoprotein and cytochrome P450 3A by HIV protease inhibitors. Drug Metab Dispos. 2001;29:754–760. [PubMed] [Google Scholar]
- 20.Lee CG, Ramachandra M, Jeang KT, Martin MA, Pastan I, Gottesman MM. Effect of ABC transporters on HIV-1 infection: inhibition of virus production by the MDR1 transporter. Faseb J. 2000;14:516–522. doi: 10.1096/fasebj.14.3.516. [DOI] [PubMed] [Google Scholar]
- 21.Speck RR, Yu XF, Hildreth J, Flexner C. Differential effects of p-glycoprotein and multidrug resistance protein-1 on productive human immunodeficiency virus infection. J Infect Dis. 2002;186:332–340. doi: 10.1086/341464. [DOI] [PubMed] [Google Scholar]
- 22.Johnstone RW, Cretney E, Smyth MJ. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood. 1999;93:1075–1085. [PubMed] [Google Scholar]
- 23.Smyth MJ, Krasovskis E, Sutton VR, Johnstone RW. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc Natl Acad Sci U S A. 1998;95:7024–7029. doi: 10.1073/pnas.95.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 25.Fellay J, Marzolini C, Meaden ER, Back DJ, Buclin T, Chave JP, Decosterd LA, Furrer H, Opravil M, Pantaleo G, Retelska D, Ruiz L, Schinkel AH, Vernazza P, Eap CB, Telenti A. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359:30–36. doi: 10.1016/S0140-6736(02)07276-8. [DOI] [PubMed] [Google Scholar]
- 26.Haas DW, Smeaton LM, Shafer RW, Robbins GK, Morse GD, Labbe L, Wilkinson GR, Clifford DB, D’Aquila RT, De Gruttola V, Pollard RB, Merigan TC, Hirsch MS, George AL, Jr, Donahue JP, Kim RB. Pharmacogenetics of long-term responses to antiretroviral regimens containing Efavirenz and/or Nelfinavir: an Adult Aids Clinical Trials Group Study. J Infect Dis. 2005;192:1931–1942. doi: 10.1086/497610. [DOI] [PubMed] [Google Scholar]
- 27.Saitoh A, Singh KK, Powell CA, Fenton T, Fletcher CV, Brundage R, Starr S, Spector SA. An MDR1-3435 variant is associated with higher plasma nelfinavir levels and more rapid virologic response in HIV-1 infected children. AIDS. 2005;19:371–380. doi: 10.1097/01.aids.0000161766.13782.2f. [DOI] [PubMed] [Google Scholar]
- 28.Haas DW, Bartlett JA, Andersen JW, Sanne I, Wilkinson GR, Hinkle J, Rousseau F, Ingram CD, Shaw A, Lederman MM, Kim RB. Pharmacogenetics of nevirapine-associated hepatotoxicity: an Adult AIDS Clinical Trials Group collaboration. Clin Infect Dis. 2006;43:783–786. doi: 10.1086/507097. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie MD, Haas DW, Motsinger AA, Donahue JP, Erdem H, Raffanti S, Rebeiro P, George AL, Kim RB, Haines JL, Sterling TR. Drug transporter and metabolizing enzyme gene variants and nonnucleoside reverse-transcriptase inhibitor hepatotoxicity. Clin Infect Dis. 2006;43:779–782. doi: 10.1086/507101. [DOI] [PubMed] [Google Scholar]
- 30.Ferry DR, Traunecker H, Kerr DJ. Clinical trials of P-glycoprotein reversal in solid tumours. Eur J Cancer. 1996;32A:1070–1081. doi: 10.1016/0959-8049(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi L, Harding MW, Neubauer M, Langer CJ, Moore M, Ross HJ, Johnson BE, Lynch TJ. A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer. 2007;109:924–932. doi: 10.1002/cncr.22492. [DOI] [PubMed] [Google Scholar]
- 32.Pusztai L, Wagner P, Ibrahim N, Rivera E, Theriault R, Booser D, Symmans FW, Wong F, Blumenschein G, Fleming DR, Rouzier R, Boniface G, Hortobagyi GN. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer. 2005;104:682–691. doi: 10.1002/cncr.21227. [DOI] [PubMed] [Google Scholar]
- 33.Rago RP, Einstein A, Jr, Lush R, Beer TM, Ko YJ, Henner WD, Bubley G, Merica EA, Garg V, Ette E, Harding MW, Dalton WS. Safety and efficacy of the MDR inhibitor Incel (biricodar, VX-710) in combination with mitoxantrone and prednisone in hormone-refractory prostate cancer. Cancer Chemother Pharmacol. 2003;51:297–305. doi: 10.1007/s00280-003-0573-4. [DOI] [PubMed] [Google Scholar]
- 34.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982;42:4730–4733. [PubMed] [Google Scholar]
- 35.Watanabe T, Tsuge H, Oh-Hara T, Naito M, Tsuruo T. Comparative study on reversal efficacy of SDZ PSC 833, cyclosporin A and verapamil on multidrug resistance in vitro and in vivo. Acta Oncol. 1995;34:235–241. doi: 10.3109/02841869509093961. [DOI] [PubMed] [Google Scholar]
- 36.Malingre MM, Richel DJ, Beijnen JH, Rosing H, Koopman FJ, Ten Bokkel Huinink WW, Schot ME, Schellens JH. Coadministration of cyclosporine strongly enhances the oral bioavailability of docetaxel. J Clin Oncol. 2001;19:1160–1166. doi: 10.1200/JCO.2001.19.4.1160. [DOI] [PubMed] [Google Scholar]
- 37.Frassetto L, Thai T, Aggarwal AM, Bucher P, Jacobsen W, Christians U, Benet LZ, Floren LC. Pharmacokinetic interactions between cyclosporine and protease inhibitors in HIV+ subjects. Drug Metab Pharmacokinet. 2003;18:114–120. doi: 10.2133/dmpk.18.114. [DOI] [PubMed] [Google Scholar]
- 38.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 39.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 40.Lederman MM, Smeaton L, Smith KY, Rodriguez B, Pu M, Wang H, Sevin A, Tebas P, Sieg SF, Medvik K, Margolis DM, Pollard R, Ertl HC, Valdez H. Cyclosporin A provides no sustained immunologic benefit to persons with chronic HIV-1 infection starting suppressive antiretroviral therapy: results of a randomized, controlled trial of the AIDS Clinical Trials Group A5138. J Infect Dis. 2006;194:1677–1685. doi: 10.1086/509261. [DOI] [PubMed] [Google Scholar]
- 41.Hulgan T, Donahue JP, Hawkins C, Unutmaz D, D’Aquila RT, Raffanti S, Nicotera F, Rebeiro P, Erdem H, Rueff M, Haas DW. Implications of T-cell P-glycoprotein activity during HIV-1 infection and its therapy. J Acquir Immune Defic Syndr. 2003;34:119–126. doi: 10.1097/00126334-200310010-00001. [DOI] [PubMed] [Google Scholar]
- 42.Minderman H, Vanhoefer U, Toth K, Yin MB, Minderman MD, Wrzosek C, Slovak ML, Rustum YM. DiOC2(3) is not a substrate for multidrug resistance protein (MRP)-mediated drug efflux. Cytometry. 1996;25:14–20. doi: 10.1002/(SICI)1097-0320(19960901)25:1<14::AID-CYTO2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhary PM, Mechetner EB, Roninson IB. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood. 1992;80:2735–2739. [PubMed] [Google Scholar]
- 44.Park SW, Lomri N, Simeoni LA, Fruehauf JP, Mechetner E. Analysis of P-glycoprotein-mediated membrane transport in human peripheral blood lymphocytes using the UIC2 shift assay. Cytometry A. 2003;53:67–78. doi: 10.1002/cyto.a.10039. [DOI] [PubMed] [Google Scholar]
- 45.Berruet N, Sentenac S, Auchere D, Gimenez F, Farinotti R, Fernandez C. Effect of efavirenz on intestinal p-glycoprotein and hepatic p450 function in rats. J Pharm Pharm Sci. 2005;8:226–234. [PubMed] [Google Scholar]
- 46.Shaik N, Giri N, Pan G, Elmquist WF. P-glycoprotein-mediated active efflux of the anti-HIV1 nucleoside abacavir limits cellular accumulation and brain distribution. Drug Metab Dispos. 2007;35:2076–2085. doi: 10.1124/dmd.107.017723. [DOI] [PubMed] [Google Scholar]
- 47.Storch CH, Theile D, Lindenmaier H, Haefeli WE, Weiss J. Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochem Pharmacol. 2007;73:1573–1581. doi: 10.1016/j.bcp.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 48.Giri N, Shaik N, Pan G, Terasaki T, Mukai C, Kitagaki S, Miyakoshi N, Elmquist WF. Investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) on pharmacokinetics and central nervous system penetration of abacavir and zidovudine in the mouse. Drug Metab Dispos. 2008;36:1476–1484. doi: 10.1124/dmd.108.020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motwani B, Khayr W. Pharmacoenhancement of protease inhibitors. Am J Ther. 2006;13:57–63. doi: 10.1097/00045391-200601000-00010. [DOI] [PubMed] [Google Scholar]