Abstract
We extend Spekreijse's strategy for analyzing lateral interactions in visual evoked potentials (VEPs) to clinical neurophysiologic testing of patients with epilepsy. Stimuli consisted of the radial windmill/dartboard pattern [Ratliff, F., & Zemon, V. (1982). Some new methods for the analysis of lateral interactions that influence the visual evoked potential. In: Bodis-Wollner (Ed.), Evoked potentials, Vol. 388. (pp. 113–124). New York: Annals of the New York Academy of Sciences.] and conventional checkerboards. The fundamental and 2nd-harmonic components of the steady-state responses were used to calculate indices reflecting facilitatory (FI) and suppressive (SI) cortical interactions.
We carried out two studies. In the first, VEPs in 38 patients receiving antiepileptic drug (AED) therapy were compared to those of age-matched controls. For three AEDs (tiagabine, topiramate, and felbamate), addition of the drug did not change the FI and SI compared to baseline values or those of normal controls. However, the addition of gabapentin was associated with an increase of the FI, and this change was reversed when the medication was withdrawn. This suggested a medication-specific change in cortical lateral interactions.
The second study focused on the effects of neurostimulation therapy. Eleven epilepsy patients receiving chronic vagus nerve stimulation (VNS) treatment were tested. By comparing VEPs recorded with the stimulator on (Stim-ON) and turned off (Stim-OFF) in the same session, we determined that VNS did not have a short-acting effect on lateral interactions. However, when compared with normal controls, the VNS patients had a significantly smaller SI (p < .05), but no difference in the FI, demonstrating the presence of a chronic effect. We conclude that with the appropriate stimuli, VEPs can be used as a measure of cortical lateral interactions in normals and epileptic patients, and demonstrate specific changes in these interactions associated with certain treatment modalities.
Keywords: Visual evoked potentials, Windmill-dartboard, Epilepsy, Gabapentin, VNS
1. Introduction
Our contribution to this volume has its conceptual origins in Henk Spekreijse's approach to dissecting the visual evoked potential into physiologically meaningful components. Achieving a physiologically meaningful analysis is a challenge for any surface-recorded measure of brain activity. This is particularly so for the visual evoked response, because the presence of multiple generators with spatiotemporal overlap defies a direct approach via dipole modeling and related techniques. In his classical paper (Spekreijse, van der Tweel, & Zuidema, 1973), Spekreijse provided a proof of concept of an alternative strategy. He designed pairs of stimuli for which some mechanisms (those responsive to local luminance) would respond in an identical fashion, but others (those responsive to local contrast) would respond in a differential fashion. Comparison of the responses thus isolates only those mechanisms that respond differentially. Subsequently, Zemon and Ratliff (1982) extended this idea to separate kinds of lateral cortical interactions. In the work described here, we show that these lateral interactions can be used as a noninvasive measure of the effect of antiepileptic treatments.
1.1. VEPs as a diagnostic tool
The visual evoked potential (VEP) has attained clinical validity as a useful diagnostic tool in the assessment of many ophthalmological and neurological conditions. In most applications, for example, amblyopia (Levi & Manny, 1986; Regan, 1977), glaucoma and optic neuropathies (Celesia & Kaufman, 1985; Halliday & McDonald, 1977; Towle, Moskowitz, Sokol, & Schwartz, 1983), multiple sclerosis (Bodis-Wollner, Hendley, Mylin, & Thornton, 1979; Ghilardi et al., 1991; Regan, Milner, & Heron,1977), Parkinson's disease (Bandini, Pierantozzi, & Bodis-Wollner, 2001) and retinal pathologies (Celesia, 1982; Regan, 1989), diagnosis rests on analysis of simple attributes of the VEP waveform such as amplitude and latency, rather than a detailed analysis of its dynamics. These dynamics are complex, because the VEP is the net result of mechanisms sensitive to luminance, contrast, and spatial pattern, includes contributions from many classes of neurons in multiple cortical layers and areas (Jeffreys & Axford,1972a, 1972b; Vaughan & Arezzo, 1988). For this reason, the potential utility of the VEP to reveal details of neuronal interactions is largely untapped.
One way to address this problem is to exploit the differential selectivity of neuronal interactions across spatial frequencies. In this manner, Bodis-Wollner and Yahr (1978) have identified specific processing abnormalities associated with dopaminergic deficiency. However, with typical pattern-reversal stimuli (checks or gratings), components that are driven by local luminance, local contrast and pattern information are superimposed, since they are all activated at each reversal of the checkerboard stimulus. As Spekreijse, Estevez, and Reits (1977) showed, these components can be separated via comparison of steady-state responses to spatially related stimuli in which local luminance changes have been equated but spatial contrast changes differ. Here, we extend this strategy to the clinical setting, and use it, along with frequency-domain methods, to identify changes in neural interactions associated with treatment of epilepsy. This approach (the “windmill-dartboard” method of Zemon & Ratliff, 1982, 1984) identifies interactions that are highly local – they are reduced by a small (2–3 minarc) separation of the stimulus components, corresponding to approximately one cortical hypercolumn (about 0.5 mm) in human striate cortex (Zemon & Ratliff, 1982). Neurophysiologic evidence in cat (Ts'o, Gilbert, & Wiesel, 1986; Zemon, Kaplan, & Ratliff, 1980) and in humans (Zemon & Ratliff, 1984) tells us it is likely that both excitatory and inhibitory synaptic potentials contribute to these lateral interactions.
1.2. Intra-cortical inhibition in epilepsy
Our motivation to use the VEP to probe cortical interactions in epilepsy is the result of two considerations. First, although fundamental pathophysiologic mechanisms of epilepsy are largely unknown, it is widely believed that alterations of intracortical inhibition are important. In particular, the “GABA hypothesis” proposes that reduced GABAergic inhibition leads to seizure susceptibility, while enhancement of GABAergic inhibition results in an antiepileptic effect (De Deyn, Marescau, & Macdonald, 1990; Treiman, 2001). Secondly, GABA is critical to the normal selectivity of visual neurons for orientation, spatial pattern, and motion, and can modulate the way they combine their visual inputs (Conners, 1992). Moreover, studies in which GABA antagonists are applied to the cortex demonstrate that it is a major contributor to the visual evoked potential (Daniels & Pettigrew, 1975; Rose & Blakemore, 1974; Zemon et al., 1980). For these reasons, it is reasonable to anticipate that lateral interactions mediated by GABA will be present in the VEP, and that these may be altered by epilepsy or its treatment. Here, we test this idea by comparing VEP measures of lateral interactions in patients undergoing two forms of treatment for epilepsy, antiepileptic drugs (AEDs) and vagus nerve stimulation (VNS).
1.3. Epilepsy treatment modalities
AED therapy is the first-line treatment for epilepsy, and results in satisfactory seizure control in approximately 60–70% of patients, depending on seizure type (Kwan & Brodie, 2000). However, AED treatment for epilepsy can be limited by toxicity, tolerability, and incomplete effectiveness. This has motivated the development of neurostimulation therapies for refractory epilepsy. The first clinical trials using vagus nerve stimulation (VNS) began in 1988, and, since its U.S. approval in 1997, it has been employed in more than 25,000 epilepsy patients worldwide, with substantial effectiveness. Approximately 57% of patients have seizure rates reduced by 50% after 1 year of treatment (Ben-Menachem, Hellstroem, Waldton, & Augustinsson, 1999; DeGiorgio et al., 2001; Labar, 2004). VNS treatment is not associated with a development of tolerance over time (Ben-Menachem et al., 1999; Uthman et al., 2004) and VNS responders maintain significant reductions in seizure rates in the long term (Janszky et al., 2005; Labar, 2004; Uthman et al., 2004). Very few patients become seizure-free, however, or are able to reduce or eliminate their medication entirely (Labar & Ponticello, 2003). Additional studies suggest that efficacy of VNS treatment is independent of concomitant antiepileptic medications, and of the stimulation parameters of the device (DeGiorgio et al., 2001; Labar, 2002). In patients with generalized epilepsy, responsiveness to VNS treatment is not predicted by seizure type, or epilepsy class, but has been associated with an onset of epilepsy at a later age (Uthman et al., 2004), and temporal lobe foci (Casazza, Avanzini, Ferroli, Villani, & Broggi, 2006).
The VNS device consists of a programmable pulse generator (NeuroCybernetic Prosthesis (NCP) System – Cyberonics, Inc.) implanted in the chest wall. It is powered by a lithium battery and connected by bipolar leads to the left vagus nerve. The device is programmed to deliver a biphasic current that continuously cycles between on and off periods. While AEDs work at least in part by modulating intracortical interactions (MacDonald & Kelly, 1994), the mechanism of action of VNS is unknown. The afferent fibers stimulated by VNS project mainly to the nucleus of the solitary tract in the brainstem, and therefore, have many subcortical and cortical connections, resulting in diffuse effects on many regions throughout the brain including the dorsal raphe nuclei, locus ceruleus, activation of thalamus, entorhinal cortex, orbitofrontal gyri, and anterior insular cortices (Naritoku, Terry, & Helfert, 1995; Van Laere, Vonck, Boon, Versijpt, & Dierckx, 2002). Electrophysiologic studies of the mechanism of action of VNS in humans have revealed no effect on EEG background (Hammond, Uthman, Reid, & Wilder, 1992a; Salinsky & Burchiel, 1993) or on standard visual, auditory, somatosensory, or P300 evoked potentials (Brazdil et al., 2001; Hammond, Uthman, Reid, & Wilder, 1992b; Uthman et al., 2004), although reduced spike duration and frequency has been observed (Koo, 2001) with chronic treatment. In a study of chemosensation effects with VNS treatment, the authors found prolonged P2 latencies of the olfactory event related potential (ERP) during the ON cycle of the stimulator suggesting a modulatory effect of VNS (Kirchner et al., 2004). However, none of these studies were specifically designed to look for effects of VNS on cortical lateral interactions, as we do here.
2. Methods
2.1. Subjects
Control participants were recruited from our laboratory and the Weill Cornell Medical College personnel. Study patients were recruited from the outpatient adult epilepsy population of The Comprehensive Epilepsy Center at New York Presbyterian Hospital. Informed consent was obtained from all participants and both studies were approved by the Institutional Review Board of Weill Cornell Medical College. In study 1 (the effect of add-on AEDs), eighteen age-matched normals (10M, 8F; average age: 37 yrs) served as controls. In study 2 (the effect of VNS), twenty-four participants (16M, 8F; average age: 34 yrs) served as normal controls. These studies were conducted several years apart, and, consequently, certain technical details differed, as described below. All control participants had normal or corrected-to-normal visual acuities and were free from ophthalmologic disease. All study patients were considered “responders” to their respective treatments (AEDs or VNS), in that their seizures were well controlled at the time of VEP testing.
2.1.1. Patient group 1 – Patients with refractory epilepsy receiving traditional AED medications
Fifty-five patients with epilepsy (25M, 30F; average age: 35 yrs) were recruited from separate double-blinded clinical trials conducted by the Comprehensive Epilepsy Center to determine the efficacy of add-on medications for refractory partial seizures. Gabapentin (GBP) was added in 24 patients, topiramate (TPM), felbamate (FBM), or tiagabine (TGB) was added in 14 patients, and 17 patients were lost to attrition. Baseline AED regimens in these patients included: phenytoin (14 patients), carbamazepine (29 patients), valproate (16 patients), and 6 other commonly prescribed AEDs. Of the 55 patients, 53% were on AED monotherapy, 42% were on two medications, and 5% were taking three or more medications for seizure control. AED dosages were adjusted to maintain serum concentrations within the therapeutic range. All patients had refractory partial seizures (89% with simple or complex-partial; 16% complex-partial with secondary generalization). The majority of the patients (56%) were diagnosed with cryptogenic epilepsy. All patients had visual acuities, with or without correction, of 20/25 or better OU. VEPs were recorded at baseline and after add-on AED treatment; VEP recordings during the add-on phase were obtained from 38 of the 55 patients.
2.1.2. Patient group 2 – Patients receiving adjunctive neurostimulation treatment
Eleven patients (5M, 6F; average age: 39 yrs) undergoing chronic VNS therapy (3.5–10 yrs) were recruited from the Comprehensive Epilepsy Center practice. This group only included patients who had a clinical benefit from VNS treatment: a ≥50% reduction in seizure frequency compared with pre-VNS seizure frequencies (9/11 patients) or a decrease in duration and severity of seizures (2/11 patients) as measured by the Chalfont Seizure Severity Scale (O'Donoghue, Duncan, & Sander, 1996). All patients were on stable, standard AED regimens as well (carbamazepine (5), levetiracetam (3), phenytoin (2), topiramate (2), lamotrigine (1), primidone (1), clonazepam (1), valproate (1), and zonisamide (1)). Nine of the 11 (81%) patients had simple and/or complex-partial seizures; two had primary generalized epilepsy. VNS settings were as follows: current: 0.25–2.5 mA, pulse frequency: 20–30 Hz, pulse width: 250–750 μs, on/off cycle: rapid cycling (OFF time ≤1.8 min) in 10 patients, standard cycling (OFF time ≥3 min) in one patient. All patients had normal fundus exams and visual acuities corrected to 20/40 or better OU. Exclusion criteria included photosensitive seizures, seizures within 24 h prior to VEP testing, evidence of occipital lesions on imaging studies, and evidence of ophthalmologic disease.
2.2. Stimuli and apparatus
Stimuli, subtending 8.8 × 8.8 deg, consisted of the windmill-dartboard patterns (1.0 deg ring thickness) shown in Fig. 1A, and standard contrast-reversal checkerboards (0.5 deg check size). Steady-tate stimuli were delivered at a contrast of 0.3; transient stimuli were delivered at a contrast of 1.0. Displays were linearized via lookup tables. Stimuli were presented in randomized order in both studies.
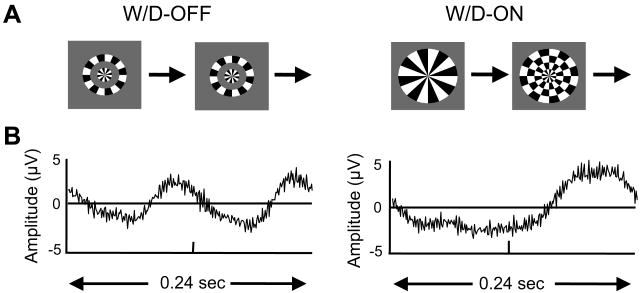
Fig. 1.
(A) Windmill–Dartboard stimuli. Left: W/D-OFF. Right: W/D-ON. The modulated regions are identical in the W/D-ON and W/D-OFF configurations, but the static component of the pattern is present only in the W/D-ON configuration. Thus, interactions between the modulated and static regions result in differences between the VEP waveforms that the two stimuli elicit. (B) Averaged steady-state responses elicited by these stimuli from one normal control. Each waveform represents the averaged response over one cycle of the stimulus. Modulation rate: 4.19 Hz, contrast: 0.3.
In study 1, the display hardware was a Textronix 608 oscilloscope at a viewing distance of 57 cm, with a mean luminance 150 cd/m2. The raster (256 × 256 pixels 270.3 Hz) was controlled by specialized electronics (Milkman et al., 1980), interfaced to a DEC 11/73 computer. Stimulation rates were 4.22 Hz for the steady-state stimuli (windmill-dartboard and checkerboard) and 1 Hz for the transient recordings (checkerboard). Pattern-appearance checkerboard and full-field flash were also used.
In study 2, the display hardware was a Multiscan 17seII monitor (Sony) at a viewing distance of 100 cm, with a mean luminance of 47 cd/m2. The raster (640 × 640, 100 Hz) was controlled by a VSG Series Three (Cambridge Research Co., UK) programmed in Delphi, housed in a Dell PC. Stimulation rates were 4.19 Hz for the steady-state stimuli and 1.07 Hz for the transient stimuli.
2.3. VEP recording procedures
Scalp signals were obtained with Grass gold-cup electrodes positioned at Oz (−) and Cz (+), with a mastoid ground. EEG activity was amplified 10,000-fold, filtered (0.01–100 Hz), and digitized (270.3 Hz, Study 1; 400 Hz, Study 2) synchronously with the display frame. Electrode impedances were 5–10kΩ. Viewing was binocular and participants were instructed to fixate on a fixation point in the center of the display. To reduce fatigue, brief breaks were taken when necessary during the recording sessions. Preliminary artifact rejection (via amplitude bounds) was automated and applied prior to signal averaging. Raw EEG was monitored by the experimenter and at the end of each trial, the first four harmonic components were calculated on-line and displayed on screen. The initial portion (5 s in Study 1, 2 s in Study 2) of each trial was discarded to avoid initial transients due to contrast adaptation and possible eye movements. Sessions were typically one hour in duration for control subjects and Study 1 patients (AEDs only).
In study 2, two additional electrodes were placed on the neck over the sternocleidomastoid muscle near the surgical scar to monitor the on/off cycling of the VNS device. VEPs were recorded twice in the same testing session (2–3 h) to obtain responses with the stimulator turned on (Stim-ON) and off (Stim-OFF). (Temporary discontinuation of the stimulator for up to one hour poses negligible risks to the patients. Stimulation is routinely discontinued for up to 12 h for surgery or procedures without complications. This is because the benefits of VNS therapy are believed to accumulate over periods of weeks.) The order of testing (Stim-ON then Stim-OFF, or Stim-OFF then Stim-ON) was counterbalanced across patients. In the Stim-OFF mode, the pulse generator current was programmed to 0 mA, and we waited one hour before recording the VEP. To record with the stimulator on, trials were initiated when the stimulator cycled off as evidenced in the EEG tracing. For patients whose off-cycle was shorter than 48 s (minimum time required to obtain a single trial), the off-cycle was programmed to 5 min to allow for several trials to be recorded before the next on-cycling of the device. No changes were made to the on-cycling time or current. At the end of the testing session, device settings were programmed back to their original values.
2.4. VEP analysis
Three minutes of averaged EEG were analyzed using a discrete Fourier transform. We focus on the amplitudes and phases of the first four harmonics because the power in higher harmonic components was negligible. Confidence limits of the Fourier components were determined using the Tcirc2 statistic (Victor & Mast, 1991). This statistic determines whether the responses are significantly different from noise and determines the 95% confidence regions of each harmonic component. In Study 1, the analysis epochs used for the Tcirc2 analysis consisted of three one-minute artifact-free trials. In Study 2, the analysis epochs used for the Tcirc2 analysis consisted of successive 10 s segments of the six 30 s trials, excluding any segments in which there were artifacts in the EEG signal, or in which the VNS device cycled on.
3. Results
3.1. Normal controls
We begin by illustrating the windmill-dartboard method of isolating intracortical lateral interactions (Ratliff & Zemon, 1982, 1984; Zemon & Ratliff, 1982) with example data from a typical normal subject. The technique relies on comparing responses to two stimuli (Fig. 1A). The first stimulus (W/D-OFF, left) consists of steady-state pattern-reversal of the center disk and annulus. This is, in effect, pattern-reversal modulation of a circular checkerboard. It elicits a response whose waveform (Fig. 1B, left) is similar to the waveform elicited by pattern-reversal checks, containing nearly identical responses at each phase of the reversal.
In the second stimulus (W/D-ON, right), the modulated components are the same as in the W/D-OFF stimulus, but two additional static annuli are present. Reversal of the modulated regions produces a transition between a “windmill,” with a small number of contours, and a “dartboard,” with a larger number of contours and edges. The critical feature of the pattern is the change in contrast across the borders of the contiguous static and dynamic regions. This stimulus results in a very different response waveform (Fig. 1B), in which the response to the two stimulus transitions differs dramatically.
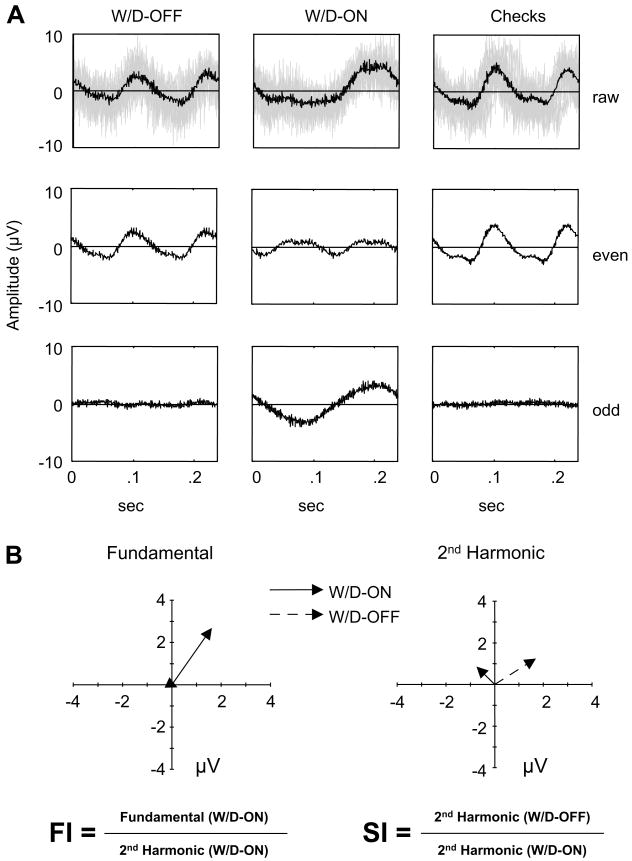
To quantify these waveforms and their differences, we proceed as illustrated in Fig. 2A. Each stimulus cycle has two transitions. For each stimulus, we decompose the raw responses into an “even” trace that contains components common to the two transitions, and an “odd” trace that contains components that differ at the two transitions. For the W/D-OFF stimulus (first column), the even trace is very nearly identical to the raw trace, and contains the bulk of the response. For the W/D-ON stimulus (second column), both the even trace and the odd trace contribute to the response, because the responses to the two stimulus transitions differ. Note that the checkerboard-reversal stimulus (third column) elicits a response that is similar to the W/D-OFF stimulus.
Fig. 2.
(A) VEPs elicited by the windmill/dartboard (W/D-ON and W/D-OFF) and checkerboard stimuli from one normal control (data from Fig. 1B). Averaged waveforms are separated into even and odd harmonic components. Top row: raw signals, middle: even harmonics, bottom: odd harmonics. The light gray shading in the top row indicates the un-averaged EEG traces. (B) Fourier components (fundamental and 2nd harmonic) represented as vectors, whose magnitude indicates amplitude and whose direction indicates response phase. The positive X-axis corresponds to an in-phase response, and counterclockwise rotation corresponds to phase advance.
Next, we quantify the even and odd traces by Fourier analysis. The even trace, so named because it contains all even Fourier components, is dominated by the component at the second harmonic of the stimulus frequency. The odd trace, so named because it contains all odd Fourier components, is dominated by the component at the fundamental stimulus frequency. Each of these Fourier components can be represented as a vector in the complex plane (Fig. 2B), whose length indicates the size of the response and whose direction indicates the timing of the response. Addition of the static component of the stimulus thus has two robust effects (Fig. 2B). A fundamental response component is elicited by the W/D-ON stimulus but not the W/D-OFF stimulus. Additionally, the second harmonic elicited by the W/D-ON stimulus is smaller than the second harmonic elicited by the W/D-OFF stimulus. These differences rely on neural interactions driven by the differentially modulated regions of the stimuli. We quantify these differences via two indices, each calculated from the Fourier components of the steady-state VEP responses:
Facilitation Index (FI) = Fundamental (W/D-ON)/2nd Harmonic (W/D-ON)
Suppression Index (SI) = 2nd Harmonic (W/D-OFF)/2nd Harmonic (W/D-ON)
We use ratios, rather than differences, to normalize for overall response size.
3.1.1. Study 1 - Patients with refractory epilepsy receiving traditional medications
The purpose of this initial study was 2-fold. Would the VEP indices of cortical interactions in epilepsy patients be different from those of normal controls and, if differences existed, could they be related to specific AEDs?
3.2. Baseline VEP measures
We found no significant differences between the normal controls and 55 patients in response measures of amplitude and latency to the standard pattern-reversal checkerboard and flash stimuli (p = .9, p = .8, respectively). Facilitation and suppression indices calculated from responses to the windmill-dartboard patterns were also not significantly different from controls. Finally, there were no significant differences in the VEP responses among the patients when they were grouped according to the three most commonly taken AED medications: phenytoin (14 patients), carbamazepine (29 patients), or valproate (16 patients). Although this was a small sample size, it suggests that epilepsy per se, as well as the above medications, do not measurably affect either standard VEP measures (in agreement with Geller, Hudnell, Vaughn, Messenheimer, & Boyes, 2005), or VEP measures of lateral interactions.
3.3. Introduction of additional medications
After introduction of an additional AED (gabapentin (GBP), topiramate (TPM), felbamate (FBM), or tiagabine (TGB)), standard VEP measures (amplitudes and latencies to the checkerboard and flash stimuli) were statistically unchanged. There was, however, a change in the FI specific to addition of GBP (dosage range: 500–3600 mg/day; median dosage: 2400 mg/day).
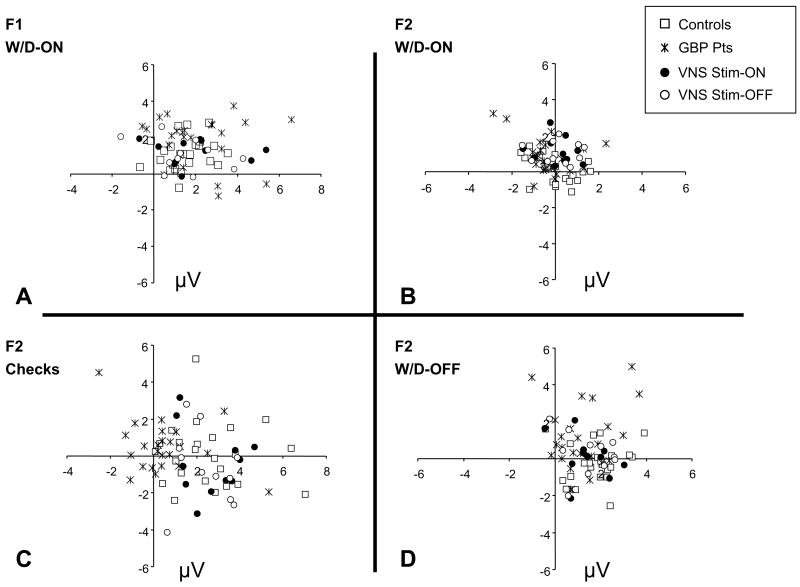
Distributions of the relevant response components are shown in Fig. 3. Response amplitudes are typically 3–5 μV. Consistent with the example subject of Figs. 1 and 2, there is a large fundamental response to the W/D-ON stimulus (Fig. 3A), and large second harmonic responses to the checkerboard stimulus (Fig. 3C) and the W/D-OFF stimulus (Fig. 3D) – but the second harmonic responses to the W/D-ON stimulus are smaller (Fig. 3B). The amplitude of the fundamental response for GBP patients appears somewhat larger than that of controls (Figs. 3A, 4 and Table 1). As measured by the FI, this approaches statistical significance (p = .062). However, there is substantial intersubject variability in the size of the Fourier components, so a within-patient test is likely to be more sensitive. Indeed, when responses from GBP-treated patients are compared to their baseline measures, the increase in the FI is statistically significant (paired t-tests, p = .016). For a small subset (6) of the GBP-treated patients, we had the opportunity to repeat VEP testing after GBP treatment was discontinued. Concomitant AEDs were unchanged during this time. We found that the facilitation indices decreased to their pre-GBP treatment values.
Fig. 3.
Polar plots of fundamental (F1) and 2nd harmonic (F2) responses from controls and both patient groups (GBP and VNS). (A) Fundamental responses to the W/D-ON stimulus. (B) Second harmonic responses to the W/D-ON, (C) Second harmonic to the checkerboard stimuli, (D) Second harmonic to the W/D-OFF. Phase convention as in Fig. 2B.
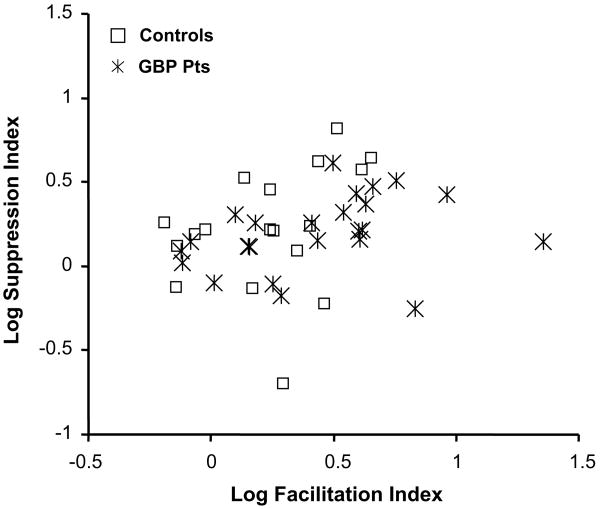
Fig. 4.
Plot of the log Facilitation versus log Suppression Indices calculated from responses obtained from 18 normal controls and 24 patients receiving add-on gabapentin (GBP) treatment.
Table 1.
Comparison of group means calculated from the log facilitation and log suppression indices
| Summary of group means | Log FI | Log SI |
|---|---|---|
| Study 1 | ||
| Controls (n = 18) | .24 ± .21 | .22 ± .26 |
| GBP Pts (n = 24) | .43 ± .29 | .20 ± .17 |
| Study 2 | ||
| Controls (n = 24) | .31 ± .20 | .34 ± .14 |
| VNS Pts (n = 11) | ||
| Stim-ON | .27 ± .28 | .17 ± .17 |
| Stim-OFF | .21 ± .22 | .19 ± .19 |
Study 1: there is no statistical difference (p = .8) in the mean SI for GBP patients compared with their control group, however, the FI approaches statistical significance (p = .06). Study 2: For VNS patients, there was no difference in the indices calculated in the Stim-ON versus Stim-OFF conditions (p = .4). There was also no statistical difference (p = .5) in the mean FI for VNS patients compared with their control group. However, the VNS patients had a significantly smaller SI, (p < .05).
For the subgroups of patients who received the three AED's other than GBP, there were no statistically significant changes in the FI or the SI. For the subgroup of patients who received GBP, there was no statistically significant change in the SI.
In sum, we found that GBP was associated with an alteration of the VEP that could be identified with the windmill-dartboard stimuli, but not with standard (flash and checkerboard) stimuli. This alteration of lateral interactions was reversible, and was not associated with the other three AEDs studied. A regression analysis showed that the change in the FI associated with GBP therapy did not correlate with age, gender, etiology of the disease, baseline medication, or seizure type and frequency. Regression analysis suggested a dependence of the W/D facilitation index on gabapentin dosage but perhaps due to the small sample size, this did not reach statistical significance. We conclude that the FI derived from windmill-dartboard VEPs can identify a change in cortical interactions in epilepsy patients that is selectively and reversibly associated with a specific AED treatment.
3.3.1. Study 2 – Patients receiving chronic VNS
The basis for the antiepileptic action of chronic vagus nerve stimulation (VNS) is currently unknown. A general hypothesis is that chronic VNS changes inhibitory circuitry throughout the cortex in a manner that lessens seizure susceptibility. Our goal was to determine if chronic VNS treatment has a measurable effect on the VEP, and if so, to compare it to the changes previously seen with GBP treatment. Furthermore, to determine if there was a short-acting component of the effect of VNS therapy on the VEP, we tested the patients with the device on and off in the same recording session.
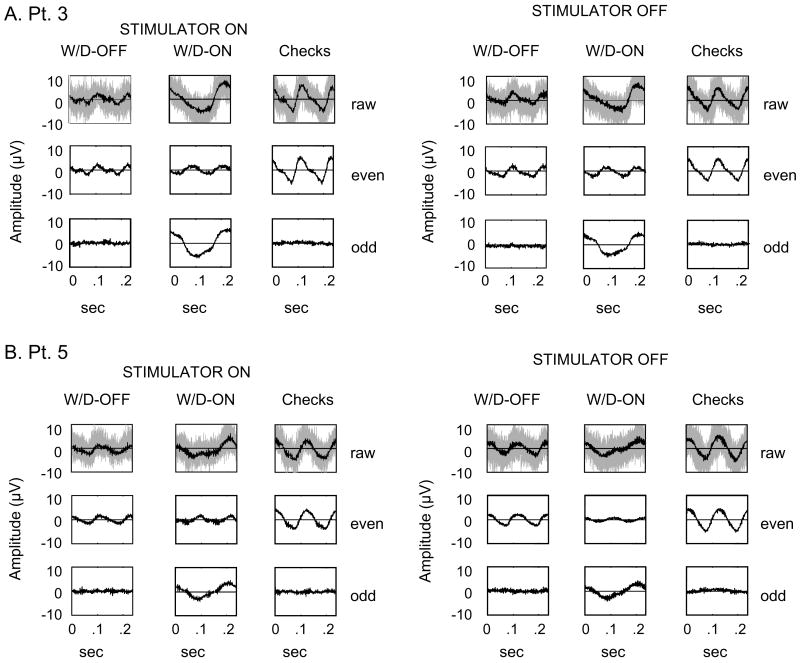
Fig. 5 illustrates typical responses obtained from two patients receiving VNS therapy in both the Stimulator on (Stim-ON) and off (Stim-OFF) conditions. There are no apparent differences in the responses obtained under these two conditions, either in the mean waveform, or in the overall variability of the signals. Thus, artifact-free VEP recordings can be reliably obtained either with the stimulator inactivated, or during the periods in between the ordinarily programmed stimulation cycles, and, at least to casual inspection, responses are remarkably similar.
Fig. 5.
Decomposition of averaged responses, as in Fig. 2A, recorded from two VNS patients in the stimulator on (Stim-ON) and off (Stim-OFF) conditions. Top row: raw signals, middle: even harmonics, bottom: odd harmonics. The light gray shading indicates the un-averaged EEG traces. (A) Patient 3 (F, age: 46, seizure type: complex-partial, duration of VNS: 5 yrs), (B) Patient 5 (M, age: 27, seizure type: complex-partial with secondary generalization, duration of VNS: 6.5 yrs).
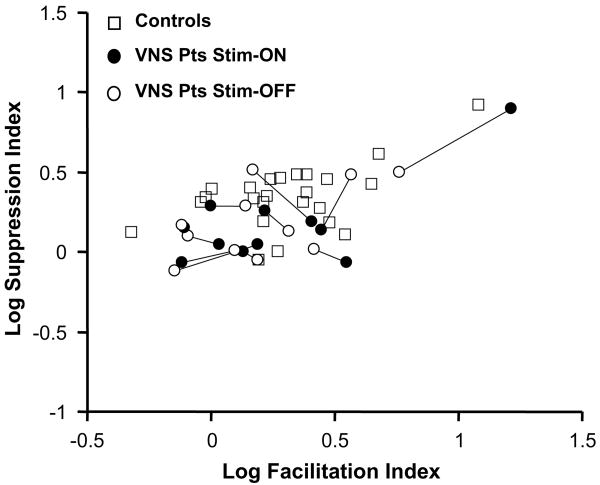
Fig. 3 shows the Fourier components of the steady-state VEPs obtained from the VNS patients, and Fig. 6 compares the corresponding FI and SI indices with those of normal controls. We first compare the indices derived from the Stim-ON and Stim-OFF conditions in individual VNS patients (Fig. 6). For each patient, a line connects the data collected in the Stim-ON (filled circle) and Stim-OFF (open circle) conditions. There were no within-subject differences (two-tailed, paired t-test, p = .4) between responses obtained in these two testing conditions. Also (not shown), there were no differences in the response variance in these two conditions, as measured by Tcirc2. This indicates the lack of a detectable short-term effect of VNS within individual patients, either on response size or its variability.
Fig. 6.
Plot of the log Facilitation versus log Suppression Indices calculated from responses obtained from 24 normal controls and 11 VNS patients. For each patient, a line connects the data collected in the Stim-ON (filled circle) and Stim-OFF (open circle) conditions. For the patients, there were no differences (paired t-tests, p = .4) in the indices obtained with the Stim-ON vs. the Stim-OFF conditions, and no differences in the facilitation index compared with normal controls (p = .5). However, there is a significant (p < .05) decrease in the suppression index.
To look for effects of chronic VNS, we compared indices obtained from Study 2 patients to those of their control group (Table 1). This revealed no change in the FI (p = .5), but a significant decrease in the SI (p < .05). Responses to the transient and steady-state checkerboard stimuli were not significantly different from that of controls, and were unchanged in Stim-ON and Stim-OFF conditions. Thus, while we identified an alteration in lateral interactions in the VNS patient group compared to controls (a decrease in SI), this alteration differed from that of the GBP group (increase in FI).
We did not identify a significant correlation between the size of the reduction of the SI and age, gender, duration of VNS therapy, or VNS dose (calculated as duty cycle × current, Harden & Labar, 2000), but the lack of a correlation must be interpreted in view of the small sample size.
4. Discussion
4.1. Summary of findings
Our main finding is that Fourier analysis of responses to the windmill/dartboard stimuli identifies alterations of the VEP associated with specific antiepileptic therapies. These alterations are not apparent from standard VEP analysis (i.e., latency and amplitude of transient checkerboard responses). We quantified the VEP changes by two indices, a facilitation index (FI) and a suppression index (SI), that reflect different kinds of lateral interactions that can be teased out of the VEP response to appropriately chosen stimuli.
In Study 1, which examined AED treatment in 55 patients, we found a significant, reversible augmentation of the FI in patients receiving add-on GBP medication. This effect appeared to be specific to GBP, and was not seen in patients receiving other add-on AEDs (TPM, FBM, TGB). In Study 2, which examined chronic VNS treatment in 11 patients, we found a significant reduction of the suppression index (SI). We also found that it was possible to record VEP's reliably during intermittent VNS, and that there was no short-term effect of discontinuing stimulation on the VEP.
4.2. Physiological correlates
Previous VEP studies in normal subjects (Zemon & Ratliff, 1982) indicate that the interactions that contribute to the FI and SI response to the windmill/dartboard stimuli reflect interactions that are highly localized in space (2–3 A″), corresponding to approximately 0.5 mm in visual cortex. Studies in the experimental animal (Zemon et al., 1980) indicate that GABAergic inhibitory mechanisms are the main contributor to the VEP. Thus, the changes quantified by the FI and SI most likely are dominated by changes in GABA-mediated local inhibitory interactions. As we review here, current views of the likely mechanisms of action of GBP and VNS are consistent with this idea.
Although gabapentin was designed as a structural analog of GABA, it does not interact directly with the GABA receptors (Crawford, Ghadiali, Lane, Blumhardt, & Chadwick, 1987). Nevertheless, several studies suggest that the action of gabapentin is mediated by GABAergic systems (Leach et al., 1997; MacDonald & Kelly, 1994; Treiman, 2001). Of particular relevance are two studies by Petroff, Hyder, Rothman, and Mattson (2000), (2006). They found that GBP increased occipital lobe GABA within 1 h of the first dose in all patients. Additionally, they found that homocarnosine (an inhibitory neuromodulator hydrolyzed into GABA) and GABA concentrations were low in patients who had frequent seizures and normal in patients with better seizure control. They hypothesized a mechanistic link; that low homocarnosine and GABA levels contribute to cortical hyperexcitability, allowing for the spread of epi-leptiform activity.
It has been suggested that VNS controls epileptic activity by increasing GABA-mediated cortical inhibition (Ben-Menachem et al., 1995, 1999; Marrosu et al., 2003; Rutecki, 1990; Van Laere et al., 2002). In 2003, Marrosu and co-workers examined the effects of VNS on cortical GABAA receptor density (GRD) in 10 patients with partial epilepsy. They found that a reduction in seizure frequency was associated with an increase in GRD to normal values (r2 = .98; p < .0001) within the first year of VNS treatment.
4.3. Future directions
Thus, while the mechanism of the antiepileptic action of GBP and VNS are both unknown, available evidence suggests that boosting of GABAergic inhibitory mechanisms plays an important role. This meshes with our observations of changes in cortical interactions that are associated with these treatments. It also suggests further directions of study. First, while Study 1 identifies alterations specific to GBP (and not some aspect of the epileptic “state” or the patient population), it is possible that the increased FI is not simply a drug effect, but rather results from an interaction of GBP and the disease state. This could be addressed by studying VEPs of patients who are receiving GBP for other indications, including chronic neuropathic pain, depression, and migraines (Pappagallo, 2003; Rogawski & Löscher, 2004). The parallel question can be addressed for VNS, since it is now approved for other indications (e.g., depression). Second, while GBP and VNS both affect lateral interactions in the VEP, the effects are different. This suggests that these two interventions have effects on different aspects of inhibitory circuitry, which could be studied in animal models. VEP studies cannot address this level of detail, but they can determine how the effects develop over the course of treatment, an issue that we are now addressing. Finally, future prospective studies of these VEP measures may provide a way to determine who is likely to benefit from VNS. This would be of considerable practical value, considering the invasive nature of VNS and its only partial success rate (Labar, 2004; Uthman et al., 2004).
Acknowledgments
We thank Drs. D. Labar and C. Harden of the Comprehensive Epilepsy Center, NY Presbyterian Hospital for patient referrals, Dr. E. Kobylarz for neuro-ophthalmologic assessment of the VNS patients, and Laura Ponticello and Helene Quinn for assistance with VNS patient testing. We also thank Ana Ashurova for assistance with data collection and analysis. A portion of this work has been presented at the 2005 meeting of the American Epilepsy Society Washington, DC (Conte, Labar, Kobylarz, Ponticello, & Victor, 2005), and at the 2006 meeting of the Vision Sciences Society Sarasota, FL (Conte et al., 2006).
This work was supported by NIH NEI EY7977.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Bandini F, Pierantozzi M, Bodis-Wollner I. Parkinson's disease changes the balance of onset and offset visual responses: An evoked potential study. Clinical Neurophysiology. 2001;112:976–983. doi: 10.1016/s1388-2457(01)00531-4. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem BE, Hambergerm JA, Hedner T, Hammond HE, Uthman RB, Slater J, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Research. 20(3):221–227. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, Hellstroem K, Waldton C, Augustinsson LE. Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurology. 1999;52:1731–1735. doi: 10.1212/wnl.52.6.1265. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Hendley CD, Mylin LH, Thornton J. Visual evoked potentials and the visuogram in multiple sclerosis. Annals of Neurology. 1979;5:40–47. doi: 10.1002/ana.410050107. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Yahr MD. Measurement of visual evoked potentials in Parkinson's disease. Brain. 1978;101:661–671. doi: 10.1093/brain/101.4.661. [DOI] [PubMed] [Google Scholar]
- Brazdil M, Chadim P, Daniel P, Kuba R, Rektor I, Novak Z, et al. Effect of vagal nerve stimulation on auditory and visual event-related potentials. European Journal of Neurology. 2001;8:457–461. doi: 10.1046/j.1468-1331.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- Casazza M, Avanzini G, Ferroli P, Villani F, Broggi G. Vagal nerve stimulation: Relationship between outcome and electroclinical seizure pattern. Seizure. 2006;15:198–207. doi: 10.1016/j.seizure.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Celesia GG. Steady-state and transient visual evoked potentials in clinical practice. In: Bodis-Wollner, editor. Evoked potentials. Vol. 388. New York: Annals of the New York Academy of Sciences; 1982. pp. 290–305. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Kaufman D. Pattern ERGs and visual evoked potentials in maculopathies and optic nerve diseases. Investigative Ophthalmology and Visual Science. 1985;26(5):726–735. [PubMed] [Google Scholar]
- Conners BW. GABAA and GABAB-mediated processes in visual cortex. In: Mize R, Marc R, Sillito A, editors. GABA in the retina and visual system. Amsterdam: Elsevier; 1992. pp. 335–348. [DOI] [PubMed] [Google Scholar]
- Conte MM, Ashurova A, Ponticello L, Kobylarz EJ, Labar DR, Victor JD. Changes in VEP indices of cortical lateral interactions with epilepsy treatment. [Abstract] Journal of Vision. 2006;6(6):204a. doi: 10.1167/6.6.204. Available from http://journalofvision.org/6/6/204/ [DOI]
- Conte MM, Labar DR, Kobylarz EJ, Ponticello LJ, Victor JD. The effects of VNS neurostimulation on the visual evoked potential. Epilepsia. 2005;46(Suppl. 8):238–239. [Google Scholar]
- Crawford P, Ghadiali E, Lane R, Blumhardt L, Chadwick D. Gabapentin as an antiepileptic drug in man. Journal of Neurology, Neurosurgery, and Psychiatry. 1987;50:682–686. doi: 10.1136/jnnp.50.6.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JD, Pettigrew JD. A study of inhibitory antagonism in cat visual cortex. Brain Research. 1975;93:41–62. doi: 10.1016/0006-8993(75)90285-1. [DOI] [PubMed] [Google Scholar]
- De Deyn P, Marescau B, Macdonald R. Epilepsy and the GABA-hypothesis: A brief review and some examples. Acta Neurologica Belgica. 1990;90(2):65–81. [PubMed] [Google Scholar]
- DeGiorgio C, Thompson J, Lewis P, Arrambide S, Naritoku D, Handforth A, et al. Vagus Nerve Stimulation: Analysis of Device Parameters in 154 Patients during the Long-Term XE5 Study. Epilepsia. 2001;42(8):1017–1020. doi: 10.1046/j.1528-1157.2001.0420081017.x. [DOI] [PubMed] [Google Scholar]
- Geller AM, Hudnell HK, Vaughn BV, Messenheimer JA, Boyes WK. Epilepsy and medication effects on the pattern visual evoked potential. Documenta Ophthalmologica. 2005;110:121–131. doi: 10.1007/s10633-005-7350-0. [DOI] [PubMed] [Google Scholar]
- Ghilardi M, Sartucci F, Brannan J, Onofrj M, Bodis-Wollner I, Mylin L, et al. N70 and P100 can be independently affected in multiple sclerosis. Electroencephalography and Clinical Neurophysiology. 1991;80(1):1–7. doi: 10.1016/0168-5597(91)90035-v. [DOI] [PubMed] [Google Scholar]
- Halliday AM, McDonald WI. Pathophysiology of demyelinating disease. British Medical Bulletin. 1977;33:21–29. doi: 10.1093/oxfordjournals.bmb.a071390. [DOI] [PubMed] [Google Scholar]
- Hammond EJ, Uthman BM, Reid SA, Wilder BJ. Electrophysiologic studies of cervical vagus nerve stimulation in humans: I. EEG effects. Epilepsia. 1992a;33:1013–1020. doi: 10.1111/j.1528-1157.1992.tb01752.x. [DOI] [PubMed] [Google Scholar]
- Hammond EJ, Uthman BM, Reid SA, Wilder BJ. Electrophysiologic studies of cervical vagus nerve stimulation in humans: II. Evoked potentials. Epilepsia. 1992b;33:1021–1028. doi: 10.1111/j.1528-1157.1992.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Harden C, Labar DR. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy & Behavior. 2000;1:93–99. doi: 10.1006/ebeh.2000.0046. [DOI] [PubMed] [Google Scholar]
- Janszky J, Hoppe M, Behne F, Tuxhorn I, Pannek HW, Ebner A. Vagus nerve stimulation: Predictors of seizure freedom. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:384–389. doi: 10.1136/jnnp.2004.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys DA, Axford JG. Source location of pattern specific components of human visual evoked potentials. I. Components of striate cortical origin. Experimental Brain Research. 1972a;16:1–21. doi: 10.1007/BF00233371. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA, Axford JG. Source location of pattern specific components of human visual evoked potentials. I. Component of extrastriate cortical origin. Experimental Brain Research. 1972b;16:22–40. doi: 10.1007/BF00233372. [DOI] [PubMed] [Google Scholar]
- Kirchner A, Landis B, Haslbeck M, Stefan H, Renner B, Hummel T. Chemosensory function in patients with vagal nerve stimulation. Journal of Clinical Neurophysiology. 2004;21(6):418–425. doi: 10.1097/01.wnp.0000141755.28070.14. [DOI] [PubMed] [Google Scholar]
- Koo B. EEG changes with vagus nerve stimulation. Journal of Clinical Neurophysiology. 2001;18(5):434–441. doi: 10.1097/00004691-200109000-00008. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New England Journal of Medicine. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Labar DR. Antiepileptic drug use during the first 12 months of vagus nerve stimulation therapy. A registry study. Neurology. 2002;59(Suppl. 4):S38–S43. doi: 10.1212/wnl.59.6_suppl_4.s38. [DOI] [PubMed] [Google Scholar]
- Labar DR. Vagus nerve stimulation for 1 year in 269 patients on unchanged antiepileptic drugs. Seizure. 2004;13:392–398. doi: 10.1016/j.seizure.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Labar DR, Ponticello L. Persistent antiepileptic effects after vagus nerve stimulation ends? Neurology. 2003;61:1818. doi: 10.1212/01.wnl.0000098995.74791.fd. [DOI] [PubMed] [Google Scholar]
- Leach JP, Sills GJ, Butler E, Forest G, Thompson GG, Brodie MJ. Neurochemical actions of gabapentin in mouse brain. Epilepsy Research. 1997;27:175–180. doi: 10.1016/s0920-1211(97)01034-6. [DOI] [PubMed] [Google Scholar]
- Levi DM, Manny RE. The VEP in the diagnostic evaluation of amblyopia. In: Cracco R, Bodis-Wollner I, editors. Evoked Potentials. New York: Alan R. Liss; 1986. pp. 437–446. [Google Scholar]
- MacDonald RL, Kelly KM. Mechanisms of action of currently prescribed and newly developed antiepileptic drugs. Epilepsia. 1994;35(Suppl. 4):S41–S50. doi: 10.1111/j.1528-1157.1994.tb05955.x. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Serra A, Maleci A, Puligheddu M, Biggio G, Piga M. Correlation between GABAA receptor density and vagus nerve stimulation in individuals with drug-resistant partial epilepsy. Epilepsy Research. 2003;55:59–70. doi: 10.1016/s0920-1211(03)00107-4. [DOI] [PubMed] [Google Scholar]
- Milkman N, Schick G, Rossetto M, Ratliff F, Shapley R, Victor J. A two-dimensional computer-controlled visual stimulator. Behavioral Research Methods and Instrumentation. 1980;12:283–292. [Google Scholar]
- Naritoku DK, Terry WJ, Helfert RB. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Research. 1995;22:53–62. doi: 10.1016/0920-1211(95)00035-9. [DOI] [PubMed] [Google Scholar]
- O'Donoghue MF, Duncan JS, Sander JW. The national hospital seizure severity scale: A further development of the chalfont seizure severity scale. Epilepsia. 1996;37:563–571. doi: 10.1111/j.1528-1157.1996.tb00610.x. [DOI] [PubMed] [Google Scholar]
- Pappagallo M. Newer antiepileptic drugs: possible uses in the treatment of neuropathic pain and migraine. Clinical Therapeutics. 2003;25(10):2506–2538. doi: 10.1016/s0149-2918(03)80314-4. [DOI] [PubMed] [Google Scholar]
- Petroff O, Hyder F, Rothman DL, Mattson RH. Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia. 2000;41(6):675–680. doi: 10.1111/j.1528-1157.2000.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Petroff O, Hyder F, Rothman DL, Mattson RH. Brain homocarnosine and seizure control of patients taking gabapentin or topiramate. Epilepsia. 2006;47(3):495–498. doi: 10.1111/j.1528-1167.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- Ratliff F, Zemon V. Some new methods for the analysis of lateral interactions that influence the visual evoked potential. In: Bodis-Wollner, editor. Evoked Potentials. Vol. 388. New York: Annals of the New York Academy of Sciences; 1982. pp. 113–124. [DOI] [PubMed] [Google Scholar]
- Ratliff F, Zemon V. Visual evoked potentials elicited in normal subjects and in epileptic patients by windmill-dartboard stimuli. In: Nodar R, Barber C, editors. Evoked potentials II. Stoneham: Butterworth Publishers; 1984. pp. 251–259. [Google Scholar]
- Regan D. Rapid methods for refracting the eye and for assessing visual acuity in amblyopia, using steady-state visual evoked potentials. In: Desmedt JE, editor. Visual evoked potentials in man: New developments. Oxford: Clarendon Press; 1977. pp. 418–426. [Google Scholar]
- Regan D. Human brain electrophysiology evoked potentials and evoked magnetic fields in science and medicine. New York: Elsevier; 1989. [Google Scholar]
- Regan D, Milner BA, Heron JR. Slowing of visual signals in multiple sclerosis, measured psychophysically and by steady-state evoked potentials. In: Desmedt JE, editor. Visual evoked potentials in man: new developments. Oxford: Clarendon Press; 1977. pp. 461–469. [Google Scholar]
- Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nature Medicine. 2004;10(7):685–692. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- Rose D, Blakemore C. Effects of bicuculline on functions of inhibition in visual cortex. Nature. 1974;249:375–377. doi: 10.1038/249375a0. [DOI] [PubMed] [Google Scholar]
- Rutecki P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia. 1990;31(Suppl. 2):S1–S6. doi: 10.1111/j.1528-1157.1990.tb05843.x. [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Burchiel KJ. Vagus nerve stimulation has no effect on awake EEG rhythms in humans. Epilepsia. 1993;34:299–304. doi: 10.1111/j.1528-1157.1993.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Spekreijse H, Estevez O, Reits D. Visual evoked potentials and the physiological analysis of visual processes in man. In: Desmedt JE, editor. Visual evoked potentials in man: New developments. Oxford: Clarendon Press; 1977. pp. 16–89. [Google Scholar]
- Spekreijse H, van der Tweel LH, Zuidema T. Contrast evoked responses in man. Vision Research. 1973;13:1577–1601. doi: 10.1016/0042-6989(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Towle VL, Moskowitz A, Sokol S, Schwartz B. The visual evoked potential in glaucoma and ocular hypertension: Effects of check size, field size, and stimulation rate. Investigative Ophthalmology and Visual Science. 1983;24:175–183. [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;(Suppl 3):8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- Ts'o DY, Gilbert CD, Wiesel TN. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. The Journal of Neuroscience. 1986;6(4):1160–1170. doi: 10.1523/JNEUROSCI.06-04-01160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthman B, Reichl A, Dean J, Eisenchenk S, Gilmore R, Reid S, et al. Effectiveness of vagus nerve stimulation in epilepsy patients: A 12-year observation. Neurology. 2004;63:1124–1126. doi: 10.1212/01.wnl.0000138499.87068.c0. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Vonck K, Boon P, Versijpt J, Dierckx R. Perfusion SPECT changes after acute and chronic vagus nerve stimulation in relation to prestimulus condition and long-term clinical efficacy. Journal of Nuclear Medicine. 2002;43(6):733–744. [PubMed] [Google Scholar]
- Vaughan H, Arezzo J. The neural basis of event-related potentials. In: Picton T, editor. The EEG handbook. Vol. 3. New York: Elsevier; 1988. pp. 45–96. revised series. [Google Scholar]
- Victor JD, Mast J. A new statistics for steady-state evoked potentials. Electroencephalography and Clinical Neurophysiology. 1991;78:378–388. doi: 10.1016/0013-4694(91)90099-p. [DOI] [PubMed] [Google Scholar]
- Zemon V, Ratliff F. Visual evoked potentials: Evidence for lateral interactions. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:5723–5726. doi: 10.1073/pnas.79.18.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemon V, Kaplan E, Ratliff F. Bicuculline enhances a negative component and diminishes a positive component of the visual evoked cortical potential in the cat. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:7476–7478. doi: 10.1073/pnas.77.12.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemon V, Ratliff F. Intermodulation components of the visual evoked potential: Responses to lateral and superimposed stimuli. Biological Cybernetics. 1984;50:401–408. doi: 10.1007/BF00335197. [DOI] [PubMed] [Google Scholar]