Abstract
Objectives:
Over the last decade, various groups have proposed prognostic scoring systems for patients with colorectal liver metastasis (CLM) treated with hepatic resection. The aims of the current study were to evaluate the differences between and clinical importance of these prognostic scoring systems and to determine their clinical applicability.
Methods:
Relevant articles were reviewed from the published literature using the MEDLINE database. The search was performed using the keywords ‘colorectal cancer’, ‘metastases’, ‘liver resection’ and ‘hepatectomy’.
Results:
Twelve prognostic scoring systems were identified from 1996 to 2009. Six of these originated from European institutions, three from Asian and three from North American centres. The median study sample was 288 patients (range 81–1568 patients) and median follow-up was 35 months (range 16–52 months). All studies were retrospective in nature and the numbers of groups proposed by the various scoring systems ranged from three to six. All the studies used the Cox proportional hazard model for multi-variable analysis.
Conclusions:
There is no ‘ideal’ prognostic scoring system for the clinical management of patients with CLM for hepatic resection. These prognostic scoring systems are clinically relevant with respect to survival but have not been used for risk stratification in controversial areas such as the administration of chemotherapy or surveillance programmes.
Keywords: hepatectomy, hepatic resection, liver metastases, colorectal cancer
Introduction
At present, hepatic resection has become the optimal and only treatment modality associated with longterm survival in patients with colorectal liver metastasis (CLM). Despite the variability in the selection criteria of patients with CLM for hepatic resection, 5-year survival rates up to 58% have been reported,1–3 and a recent meta-analysis reported 10-year survival at 17–33%.4 These results have repeatedly supported the clinical ‘controversy’ that both longterm disease-free (>5 years)3 periods and potential cure (>10 years)2 can be achieved in certain patient groups following hepatic resection compared with the survival outcome of patients with non-resectable disease.5–7
In 1986, Ekberg et al. concluded that resection for CLM was indicated only in patients with less than four liver metastases including bilobar disease, no evidence of extrahepatic disease and in whom a resection margin ≥10 mm could be achieved.8 Better understanding of hepatic segmental anatomy, refined haemostatic techniques,9,10‘down-sizing’ chemotherapy and portal vein embolization11 have increased the numbers of patients undergoing hepatic resection to include some patients previously considered to be non-resectable. The current guidelines state that the aims of liver resection in patients with CLM are to remove all macroscopic disease, to achieve clear resection margins and to leave sufficient functioning liver. These criteria apply to patients with solitary, multiple and bilobar disease as well as patients with extrahepatic disease that is confined to the lungs, spleen or adrenal glands.12 Although some authorities may consider radical surgery to be cyto-reductive only, the combination of resection of all macroscopic disease with chemotherapy may improve survival.13 The use of chemotherapy in this setting complements the effect of hepatic resection in removing all macroscopic disease.
Furthermore, studies have reported up to 40% survival at 5 years following repeat hepatic resection for recurrence of CLM, with acceptable morbidity and mortality rates.14–17 Recent data have suggested that patients with lung metastases of colorectal origin have a 5-year survival following thoracotomy similar to that observed in patients following resection of CLM.18,19 These results reflect the adoption of a more aggressive approach towards the treatment of metastatic colorectal disease.
To address the controversial topic of patient selection, various groups have proposed using prognostic scoring systems to stratify patients into risk categories in order to identify the optimal clinical management strategy for each patient. Prognostic scoring systems are believed to be advantageous with respect to high-quality analyses, reproducibility between institutions and clinical applicability, as well as being subject to consistent external validation. The aims of the present systematic review of all studies of prognostic scoring systems were to evaluate the differences between and relevance of these prognostic scoring systems and to determine their respective clinical applicability.
Materials and methods
An electronic search was performed of the MEDLINE database for the period 1980 to the present using the MeSH headings: ‘colorectal cancer’, ‘liver metastases’, ‘liver resection’ and ‘hepatectomy’. The search was limited to English-language publications and papers reporting studies using human subjects. All titles and abstracts were reviewed and appropriate papers were further assessed. The reference sections of all papers deemed appropriate were further reviewed to identify papers that might have been missed on the primary search criteria.
Studies were included if they described or formulated a prognostic scoring system based on demographic, clinical and/or pathological variables that influenced the survival outcome for patients undergoing liver resection for CLM (Fig. 1). All series fitting the search specifications independent of the size of the study population were included. The minimal dataset necessary for inclusion required: details of CLM patients treated with hepatic resection; documentation of survival, and a description of the prognostic scoring system(s). Papers that provided adequate survival data and identified prognostic variables but did not include a prognostic scoring system were excluded. Case reports, editorials, abstracts and reviews were also excluded.
Figure 1.
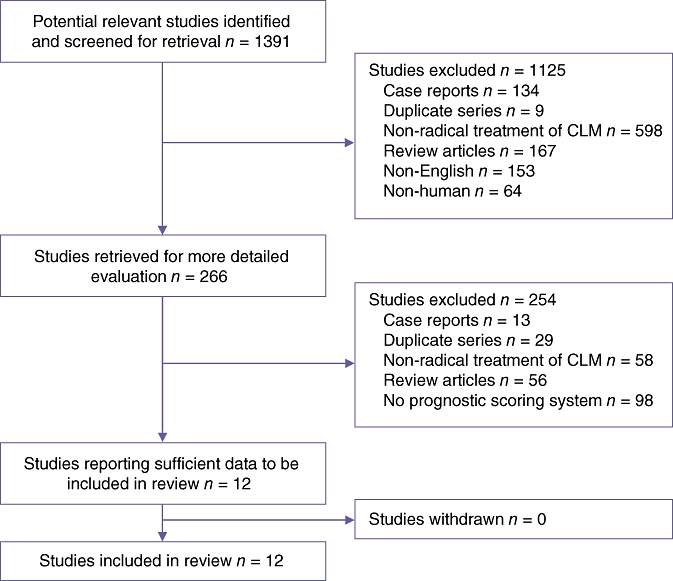
Diagram demonstrating the search strategy used to identify studies to be included in this review. CLM, colorectal liver metastasis
Prognostic scoring systems
To date, numerous studies have correlated a number of demographic, clinical, operative and pathological factors with disease-free and overall survival of patients treated with hepatic resection for CLM using statistical analysis comprising univariable and multi-variable analyses.20–23 In comparison, there have been limited publications on prognostic scoring systems.24–35 Based on the current search criteria, 12 prognostic scoring systems were identified from 1996 to 2009 (Table 1). The majority of the proposed scoring systems originated from European institutions (n= 6; UK = 3, France = 1, Italy = 1, Germany = 1), followed by Asian (n= 3; Japan = 2, South Korea = 1) and North American (n= 3) centres. The median study sample was 288 patients (range 81–1568 patients) and the median follow-up was 35 months (range 16–52 months). All studies were retrospective in nature and the numbers of groups proposed by the various scoring systems ranged from three to six. All the studies used the Cox proportional hazard model for multi-variable analysis.
Table 1.
Current published prognostic scoring systems for patients with colorectal liver metastasis
| Authors, year | Patients, n (period) | Centre | Endpoint | Independent prognostic factors |
Prognostic analyses |
|||
|---|---|---|---|---|---|---|---|---|
| Groups | Score | Outcome (5-year)a | ||||||
| Nordlinger et al.,24 1996 | 1568 (1968–1990) | 85 centres, France | Overall survival | Age ≥60 years Serosal invasion of primary tumour Node-positive primary Liver metastasis <2 years Metastasis size ≥5 cm Liver metastases ≥4 Margin ≤1 cm | Low risk (n= 305) Intermediate risk (n= 738) High risk (n= 230) | 0–2 3–4 5–7 | 2-year: 79% 60% 43% | |
| Fong et al.,25 1999 | 1001 (1985–1998) | New York, USA | Disease-free survival | Margin involved Extrahepatic disease Node-positive primary Liver metastasis <1 year Liver metastasis >1 Metastasis size ≥5 cm CEA >200 ng/ml | 0 (n= 52) 1 (n= 262) 2 (n= 350) 3 (n= 243) 4 (n= 80) 5 (n= 14) | 0 1 2 3 4 5 | 60% 44% 40% 20% 25% 14% | |
| Iwatsuki et al.,26 1999 | 305 (1981–1996) | Pittsburgh, USA | Overall and disease-free survival | Liver metastases ≥2 Metastasis size ≥8 cm Bilobar distribution Interval between colorectal and liver resection ≤30 months Margin involved Extrahepatic disease | Grade 1 (n= 32) Grade 2 (n= 112) Grade 3 (n= 47) Grade 4 (n= 47) Grade 5 (n= 5) Grade 6 (n= 62) | 0 1 2 3 4 R1 EHD | 48% 34% 18% 6% 1% 0% | |
| Ueno et al.,27 2000 | 85 (1985–1996) | Saitama, Japan | Overall survival, recurrence within 6 months | Primary tumour – marked tumour budding / node-positive Liver metastasis <1 year Liver metastases ≥3 | Stage A (n= 30) Stage B (n= 46) Stage C (n= 9) | 0–1 2 3 | 55% 14% 0% | |
| Lise et al.,28 2001 | 132 (1977–1997) | Padova, Italy | Disease-free survival, diffuse recurrence within 6 months, recurrence suitable for surgery | >30% liver invasion Node-positive primary Liver metastases >1 GPT levels ≥55 U/l Non-anatomical resection | Group A (n= 38) Group B (n= 36) Group C (n= 58) | 0–2 3–5 ≥6 | 3-year: 80% 55% 10% | |
| Nagashima et al.,29 2004 | 81 (1981–1997) | Tokyo, Japan | Overall survival | Serosal invasion of primary tumour Node-positive primary Resectable extrahepatic disease Liver metastases >1 Metastasis size 5 cm | Grade A (n= 28) Grade B (n= 14) Grade C (n= 13) | 0–1 2–3 >3 | 85% 56% 0% | |
| Schindl et al.,30 2005 | 270 (1988–2002) | Edinburgh, UK | Overall survival | Duke's stage C CEA level Alkaline phosphatase Albumin Liver metastasis >3 | Good (n= 33) Moderate (n= 172) Poor (n= 65) | 0–10 11–25 >25 | Median survival: 60 months 32 months 22 months | |
| Malik et al.,31 2007 | 687 (1993–2006) | Leeds, UK | Overall and disease-free survival | Inflammatory response to tumour Liver metastases ≥8 | 0 (n= 382) 1 (n= 155) 2 (n= 23) | 0 1 2 | 49% 34% 0% | |
| Zakaria et al.,32 2007 | 662 (1960–1995) | Rochester, USA | Disease-free survival Recurrence | Liver metastasis ≤30 months Metastasis size ≥8 cm Blood transfusion Positive hepatoduodenal nodes | Group 1 Group 2 Group 3 (Note: n was not stated) | 0 BT HDN | 55% 39% 20% | |
| Lee et al.,33 2008 | 135 (1994–2005) | Seoul, South Korea | Overall survival | Margin ≤5 mm CEA >5 ng/ml Node-positive primary ≥4 Liver metastases >1 | Low risk (n= 85) Intermediate risk (n= 36) High risk (n= 14) | 0–1 2 3–4 | 46% 41% 11% | |
| Rees et al.,34 2008 | 929 (1987–2005) | Basingstoke, UK | Overall survival | Liver metastases >1 Node-positive primary Poorly differentiated primary Extrahepatic disease Metastasis size ≥5 cm CEA >60 ng/ml Margin involved | 0 1–5 6–10 11–15 >15 (Note: n was not stated) | 0 1–5 6–10 11–15 >15 | Preop |
Postop |
| 66% 51% 35% 21% 2% | 64%49%34%21%2% | |||||||
| Konopke et al.,35 2009 | 201 (1993–2006) | Dresden, Germany | Overall and disease-free survival | Liver metastasis ≥4 Synchronous liver metastases CEA >200 ng/ml | Low risk (n= 112) Intermediate risk (n= 74) High risk (n= 15) | 0 1 ≥2 | Median survival: 67 months 47 months 38 months | |
Survival data are presented as 5-year survival rates unless otherwise stated
CEA, carcino-embryonic antigen; GPT, glutamic pyruvic transaminase; R1, involved margin; EHD, extrahepatic disease; BT, blood transfusion; HDN, hepatoduodenal nodes
Nordlinger and co-workers were the first group to propose a prognostic scoring system.24 It was based on their analysis of 1568 patients drawn from 85 institutions over a study period of 22 years (1968–1990). The median follow-up in this study was 19 months (range 9–30 months) and the majority of liver resections were performed after 1987 (n= 918). Multi-variable analysis revealed seven independent prognostic factors that included: age ≥60 years; serosal invasion of primary tumour; lymph node-positive in the primary tumour; liver metastasis presenting within 2 years of the primary tumour; liver metastasis sized ≥5 cm; more than four liver metastases, and a resection margin ≤1 cm. Their prognostic scoring system divided patients into three groups and showed 2-year survival rates that decreased from 79% in the low-risk group to 43% in the high-risk group. In addition, Nordlinger et al.24 performed a bootstrap analysis for internal validation of their prognostic scoring system.
Fong's clinical risk score from the Memorial Sloan-Kettering Cancer Center, New York is arguably the most well-known prognostic scoring system. Fong and colleagues25 included 1001 patients in their study (1985–1998), which involved a median follow-up of 22 months (range 0–89 months). The study identified seven independent prognostic variables derived from their multi-variable analysis: lymph node-positive in the primary tumour; a disease-free interval from primary to liver metastasis ≤12 months; more than one hepatic tumour; largest hepatic metastasis ≥5 cm, carcino-embryonic antigen (CEA) level >200 ng/ml; the presence of extrahepatic disease, and an involved resection margin. Of these, margin involvement and the presence of extrahepatic disease were the most influential and the authors suggested that these characteristics should be considered contraindications to liver resection. In formulating their preoperative scoring system, Fong et al.25 excluded patients with tumour involvement at the resection margin and extrahepatic disease. Using the first five preoperative variables described above, Fong's clinical scoring system showed a declining 5-year disease-free survival in patients scoring 0 (60%) compared with patients scoring 5 (14%).
Using a dataset that included 305 patients with CLM recorded over 15 years, Iwatsuki and co-workers suggested a graded scoring system using six groups and included both disease-free and overall survival in their analyses.26 The median follow-up was 32 months. Multi-variable analysis indicated that margin involvement, extrahepatic disease including lymph node involvement, more than three liver metastases, bilobar distribution and an interval of ≤30 months between colorectal and liver resection were independent prognostic variables. When 62 (20%) patients with margin involvement or extrahepatic disease including lymph node involvement were excluded, re-analysis revealed that more than two liver metastases, tumour size ≥8 cm, bilobar distribution and an interval between colorectal and liver resection of ≤30 months were independent predictors of poorer disease-free survival. These four variables were used in the scoring system. The authors identified six groups in total: grade 1 patients showed no risk factors; grade 2 patients had one risk factor; grade 3 patients had two risk factors, and so forth. The last group (grade 6) consisted of patients with either margin involvement or extrahepatic disease. This group of patients had no 5-year survivors, whereas 48% of grade 1 patients survived to 5 years.
Ueno et al. proposed a prognostic scoring system comprising three stages (A–C) following analysis of 85 patients with CLM over an 11-year study period.27 This study had a median follow-up of 52 months (range 13–118 months) and 5-year disease-free and overall survival rates of 21% and 28%, respectively. Multi-variable analysis identified marked tumour budding and lymph node positivity of the primary tumour, liver metastasis at <1 year after primary resection and three or more liver metastases as independent predictors of poorer overall survival. Tumour budding was defined as micro-tubular cancer nests or microscopic clusters of undifferentiated cancer cells. Hence, the scores were calculated as follows: stage A (none or either liver metastasis at <1 year or three or more liver metastases); stage B (primary tumour aggressiveness or both liver metastasis at <1 year and three or more liver metastases), and stage C (all three risk factors). There were no 5-year survivors among patients (n= 9) in stage C. In addition, rates of disease recurrence within 6 months were reported at 7%, 30% and 44%, in stages A, B and C, respectively.
Lise and colleagues published their scoring system in 2001 based on a survey of patients conducted over 20 years (1977–1997).28 The median follow-up was 52 months. The endpoint of this study was the identification of variables influencing disease-free survival, diffuse recurrence within 6 months and recurrences suitable for surgery. Multi-variable analysis of 20 variables identified five adverse prognostic variables: >30% liver invasion; lymph node-positive primary tumour; more than one liver metastasis; serum glutamic pyruvic transaminase (GPT) levels ≥55 U/l, and non-anatomical hepatic resection. Of note, this study did not include pathological data with respect to resection margin because histological samples were available for only 31% (n= 42) of cases. The scoring system devised by Lise et al.28 resulted in three groups, of which group A included patients with the best prognosis (80%), in whom the authors observed a declining 3-year disease-free survival, and group C included patients with the worst prognosis (10%).
Nagashima et al. developed a formula for a prognostic scoring system in 2004 and divided patients into three grades (A–C), with grade C having the worst prognosis.29 The median follow-up of 81 patients undergoing hepatectomy from 1981 to 1997 was 36 months (range 4–156 months). Cox regression analysis indicated that serosal invasion of the primary tumour, lymph node-positive primary disease, non-resectable extrahepatic disease, more than one liver metastasis and tumour size ≥5 cm were independent prognostic variables. Grade A patients had a 5-year overall survival of 85%, by contrast with grade C patients, none of whom survived to 5 years. In addition, the study by Nagashima et al.29 was the first to undergo external validation of its prognostic scoring system, using 70 patients undergoing hepatectomy in another Tokyo centre, which concluded similar findings.
Schindl et al. published a suggested prognostic scoring system in 2005 following retrospective analysis of 270 patients referred to their institution in Edinburgh, UK over a 14-year period.30 Schindl et al. included non-resectable cases in their scoring system and 150 (56%) of their patients were treated with hepatic resection.30 This study had a median follow-up of 16 months and reported overall 5-year survival rates for the original cohort and for patients who underwent hepatic resection of 15% and 36%, respectively. The independent prognostic variables identified included Duke's stage of primary tumour, serum CEA, alkaline phosphatase and albumin levels and number of liver metastases. Patients were divided into three groups based on their calculated score, namely, the good, moderate and poor prognosis groups. Irrespective of their groups, patients who underwent hepatic resection had significantly better outcomes compared with patients treated palliatively. Nevertheless, the poor prognosis group (n= 12) did not have any 5-year survivors following hepatectomy, and the good and moderate prognosis groups demonstrated 5-year survival rates of 63% and 20%, respectively. This prognostic scoring system was externally validated in 193 patients referred to another institution (in Vienna, Austria) and similar findings were reported.
Malik and co-workers developed a preoperative scoring system following analysis of 687 patients who underwent hepatectomy over a period of 13 years.31 The endpoint of this study was the identification of variables influencing both disease-free and overall survival. The median follow-up was 34 months (range 12–168 months) and overall 5-year survival was 45%. Multi-variable analysis revealed the presence of eight or more liver metastases and an inflammatory response to tumour (IRT) to be independent predictors of poorer disease-free and overall survival. Inflammatory response to tumour was defined by an elevated C-reactive protein (C-RP) level (>10 mg/l) or raised neutrophils : lymphocyte ratio (≥5). Based on these results, a preoperative prognostic score was developed, on which 0 = no risk factors, 1 = either IRT or eight or more liver metastases, and 2 = both IRT and eight or more liver metastases. Patients who scored 0 and 1 had 5-year overall survival rates of 49% and 34%, respectively. By contrast, there were no 5-year survivors among patients who had both prognostic factors (n= 23, median survival = 21 months).
Zakaria and co-authors proposed their ‘Mayo scoring system’ following retrospective analysis of 662 consecutive patients who underwent hepatectomy over 35 years.32 The endpoint of this study was to determine prognostic variables that influenced both disease-free survival and recurrences. The median follow-up was 3 years (range 5 days–37 years) and overall 5-year survival was 37%. This study also reported a 65% probability of recurrences at any site at 5 years. Multi-variable analysis showed that a poorer disease-free survival was significantly associated with liver metastasis within 30 months of primary colorectal tumour diagnosis, hepatic metastases sized ≥8 cm, perioperative blood transfusion and positive hepatoduodenal lymph nodes. Zakaria et al.32 divided patients into three groups: group 1 (patients with any risk factor except blood transfusion and positive hepatoduodenal lymph nodes); group 2 (patients with any risk factor except positive hepatoduodenal lymph nodes), and group 3 (patients with positive hepatoduodenal lymph nodes with or without other risk factors). Five-year disease-free survival was 55% in group 1, 39% in group 2 and 20% in group 3. Zakaria et al.32 further analysed the applicability of three other scoring systems (by Nordlinger et al.,24 Fong et al.25 and Iwatsuki et al.26) using their patient population and observed that neither survival nor recurrence among their population sample was stratified discretely by any of these three scoring systems.
Lee and colleagues devised a prognostic scoring system for patients undergoing simultaneous resection of the colorectal primary and synchronous liver metastases based on their 11-year experience.33 All 135 patients included in their survival analysis had a clear liver resection margin. The median follow-up was 47 months (range 12–134 months). Multi-variable analysis showed a resection margin ≤5 mm, CEA levels >5 ng/ml, four or more node-positive primaries and more than one liver metastasis to be independent adverse prognostic variables. Lee et al.'s33 prognostic scoring system comprised low (0–1), intermediate (2) and high (3–4) risk groups depending on the number of prognostic variables. Declining 5-year overall survival rates of 46%, 41% and 11% were observed in the low-, intermediate- and high-risk groups, respectively.
Rees et al. formulated a prognostic scoring system incorporating both pre- and postoperative variables based on a series of 929 patients who underwent hepatic resection for CLM over 18 years.34 In this series, median survival was 42 months and 5- and 10-year cancer-specific survival rates were 36% and 23%, respectively. Statistical analysis included 80 patients who underwent re-resection. The multi-variable analysis included prognostic variables with a P-value <0.25 on univariate analysis. Prognostic factors found to be significant on multi-variable analysis were more than one liver metastasis, a lymph node-positive primary, a poorly differentiated primary, extrahepatic disease, tumour size ≥5 cm, CEA >60 ng/ml and an involved resection margin. The first six variables were used in the preoperative score and the last six in the postoperative score. Patients were divided into five groups according to their scores. Patients showed similar 5-year overall survival rates for pre- and postoperative scores in all five groups. For example, patients with the lowest scores (0) had a pre- and postoperative 5-year survival of 64%, whereas patients with the highest scores (>15) had a pre- and postoperative 5-year survival of 2%. Similarly to Nordlinger et al.,24 Rees and co-workers34 also performed internal validation of their scoring system using bootstrap analysis with 50% of their study sample.
The most recent prognostic scoring system was devised by Konopke and co-authors based on their analysis of 201 patients over 13 years.35 The median follow-up was 31 months (range 6–143 months) and the authors included both disease-free and overall survival in their analyses. The disease-free and overall 5-year survival rates were 28% and 43%, respectively. Only patients with a clear resection margin were included in the analysis. Multi-variable analysis identified synchronous colorectal primary and liver metastases, the presence of four or more liver metastases and CEA >200 ng/ml as adverse prognostic factors. Patients were divided into three groups, of which the high-risk group (patients with two or more of these variables) had a median survival of 38 months, whereas the low-risk group (patients who did not exhibit any of these variables) had a median survival of 67 months. Internal validation of this scoring system was performed using bootstrap analysis.
Discussion
The ‘ideal’ scoring system
None of the studies based on the current available prognostic scoring systems were without limitations; hence the ‘ideal’ scoring system has not yet been established. All these studies were based on retrospective data and many series included patients operated on during the 1970s and 1980s. Selection of patients for hepatic resection was previously stringent because the risk for postoperative morbidity and mortality was significant, the accuracy of radiological imaging for preoperative staging for metastatic disease was limited and the survival outcome following surgery was undetermined. During this time period there were many major developments in hepatic resectional surgery, not only in terms of surgical technique, but also, importantly, in relation to anaesthesia and perioperative care, and thus the overall results may underestimate true outcomes following resection in CLM. This has cemented hepatic resection as the treatment of choice for CLM, including staged hepatic resection following neoadjuvant chemotherapy36 and portal vein embolization.11
A number of risk factors have been consistently reported as important in predicting a poorer outcome following resection for CLM, including: number and size of hepatic metastases; presence of extrahepatic disease; high serum CEA levels; synchronous primary colorectal carcinoma and hepatic metastases; lymph node-positive primary tumour; presence of IRT, and involved resection margin. Nevertheless, the published studies have been inconsistent in identifying these variables as independent predictors of survival on multi-variable analyses in different datasets, which suggests that certain prognostic variables may be more significant in certain patient groups. One possible explanation for this observation may involve the sample size of these studies. All the published studies were retrospective and therefore none included a power calculation. Although some studies did include substantial numbers of patients, many of them were left with very small sample sizes when the patients were divided into subgroups according to the proposed scoring systems, especially those with more than three risk groups. Length of follow-up may be another factor contributing to this inconsistency in identifying significant prognostic variables. The median follow-up for the 12 studies reviewed here was 35 months. Certain prognostic factors that are significant in studies with shorter follow-up periods tend to be less accurate in predicting overall survival and recurrences in studies with longer follow-up data. Hence, prognostic models that are based on shorter durations of follow-up may not be accurate or representative in predicting survival and recurrences. Another reason for this discrepancy between institutions in identifying significant prognostic factors may involve limitations of the data available for analysis. Certain studies, such as that by Lee et al., included multiple variables for survival analysis, among which were various factors related mainly to the primary tumour.33 Certain ‘unique’ prognostic factors were analysed only by certain authors, such as tumour budding, analysed by Ueno et al.,27 and IRT, analysed by Malik et al.,31 whereas some important prognostic markers were omitted from statistical analysis by other authors, such as resection margin, which was omitted by Lise et al.28 In addition, well-recognized prognostic factors, such as number of liver metastases, were grouped differently by the various studies. Nordlinger et al.24 observed that more than four liver metastases was a significant prognostic factor, whereas Lise et al.28 and Nagashima et al.29 reported the presence of more than one liver metastasis as an adverse prognostic variable. It should also be noted that not all studies had the same endpoint when analysing these prognostic variables. The majority of authors analysed variables with overall patient survival as their endpoint, with the exception of Fong et al.,25 Lise et al.28 and Zakaria et al.,32 who focused on disease-free survival only. Three groups (Iwatsuki et al.,26 Malik et al.31 and Konopke et al.35) addressed a combination of both disease-free and overall survival in their analyses.
These scoring systems also differed with respect to their inclusion and exclusion criteria. Fong et al.25 excluded patients with a positive resection margin and extrahepatic disease from their preoperative scoring system. Similarly, Iwatsuki and co-workers26 excluded patients with a positive resection margin and/or extrahepatic disease, including lymph nodes, as these patients had the worst survival outcome. Lee et al.33 and Konopke et al.35 included only patients with a clear resection margin. Schindl and colleagues30 described the only series to include both surgically managed and non-resectable cases in their scoring system. Only the studies by Fong et al.25 and Rees et al.34 included patients who underwent re-resection. The exclusion of patients with a positive resection margin following resection for CLM is controversial. Recently, Bodingbauer et al. observed that resection margin and size of margin width did not correlate significantly with survival following resection for CLM.37 In a series of 1019 patients, Are and co-investigators demonstrated that a resection margin >1 cm was an independent predictor of survival following resection for CLM.21 However, Figueras et al. showed that a margin width <1 cm in patients who underwent resection for CLM did not significantly influence recurrence of disease in a cohort of 609 patients.38 Other authors have reported similar findings.39 In cases with multiple and/or large hepatic metastases, it may be difficult to obtain a clear resection margin of 1 cm even with procedures such as hemi-hepatectomy or tri-sectionectomy. In addition, the role of adjuvant chemotherapy in cases with microscopic tumour involvement at the resection margin is still unknown. The inclusion of re-resection patients in the same analysis as patients undergoing primary hepatic resection may skew statistical analysis, as these patients are likely to have a better survival as a result of treatable recurrences.
Like the advances in diagnostic, anaesthetic and surgical techniques over the last three decades, the use of chemotherapy in a neoadjuvant or adjuvant setting has contributed to the improved survival outcome for patients with CLM.40 Some authorities have suggested that preoperative chemotherapy can potentially improve survival, especially in cases of initially inoperable metastases, principally confined to the liver, which are converted into operable disease. However, there is concern that patients treated with neoadjuvant chemotherapy have increased morbidity and mortality following hepatic resection.41,42 Nevertheless, neoadjuvant chemotherapy has contributed to the increase in indications for resection in CLM. Five prognostic scoring systems did not include chemotherapy as part of their statistical analyses. Nordlinger et al. showed patients (35%) who received adjuvant chemotherapy did not have a significantly different overall survival compared with patients who did not have chemotherapy.24 However, the study did not provide details of its chemotherapy protocol and, given that 85 centres contributed towards this series, it is likely that there were no standard protocols for the whole population sampled. The time period of this study would also suggest that chemotherapy agents such as oxaliplatin were not used. Iwatsuki et al.26 and Zakaria et al.32 reported no significant influence of adjuvant chemotherapy with respect to survival. However, these two groups reported on long study periods and thus included patients treated in the 1970s and 1980s. Malik and co-investigators observed that neoadjuvant chemotherapy did not significantly alter the survival outcome of patients with CLM following hepatectomy.31 Nevertheless, patients who received oxaliplatin-based neoadjuvant chemotherapy represented only 10% (n= 71) of their series. Following simultaneous resection of synchronous colorectal carcinoma and hepatic metastases, Lee et al. did not show an improved overall survival in patients treated with adjuvant chemotherapy (n= 112, 81%) compared with patients who did not have chemotherapy.33 Rees et al.34 and Konopke et al.35 documented no significant survival differences with the use of adjuvant chemotherapy following resection of the primary colorectal tumour.34 Rees and co-workers34 did not perform a subgroup analysis of CLM patients who underwent down-sizing chemotherapy (n= 105, 21%) with respect to survival. Further subgroup analysis of patients who underwent neoadjuvant 5-fluorouracil-based chemotherapy (n= 43, 21%) by Konopke et al.35 revealed a significant survival difference in patients in the ‘high-risk’ group, who were given preoperative chemotherapy and hepatic resection, compared with patients treated with resection only. Based on these results, chemotherapy, irrespective of whether it is delivered pre- or postoperatively, does not appear to influence the longterm outcome of patients with CLM treated with hepatic resection and hence its inclusion as a variable within the prognostic scoring system does not seem justified. However, these studies involved long study periods, different chemotherapy regimens and non-standard protocols, which may have contributed to the non-significance of chemotherapy on statistical analysis. Certainly, more studies are required to confirm this and hence the role of chemotherapy in a prognostic scoring system should not be discounted.
Prognostic scoring models for patients with CLM require internal and external validation before they can be widely implemented. With respect to data validation, only Nordlinger et al.,24 Rees et al.34 and Konopke et al.35 performed internal validation of their scoring systems based on a data re-sampling technique with bootstrap analysis. Bootstrapping is a widely accepted method for internal validation, but is known to be labour-intensive. Nagashima et al.29 and Schindl et al.30 performed external but not internal validation of their results. External validity of a prognostic scoring system can be analysed by a variety of methods, including prospective, independent, multi-institutional and multiple validation with different follow-up periods.43 The remaining studies reported no data validation. All but one study suggesting a prognostic scoring system was based on a single-centre experience. Nordlinger et al.24 was the only study to propose a scoring system based on analysis of a multi-institution dataset. Since these scoring systems were proposed, various authors have attempted to address their usefulness in clinical practice. Mann et al.44 observed that their study population validated the clinical risk score devised by Fong et al.25 and found it to be highly predictive of patient survival. Other authors have documented similar results.45,46 However, there have also been conflicting results with regard to the usefulness of these scoring systems. Zakaria et al.32 failed to confirm predictive stratification of their patients with CLM undergoing hepatic resection using the prognostic scoring systems proposed by Nordlinger et al.,24 Fong et al.25 and Iwatsuki et al.26 Although robust statistical analysis may ensure prognostic scoring system reliability, this does not guarantee the applicability of predictive stratification of patients with the same disease between different institutions.
Clinical application
In terms of clinical application, the principal issues concern the accuracy of the scoring systems proposed by the various groups in the clinical management of patients with CLM. These prognostic scoring systems were designed to refine patient selection for resection of CLM and to facilitate patient stratification into risk or prognostic groups for clinical management. All the prognostic scoring systems proposed are based on a solid foundation of statistical analyses of their respective datasets. Nevertheless, this does not guarantee ‘generalizability’. The clinical usefulness of a prognostic system is established by its being tested and found accurate across increasingly diverse settings. Furthermore, however accurate statistical analysis may be, it does not mean that data or a prognostic model from one institution will fit into another institution's dataset addressing the same disease. Invariably, selection biases, population differences, differences in groups of variables, differences in length of follow-up, changes in radiological imaging and improvements in surgical techniques will come into play. In studies that analyse disease-free survival and recurrences, this can vary considerably between institutions according to the different surveillance protocols implemented. The time-point at which recurrences are detected will invariably depend on the frequency of surveillance following resection in patients with CLM.
In accordance with current guidelines, patients with CLM suitable for surgery would be offered surgery with the aim of resecting all macroscopic disease with a clear margin and leaving sufficient liver remnant. Hence, prognostic scoring systems to select patients for resection would be considered obsolete. All patients with resectable disease, irrespective of the number and size of hepatic metastases, should be treated with hepatic resection, and a subgroup of patients who may be suitable for further pre-surgical treatment, such as down-sizing chemotherapy or portal vein embolization, should be treated accordingly prior to reassessment for surgery. Prognostic scoring systems allow the identification of patient groups at higher risk of recurrence and poorer survival, and their main role in clinical management may be to identify patients who would benefit from further intensive postoperative radiological surveillance.
Chemotherapy
The exact timing and role of chemotherapy in patients with resectable metachronous liver metastases represent an area of intense debate. With respect to synchronous liver metastasis, the optimal timing of and indication for surgical resection are even less defined, especially with regard to the unclear boundary between simultaneous resection followed by adjuvant chemotherapy vs. neoadjuvant chemotherapy followed by liver resection. Although most authorities would agree that patients with CLM would benefit from chemotherapy, there is controversy as to preferences for preoperative vs. postoperative chemotherapy.47 This is partly in response to studies that have reported a significant association between the administration of neoadjuvant chemotherapy and increased morbidity and mortality following hepatectomy for CLM.41 Oxaliplatin-based regimes are known to cause sinusoidal obstruction syndrome, which leads to impaired regeneration and increases the risk for liver failure after major liver resections.48 Vauthey et al. observed that administration of irinotecan caused chemotherapy-associated steatohepatitis (CASH) that was associated with increased postoperative mortality, specifically deaths from postoperative liver failure.41 Nevertheless, a recent randomized trial by Nordlinger and co-authors observed that patients who received chemotherapy (FOLFOX4: oxaliplatin, leucovorin and fluorouracil regimen), either prior to or following hepatic resection for CLM, had a significant reduction in the risk for progression compared with patients who had surgery only.40 In the subgroup of patients with initially non-resectable disease, the use of neoadjuvant chemotherapy can be used as a down-sizing technique to enable resection.13
The management of patients with potentially curative CLM requires a multidisciplinary approach. A sub-set of patients would benefit from enrolment into multimodal treatment procedures with chemotherapy and resection. However, it is unclear whether to prefer simultaneous resection followed by adjuvant chemotherapy or neoadjuvant chemotherapy followed by liver resection. Ultimately, the decision about which therapeutic procedure to follow in synchronous CLM remains subjective and depends on the expertise and aggressiveness of the surgeon. None of the current prognostic scoring systems included chemotherapy as a prognostic factor because of its failure to achieve statistical significance. However, Konopke et al.35 did report that neoadjuvant chemotherapy improved the survival of high-risk patients who underwent both chemotherapy and resection compared with those who underwent resection only. This is an important area in which a preoperative prognostic scoring system would be clinically useful in stratifying patients who would benefit from either neoadjuvant or adjuvant chemotherapy. More studies are needed to determine the suitability of chemotherapy in an adjuvant or neoadjuvant setting.
Surveillance
Historically, patients with disease recurrence following resection for CLM were considered to have a poor prognosis, few patients were offered repeat liver resections49,50 and, hence, intensive surveillance follow-up could not be justified. However, as more hepatobiliary units are adopting an aggressive surgical approach towards hepatic and/or extrahepatic recurrences following curative resection for CLM, an intensive surveillance programme designed to detect recurrences where surgical intervention can alter survival outcome is crucial. Prognostic scoring systems could play a role in determining the frequency of radiological imaging required by patients with CLM following hepatectomy. Not all patients would require the same intensive follow-up protocol. Beard et al.51 and Gazelle et al.52 have suggested a selective approach in surveillance based on cost : benefit ratio analysis. Bhattacharjya et al. recently evaluated a prospective intensive follow-up programme using serial tumour marker estimations and contrast-enhanced computed tomography (CT) of the chest and abdomen in patients undergoing potentially curative resection of colorectal liver metastases.53 This series showed that none of their eight patients with recurrent liver metastases within 6 months of hepatic surgery had resectable disease.53 Similarly, Metcalfe et al. identified only one of 22 potentially curable recurrences within 12 months of liver resection using a 6-monthly CT surveillance protocol.54 However, both these studies were limited by small sample sizes. Although the first 2 years of follow-up after resection for CLM have been recognized as representing the period when disease is most likely to recur,55 the frequency of radiological imaging during this period is controversial. The majority of hepatobiliary units offer either 3- or 6-monthly surveillance follow-up during this period.14,25,37,38,53 Nevertheless, cost : benefit analysis must be considered carefully when designing surveillance protocols. The timing and pattern of disease recurrence, its implication for surgical and/or medical intervention and its subsequent effect on patient outcomes are crucial to the design of surveillance protocols. Positron emission tomography (PET) may be an imaging modality of choice in future protocols for surveillance after surgery for CLM. A recent study by Fernandez and co-workers reported that patients staged preoperatively with PET had a 5-year overall survival of 58% following hepatic resection.1 In addition, other authors have found that PET frequently detects recurrent and metastatic colorectal cancer in patients with normal CT scans, but rising CEA levels.56,57 There are few considerations with regard to the cost and availability of PET to be taken into account prior to its widespread use. To date, no published studies have assessed the intervals and duration of surveillance with respect to prognostic variables following hepatic resection for CLM.
Conclusions
Currently, there is no ideal prognostic scoring system for the clinical management of patients with CLM for hepatic resection. Because of its potential for cure and longterm disease-free survival, hepatic resection should be offered to all patients with CLM in whom macroscopic disease can be addressed. Although these prognostic scoring systems are clinically relevant with respect to survival, their broad application currently has limited value with respect to patient stratification for clinical management. Any future prognostic scoring system should address patient selection for neoadjuvant or adjuvant chemotherapy and determine the surveillance protocol required by patients following hepatic resection for CLM. Designing the ‘ideal’ prognostic scoring system is clearly the next step.
References
- 1.Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. discussion 447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 3.Gomez D, Morris-Stiff G, Wyatt J, Toogood GJ, Lodge JP, Prasad KR. Surgical technique and systemic inflammation influences longterm disease-free survival following hepatic resection for colorectal metastasis. J Surg Oncol. 2008;98:371–376. doi: 10.1002/jso.21103. [DOI] [PubMed] [Google Scholar]
- 4.Memon MA, Beckingham IJ. Surgical resection of colorectal liver metastases. Colorectal Dis. 2001;3:361–373. doi: 10.1046/j.1463-1318.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 6.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 7.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 8.Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hagerstrand I, Ranstam J, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. doi: 10.1002/bjs.1800730917. [DOI] [PubMed] [Google Scholar]
- 9.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 10.Heriot AG, Karanjia ND. A review of techniques for liver resection. Ann R Coll Surg Engl. 2002;84:371–380. doi: 10.1308/003588402760978148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeck D, Bachellier P, Nakano H, Oussoultzoglou E, Weber JC, Wolf P, et al. One or two-stage hepatectomy combined with portal vein embolization for initially non-resectable colorectal liver metastases. Am J Surg. 2003;185:221–229. doi: 10.1016/s0002-9610(02)01373-9. [DOI] [PubMed] [Google Scholar]
- 12.Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(Suppl. 3):1–8. doi: 10.1136/gut.2006.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, et al. Five-year survival following hepatic resection after neoadjuvant therapy for non-resectable colorectal [liver] metastases. Ann Surg Oncol. 2001;8:347–353. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- 14.Muratore A, Polastri R, Bouzari H, Vergara V, Ferrero A, Capussotti L. Repeat hepatectomy for colorectal liver metastases: a worthwhile operation? J Surg Oncol. 2001;76:127–132. doi: 10.1002/1096-9098(200102)76:2<127::aid-jso1023>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Sakaguchi T, Yokoi Y, Kurachi K, Okamoto K, Okumura T, et al. Impact of repeat hepatectomy on recurrent colorectal liver metastases. Surgery. 2001;129:421–428. doi: 10.1067/msy.2001.112486. [DOI] [PubMed] [Google Scholar]
- 16.Yamada H, Katoh H, Kondo S, Okushiba S, Morikawa T. Repeat hepatectomy for recurrent hepatic metastases from colorectal cancer. Hepatogastroenterology. 2001;48:828–830. [PubMed] [Google Scholar]
- 17.Nishio H, Hamady ZZ, Malik HZ, Fenwick S, Rajendra Prasad K, Toogood GJ, et al. Outcome following repeat liver resection for colorectal liver metastases. Eur J Surg Oncol. 2007;33:729–734. doi: 10.1016/j.ejso.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Kanemitsu Y, Kato T, Hirai T, Yasui K. Preoperative probability model for predicting overall survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:112–120. doi: 10.1002/bjs.4370. [DOI] [PubMed] [Google Scholar]
- 19.Vogelsang H, Haas S, Hierholzer C, Berger U, Siewert JR, Prauer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:1066–1071. doi: 10.1002/bjs.4602. [DOI] [PubMed] [Google Scholar]
- 20.Wong VK, Malik HZ, Hamady ZZ, Al-Mukhtar A, Gomez D, Prasad KR, et al. C-reactive protein as a predictor of prognosis following curative resection for colorectal liver metastases. Br J Cancer. 2007;96:222–225. doi: 10.1038/sj.bjc.6603558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Are C, Gonen M, Zazzali K, Dematteo RP, Jarnagin WR, Fong Y, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in longterm survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elias D, Ouellet JF, Bellon N, Pignon JP, Pocard M, Lasser P. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg. 2003;90:567–574. doi: 10.1002/bjs.4071. [DOI] [PubMed] [Google Scholar]
- 24.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 25.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno H, Mochizuki H, Hatsuse K, Hase K, Yamamoto T. Indicators for treatment strategies of colorectal liver metastases. Ann Surg. 2000;231:59–66. doi: 10.1097/00000658-200001000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lise M, Bacchetti S, Da Pian P, Nitti D, Pilati P. Patterns of recurrence after resection of colorectal liver metastases: prediction by models of outcome analysis. World J Surg. 2001;25:638–644. doi: 10.1007/s002680020138. [DOI] [PubMed] [Google Scholar]
- 29.Nagashima I, Takada T, Matsuda K, Adachi M, Nagawa H, Muto T, et al. A new scoring system to classify patients with colorectal liver metastases: proposal of criteria to select candidates for hepatic resection. J Hepatobiliary Pancreat Surg. 2004;11:79–83. doi: 10.1007/s00534-002-0778-7. [DOI] [PubMed] [Google Scholar]
- 30.Schindl M, Wigmore SJ, Currie EJ, Laengle F, Garden OJ. Prognostic scoring in colorectal cancer liver metastases: development and validation. Arch Surg. 2005;140:183–189. doi: 10.1001/archsurg.140.2.183. [DOI] [PubMed] [Google Scholar]
- 31.Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806–814. doi: 10.1097/SLA.0b013e318142d964. [DOI] [PubMed] [Google Scholar]
- 32.Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–191. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee WS, Kim MJ, Yun SH, Chun HK, Lee WY, Kim SJ, et al. Risk factor stratification after simultaneous liver and colorectal resection for synchronous colorectal metastasis. Langenbecks Arch Surg. 2008;393:13–19. doi: 10.1007/s00423-007-0231-0. [DOI] [PubMed] [Google Scholar]
- 34.Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of longterm survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 35.Konopke R, Kersting S, Distler M, Dietrich J, Gastmeier J, Heller A, et al. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009;29:89–102. doi: 10.1111/j.1478-3231.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 36.Meric F, Patt YZ, Curley SA, Chase J, Roh MS, Vauthey JN, et al. Surgery after downstaging of unresectable hepatic tumours with intra-arterial chemotherapy. Ann Surg Oncol. 2000;7:490–495. doi: 10.1007/s10434-000-0490-2. [DOI] [PubMed] [Google Scholar]
- 37.Bodingbauer M, Tamandl D, Schmid K, Plank C, Schima W, Gruenberger T. Size of surgical margin does not influence recurrence rates after curative liver resection for colorectal cancer liver metastases. Br J Surg. 2007;94:1133–1138. doi: 10.1002/bjs.5762. [DOI] [PubMed] [Google Scholar]
- 38.Figueras J, Burdio F, Ramos E, Torras J, Llado L, Lopez-Ben S, et al. Effect of subcentimetre non-positive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol. 2007;18:1190–1195. doi: 10.1093/annonc/mdm106. [DOI] [PubMed] [Google Scholar]
- 39.Hamady ZZ, Cameron IC, Wyatt J, Prasad RK, Toogood GJ, Lodge JP. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1-cm rule. Eur J Surg Oncol. 2006;32:557–563. doi: 10.1016/j.ejso.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomized controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130:515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 44.Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168–1172. doi: 10.1001/archsurg.139.11.1168. [DOI] [PubMed] [Google Scholar]
- 45.Mala T, Bohler G, Mathisen O, Bergan A, Soreide O. Hepatic resection for colorectal metastases: can preoperative scoring predict patient outcome? World J Surg. 2002;26:1348–1353. doi: 10.1007/s00268-002-6231-x. [DOI] [PubMed] [Google Scholar]
- 46.Merkel S, Bialecki D, Meyer T, Muller V, Papadopoulos T, Hohenberger W. Comparison of clinical risk scores predicting prognosis after resection of colorectal liver metastases. J Surg Oncol. 2009;100:349–357. doi: 10.1002/jso.21346. [DOI] [PubMed] [Google Scholar]
- 47.Malik HZ, Farid S, Al-Mukthar A, Anthoney A, Toogood GJ, Lodge JP, et al. A critical appraisal of the role of neoadjuvant chemotherapy for colorectal liver metastases: a case-controlled study. Ann Surg Oncol. 2007;14:3519–3526. doi: 10.1245/s10434-007-9533-2. [DOI] [PubMed] [Google Scholar]
- 48.Mehta NN, Ravikumar R, Coldham CA, Buckels JA, Hubscher SG, Bramhall SR, et al. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:782–786. doi: 10.1016/j.ejso.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Fong Y, Blumgart LH, Cohen A, Fortner J, Brennan MF. Repeat hepatic resections for metastatic colorectal cancer. Ann Surg. 1994;220:657–662. doi: 10.1097/00000658-199411000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinson CW, Wright JK, Chapman WC, Garrard CL, Blair TK, Sawyers JL. Repeat hepatic surgery for colorectal cancer metastasis to the liver. Ann Surg. 1996;223:765–773. doi: 10.1097/00000658-199606000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beard SM, Holmes M, Price C, Majeed AW. Hepatic resection for colorectal liver metastases: a cost-effectiveness analysis. Ann Surg. 2000;232:763–776. doi: 10.1097/00000658-200012000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gazelle GS, Hunink MG, Kuntz KM, McMahon PM, Halpern EF, Beinfeld M, et al. Cost-effectiveness of hepatic metastasectomy in patients with metastatic colorectal carcinoma: a state-transition Monte Carlo decision analysis. Ann Surg. 2003;237:544–555. doi: 10.1097/01.SLA.0000059989.55280.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhattacharjya S, Aggarwal R, Davidson BR. Intensive follow-up after liver resection for colorectal liver metastases: results of combined serial tumour marker estimations and computed tomography of the chest and abdomen – a prospective study. Br J Cancer. 2006;95:21–26. doi: 10.1038/sj.bjc.6603219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metcalfe M, Mann C, Mullin E, Maddern G. Detecting curable disease following hepatectomy for colorectal metastases. Aust N Z J Surg. 2005;75:524–527. doi: 10.1111/j.1445-2197.2005.03421.x. [DOI] [PubMed] [Google Scholar]
- 55.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 56.Valk PE, Abella-Columna E, Haseman MK, Pounds TR, Tesar RD, Myers RW, et al. Whole-body PET imaging with [18F]fluorodeoxyglucose in management of recurrent colorectal cancer. Arch Surg. 1999;134:503–511. doi: 10.1001/archsurg.134.5.503. [DOI] [PubMed] [Google Scholar]
- 57.Flanagan FL, Dehdashti F, Ogunbiyi OA, Kodner IJ, Siegel BA. Utility of FDG-PET for investigating unexplained plasma CEA elevation in patients with colorectal cancer. Ann Surg. 1998;227:319–323. doi: 10.1097/00000658-199803000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]


