Abstract
Background:
During the last two decades, resection of colorectal liver metastases (CLM) in selected patients has become the standard of care, with 5-year survival rates of 25–58%. Although a substantial number of actual 5-year survivors are reported after resection, 5-year survival rates may be inadequate to evaluate surgical outcomes because a significant number of patients experience a recurrence at some point.
Objectives:
This study aimed to analyse longterm results and prognostic factors in liver resection for CLM in patients with complete 10-year follow-up data.
Methods:
A total of 369 patients who underwent liver resection for CLM between 1985 and 1998 were identified from a bi-institutional database. Postoperative deaths and patients with extrahepatic disease were excluded. Clinicopathological prognostic factors were analysed using univariate and multivariate analyses.
Results:
The sample included 309 consecutive patients with complete 10-year follow-up data. Five- and 10-year overall survival rates were 32% and 23%, respectively. Overall, 93% of recurrences occurred within the first 5 years of follow-up, but 11% of patients who were disease-free at 5 years developed later recurrence. Multivariate analysis demonstrated four independent negative prognostic factors for survival: more than three metastases; a positive surgical margin; tumour size >5 cm, and a clinical risk score >2.
Conclusions:
Five-year survival rates are not adequate to evaluate surgical outcomes of patients with CLM. Approximately one-third of actual 5-year survivors suffer cancer-related death, whereas patients who survive 10 years appear to be cured of disease.
Keywords: colorectal cancer, liver metastasis, disease-free survival
Introduction
During the last two decades, resection of colorectal liver metastases (CLM) in selected patients has been increasingly accepted as the reference standard treatment.1 This is a consequence of the improved safety of liver resection and the increasing evidence in favour of an associated survival benefit. The evidence for such survival benefit is based on retrospective studies reporting actuarial 5-year survival rates of 25–58% for patients undergoing resection compared with patients treated with chemotherapy (6–12 months) or untreated patients (12–20 months).2–9 However, the real cure rate associated with liver resection remains poorly defined as previous studies have restricted their follow-up to 5 years.
Five-year survival rates may be insufficient as evidence of an adequate oncological outcome and in fact there is increasing evidence that a significant number of patients experience recurrence after 5 years.10,11 In order to evaluate the real curative efficacy of surgery, longer-term follow-up data are necessary. The aim of this study was to analyse longterm outcomes of surgery, in terms of prognostic factors and incidence of late recurrences, in patients with CLM and complete 10-year follow-up data.
Materials and methods
Between September 1985 and May 1998, 369 patients treated with curative intent surgery for CLM were identified at two major hepatobiliary centres: the Royal Infirmary of Edinburgh (RIE), Edinburgh, UK, and the Department of Surgery–Liver Unit, Scientific Institute San Raffaele (HSR), Milan, Italy.
Prior to surgery, all patients were evaluated with a baseline history and physical examination, serum laboratory tests, and appropriate imaging studies (computed tomography [CT] or magnetic resonance imaging [MRI] scan of the abdomen and pelvis, and chest radiography or CT) at the discretion of the treating doctor. Patients were deemed to have resectable disease only if it was anticipated that the metastases could be completely resected, at least two adjacent liver segments could be spared, vascular inflow and outflow could be preserved, and the volume of the liver remaining after resection would be adequate.
The follow-up included clinical examination, estimation of serum carcinoembryonic antigen (CEA) level, and CT or MRI scanning at 6 months and then yearly. Follow-up was performed by assessing the records of the respective institutions for laboratory, interventional and pathological data, and by postal or telephone contact with the local referring clinician (surgeon or oncologist) for patients residing at a distance from the hospital. Data relating to the patients' well-being and oncological status were pursued until the endpoints of demonstrable tumour recurrence, cancer-specific survival and overall survival were established.
Data collection
Standard demographic and clinicopathological data were collected on each patient, including data on sex, age and CEA level, as well as on treatment-related variables including history of chemotherapy. Data were also collected on tumour characteristics. Specifically, data were collected on primary tumour location, stage (American Liver Tumour Study Group modified Tumor-Node-Metastasis [TNM] classification) and presentation (synchronous vs. metachronous). The number, size and distribution of the hepatic metastases were also recorded. Resection was classified as less than a hemi-hepatectomy (e.g. segmentectomy or subsegmentectomy), hemi-hepatectomy or extended hepatectomy (more than five liver segments). Date of last follow-up, vital status and recurrence-related information were collected. With regard to recurrence, both the sequence and overall pattern of recurrence were noted. Information regarding the location and number of lesions, as well as the disease-free interval from the date of the initial operation to the development of recurrent disease was recorded.
Disease-specific survival (DSS) was calculated from the time of initial hepatectomy until cancer-related death. For the purposes of analysis, recurrences were classified as intrahepatic only, extrahepatic only, or intra- and extrahepatic. Patients with synchronous extrahepatic disease at the time of liver resection or with incomplete 10-year follow-up were excluded from the study. Patient survival analysis was also performed with stratification by the clinical risk score (CRS). This is a staging system that grades risk for recurrence by five clinical parameters.1 The five clinical criteria are: nodal status of the primary tumour; a disease-free interval from the primary to discovery of the liver metastases of <12 months; more than one tumour; preoperative CEA level >200 ng/ml, and size of the largest tumour >5 cm. Each criterion was assigned 1 point, and the total points were used to correlate to the clinical outcomes of each patient after liver resection. The total score has been shown to be highly predictive of longterm outcomes.1,12,13
Statistical analyses
Summary statistics were obtained using established methods and presented as percentages or median values. Disease-specific survival was calculated from the time of initial hepatectomy until cancer-related death.
Rates for DSS and time to recurrence were estimated using the non-parametric product limit method (Kaplan–Meier).14 Differences in recurrence and survival were examined using the log-rank test. Factors associated with overall survival were examined using univariate and multivariate Cox regression analyses. The hazard ratio and 95% confidence intervals (CIs) were estimated and a P-value of <0.05 was considered significant. All statistical analyses were performed using spss Version 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 369 patients underwent liver resection for CLM during the study period. Thirty patients (8.1%) were lost to follow-up before 10 years and thus were excluded from the study. Five patients (1.4%) who died in the perioperative period and 25 patients (6.8%) with synchronous extrahepatic disease were also excluded from the study. Therefore, analysis focused on a cohort of 309 patients with a median age at the time of resection of 65 years (range 22–85 years) and complete 10-year follow-up. Complete information on the use of adjuvant chemotherapy was not available for all patients. Of the 72 patients for whom the chemotherapy regimen was known, some patients were treated with 5-fluorouracil-based monotherapy (n= 44) and others received hepatic artery infusional chemotherapy (n= 28). Table 1 shows the clinicopathological features of the 309 patients in the study.
Table 1.
Characteristics of patients and survival (n= 309)
| n, (%) | 5-year survival, % | 10-year survival, % | |
|---|---|---|---|
| Age >70 years | |||
| No | 227 (73) | 35 | 21 |
| Yes | 82 (27) | 32 | 19 |
| Gender | |||
| Male | 195 (63) | 33 | 20 |
| Female | 114 (37) | 36 | 25 |
| Primary tumour | |||
| Colon | 268 (87) | 37 | 20 |
| Rectum | 41 (13) | 31 | 18 |
| Primary tumour | |||
| Node-negative | 111 (36) | 42 | 21 |
| Node-positive | 198 (64) | 31 | 17 |
| Metastases presentation | |||
| Synchronous | 79 (25) | 32 | 14 |
| Metachronous | 230 (75) | 43 | 20 |
| Disease-free interval | |||
| <12 months | 125 (41) | 35 | 14 |
| >12 months | 184 (59) | 42 | 20 |
| Largest tumour size | |||
| <5 cm | 178 (58) | 39 | 19 |
| >5 cm | 131 (42) | 29 | 6 |
| CEA, median | |||
| <24 ng/ml | 170 (55) | 37 | 20 |
| >24 ng/ml | 139 (45) | 22 | 4 |
| CEA | |||
| <200 ng/ml | 246 (79) | 37 | 20 |
| >200 ng/ml | 40 (21) | 22 | 4 |
| Extent of resection | |||
| Wedge resection | 100 (32) | 36 | 24 |
| Segmentectomy (1) | 65 (21) | 35 | 21 |
| Segmentectomy (>1) | 68 (22) | 37 | 17 |
| Extra right hepatectomy | 46 (15) | 32 | 16 |
| Extra left hepatectomy | 30 (10) | 30 | 18 |
| Number of lesions | |||
| 1 | 155 (50) | 38 | 25 |
| 2 | 67 (21) | 36 | 20 |
| 3 | 45 (15) | 32 | 15 |
| ≥4 | 42 (14) | 27 | 10 |
| Surgical margin | |||
| Negative | 275 (89) | 38 | 22 |
| Positive | 34 (11) | 26 | 11 |
| Blood loss >2000 cc | |||
| No | 275 (90) | 37 | 20 |
| Yes | 34 (10) | 33 | 15 |
| Portal clamping | |||
| No | 130 (46) | 38 | 18 |
| Yes | 149 (54) | 32 | 15 |
| Portal clamping | |||
| <30 min | 79 (53) | 31 | 16 |
| >30 min | 70 (47) | 28 | 13 |
| Adjuvant chemotherapy | |||
| No | 207 (74) | 34 | 18 |
| Yes | 72 (26) | 36 | 19 |
Disease-specific and disease-free survival
One-, 3-, 5- and 10-year overall survival rates for the entire cohort of patients were 82.2%, 45.3%, 32% and 23.6%, respectively (Fig. 1). The median survival was 49 months (range 1–250 months) and the survival curve reached a plateau after 10 years from the time of hepatic resection. This plateau represents 71 actual 10-year survivors, demonstrating a minimum cure rate of 23%. Disease status at last follow-up identified 69 patients (22%) with no evidence of disease, two patients alive with disease, 220 patients (71%) dead as a result of disease and 18 patients (6%) dead as a result of other causes.
Figure 1.

Kaplan–Meier plot of observed overall survival rates
One-, 3-, 5- and 10-year DSS rates were 78.4%, 47.3%, 36.1% and 29%, respectively (Fig. 2). The majority of the recurrences occurred within the first 5 years of follow-up, but nine of the 77 patients (12%) who were disease-free at 5 years developed late recurrence.
Figure 2.
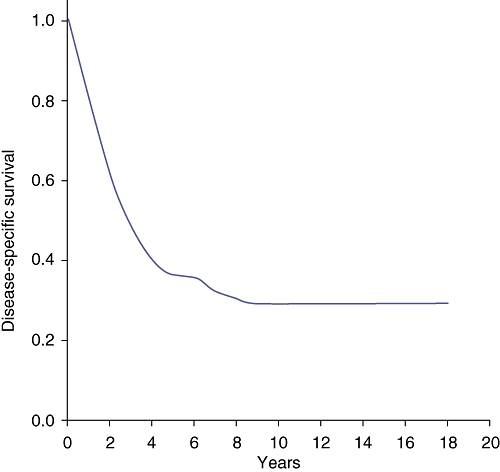
Kaplan–Meier plot of observed disease-specific survival rates
Isolated liver recurrence was observed in 111 patients (36%), of whom 36 (32%) underwent liver re-resection. Of these 36 re-resected patients, 11 patients are alive without any recurrence; in the remaining 25 patients isolated hepatic recurrence was observed in 12 patients, isolated extrahepatic recurrence in eight and associated hepatic and extrahepatic recurrence in five patients. Four of the 25 patients with liver recurrence underwent a third hepatic resection and one patient is alive without any recurrence.
Forty-five patients (15%) experienced associated hepatic and extrahepatic recurrence. Isolated extrahepatic recurrence was recorded in 64 patients (21%). Of 32 patients with lung metastases, seven underwent re-resection and one is alive and disease-free.
Ten-year survivors
Disease status at last follow-up identified 69 patients (22%) with no evidence of disease, and two patients alive with disease. The median follow-up for the 10-year survivors was 146 months (range 124–243 months). Fifty-six of the 71 (79%) actual 10-year survivors remained disease-free after a single hepatectomy. In 11 (16%) patients, recurrences were confined to the liver; these patients underwent a second liver resection and two (3%) of them subsequently underwent a third liver resection. In two patients recurrences were limited to the lungs. One patient experienced an additional pulmonary recurrence and is alive with disease. One patient had recurrence involving both the liver and the lung. This patient underwent second resections of both liver and lung and remains alive with disease secondary to additional pulmonary recurrences. Of the 10-year survivors, 93% (66 of 71) had liver-only disease throughout their course.
The CRS was found to be predictive of longterm outcome (P < 0.001). Ten-year survival rates for CRS of 0, 1, 2 and 3 were 41.3%, 37.6%, 21.4% and 0%, respectively.
Factors influencing probability of survival
Univariate analysis identified six factors that were significantly predictive of survival (Table 2), namely: node status of the primary tumour; preoperative CEA level >200 ng/ml; tumour size >5 cm, more than three lesions; surgical margin status, and CRS >2. Multivariate analysis on these factors revealed that a preoperative CEA level >200 ng/ml, tumour size >5 cm, surgical margin status and CRS >2 remained independent predictors of poor survival (Table 2).
Table 2.
Prognostic factors associated with 10-year overall survival
| Prognostic factor |
Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| Rectal primary tumour | 0.69 | 0.47–1.31 | 0.450 | – | – | – |
| Primary LN metastasis | 1.36 | 0.88–2.12 | 0.045 | – | – | – |
| Synchronous metastasis | 0.80 | 0.50–1.21 | 0.490 | – | – | – |
| CEA >200 ng/ml | 1.73 | 0.89–2.46 | 0.022 | 1.81 | 1.80–3.60 | 0.040 |
| Hepatic lesion >5 cm | 1.51 | 0.86–2.76 | 0.045 | 1.64 | 0.65–1.98 | 0.039 |
| Number lesions >3 | 1.57 | 0.81–2.77 | 0.021 | 1.61 | 1.10–2.49 | – |
| Blood loss >2000 ml | 0.69 | 0.43–1.11 | 0.221 | – | – | – |
| Positive surgical margin | 1.89 | 1.63–3.12 | 0.021 | 1.48 | 1.20–3.25 | 0.045 |
| ≥Hemi-hepatectomy | 0.79 | 0.49–1.48 | 0.455 | – | – | – |
| Adjuvant chemotherapy | 0.54 | 0.39–1.14 | 0.542 | |||
| Portal clamping | 0.61 | 0.41–1.21 | 0.452 | |||
| CRS >2 | 3.19 | 1.63–4.12 | <0.001 | 2.76 | 1.85–4.55 | 0.001 |
CI, confidence interval; LN, lymph node; CEA, carcinoembryonic antigen; CRS, clinical risk score
Discussion
Hepatic resection of CLM has become the treatment of choice for selected patients after resection of the primary colorectal cancer. Despite variability in criteria for patient selection, 5-year survival rates have ranged consistently from 25% to 58%2–9 and these data have supported the clinical contention that cure may be achieved in some of these patients. However, the longterm outcome of patients undergoing hepatic resection for CLM remains controversial. In fact, most studies report actual 5-year survival statistics as evidence of an adequate oncological outcome, although some 5-year survivors show evidence of disease recurrence.1,15–17
Longer survival rates are rarely available in the literature. Some authors report 10-year survival rates of 20–25%, but these are estimated actual data.1,17 The only two published series with complete follow-up data report 10-year survival rates of 17% and 16%, respectively.10,11 However, surgical resection of CLM should nowadays be focused on disease-free survival as a true measure of curative therapy, rather than on overall survival time, as it is known that modern systemic chemotherapy may prolong survival beyond 2 years in the palliative setting.18 In the literature, the recurrence rate after liver surgery for colorectal metastases is approximately 60–90%.1 The majority of recurrences usually occur within the first 3 years of follow-up,1,16 but no clear data are available about later recurrences. Tomlinson et al. analysed 10-year outcomes and late recurrences: 73 of 102 5-year survivors died of cancer-related causes, and 23% of these had a documented first recurrence after 5 years.10 Unfortunately, the data for 19% of the 5-year survivors were inadequate to evaluate the timing of recurrence. Similarly, Viganòet al. reported that 88% of recurrences occurred within the first 3 years and 98% within the first 5 years. However, 50% of patients who were disease-free at 3 years and 15% of patients who were disease-free at 5 years later developed recurrences.11 No recurrence was observed after 10 years of follow-up.
It is evident in the current series that the DSS curve reaches a plateau only after 10 years. Of the 71 actual 10-year survivors representing this plateau in the curve, no patient suffered a cancer-related death. The time-point from liver resection at which disease-specific death becomes an extremely rare event should be used to consider a patient as cured. In the current series, most of the recurrences occurred within the first 5 years. However, 11% of patients who were disease-free at 5 years later developed recurrences.
Currently, there is no consensus regarding the extent and frequency of follow-up after hepatic resection for CLM. Most patients undergo serial serum CEA level tests and CT scans for ≥5 years following liver resection in an attempt to identify early recurrence that may be amenable to further resection for cure.19 The results of this study confirm that late recurrence (>5 years) may occur and for some of these patients re-resection may still be possible. In the present series, three patients who were disease-free at 5 years and developed liver recurrences underwent liver resection and two of them were alive at the 10-year follow-up.
There is increasing evidence that modern systemic adjuvant chemotherapy may prolong survival in patients with CLM.1,18 However, patients included in our study underwent liver resection before the introduction of modern chemotherapeutic agents such as irinotecan, oxaliplatin and bevacizumab. During the time period of this study, the only available perioperative chemotherapy agents were fluorouracil-based and had minimal efficacy. Given the marginal benefit associated with fluorouracil-based therapies and the fact that patients with extrahepatic disease were not included, this cohort represents an ideal group of patients in which to investigate the true benefit of liver resection of CLM.
Previously determined prognostic factors were also analysed in the hope of identifying factors that might negate the potential for longterm survival and cure. However, no single preoperative or postoperative prognostic factor was sufficient to negate this potential. The presence of four or more tumours, CEA >200 ng/ml and large tumours were associated with an unfavourable prognosis. This supports data from previous studies.2,9,12 Similarly, the presence of a positive margin was also associated with significantly reduced survival rates. Microscopic involvement of surgical resection margins has emerged in many publications as a significant factor of poor prognosis.2,20–22 Tomlinson et al. reported that no patients who survived 10 years had a positive margin.10 However, in the present series five patients with a microscopic positive margin were disease-free at the 10-year follow-up. This finding may be secondary to the different techniques used for liver parenchyma transection. In this series, the ultrasonic dissector was used routinely. In comparison with the clamp crushing technique, the ultrasonic dissector aspirates approximately 5 mm of normal liver parenchyma during transection of liver parenchyma, overestimating the positive margin rate. This potential overestimation of the proportion of R1 resections has been recently reported by several authors.23–25 The results of this study confirm that clinicopathological factors such as tumour number, tumour size and surgical margin should no longer be used to categorically exclude patients from consideration for surgical resection, but possibly to select patients for aggressive chemotherapy programmes.
Given that no single risk factor precluded the possibility of longterm survival and cure, the CRS was analysed. A CRS ≤2 predicted a better survival compared with the higher scores. No patient with a CRS >3 was alive at the 10-year follow-up. These results confirm that the CRS is a valid staging system to predict outcome following liver resection for CLM.
In conclusion, cure after liver resection of CLM should be defined as 10-year survival after initial hepatectomy. Five-year survival rates are not adequate to evaluate the surgical outcomes of patients with CLM as recurrence is possible after this period, although re-resection may still be an option for some of these patients. On investigation of previously determined unfavourable prognostic factors, no single preoperative or postoperative factor was sufficiently discriminatory to negate the potential for cure after resection.
Conflicts of interest
None declared.
References
- 1.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 5.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in longterm survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes KS, Rosenstein RB, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, et al. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of longterm survivors. Dis Colon Rectum. 1988;31:1–4. doi: 10.1007/bf02552560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adson MA, van Heerden JA, Adson MH, Wagner JS, Ilstrup DM. Resection of hepatic metastases from colorectal cancer. Arch Surg. 1984;119:647–651. doi: 10.1001/archsurg.1984.01390180015003. [DOI] [PubMed] [Google Scholar]
- 8.Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer – competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol. 1990;16:360–365. [PubMed] [Google Scholar]
- 9.Arru M, Aldrighetti L, Castoldi R, Di Palo S, Orsenigo E, Stella M, et al. Analysis of prognostic factors influencing longterm survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93–103. doi: 10.1007/s00268-007-9285-y. [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 11.Viganò L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Longterm survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 12.Pulitanò C, Arru M, Catena M, Guzzetti E, Vitali G, Ronzoni M, et al. Results of preoperative hepatic arterial infusion chemotherapy in patients undergoing liver resection for colorectal liver metastases. Ann Surg Oncol. 2008;15:1661–1669. doi: 10.1245/s10434-008-9882-5. [DOI] [PubMed] [Google Scholar]
- 13.Parks R, Gonen M, Kemeny N, Jarnagin W, D'Angelica M, DeMatteo R, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007;204:753–761. doi: 10.1016/j.jamcollsurg.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–480. [Google Scholar]
- 15.D'Angelica M, Brennan MF, Fortner JG, Cohen AM, Blumgart LH, Fong Y. Ninety-six 5-year survivors after liver resection for metastatic colorectal cancer. J Am Coll Surg. 1997;185:554–559. doi: 10.1016/s1072-7515(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka K, Shimada H, Ueda M, Matsuo K, Endo I, Togo S. Longterm characteristics of 5-year survivors after liver resection for colorectal metastases. Ann Surg Oncol. 2007;14:1336–1346. doi: 10.1245/s10434-006-9071-3. [DOI] [PubMed] [Google Scholar]
- 17.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict longterm survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folprecht G, Lutz MP, Schöffski P, Seufferlein T, Nolting A, Pollert P, et al. Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol. 2006;17:450–456. doi: 10.1093/annonc/mdj084. [DOI] [PubMed] [Google Scholar]
- 19.Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(Suppl.3):1–8. doi: 10.1136/gut.2006.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirabe K, Takenaka K, Gion T, Fujiwara Y, Shimada M, Yanaga K, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84:1077–1080. [PubMed] [Google Scholar]
- 21.Welsh FK, Tekkis PP, O'Rourke T, John TG, Rees M. Quantification of risk of a positive (R1) resection margin following hepatic resection for metastatic colorectal cancer: an aid to clinical decision-making. Surg Oncol. 2008;17:3–13. doi: 10.1016/j.suronc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto J, Shimada K, Kosuge T, Yamasaki S, Sakamoto M, Fukuda H. Factors influencing survival of patients undergoing hepatectomy for colorectal metastases. Br J Surg. 1999;86:332–337. doi: 10.1046/j.1365-2168.1999.01030.x. [DOI] [PubMed] [Google Scholar]
- 23.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- 25.Bodingbauer M, Tamandl D, Schmid K, Plank C, Schima W, Gruenberger T. Size of surgical margin does not influence recurrence rates after curative liver resection for colorectal cancer liver metastases. Br J Surg. 2007;94:1133–1138. doi: 10.1002/bjs.5762. [DOI] [PubMed] [Google Scholar]


