Abstract
Background:
Two-stage hepatectomy has been proposed for patients with bilateral colorectal liver metastases. The present study assesses the feasibility and outcome of two-stage hepatectomy for the treatment of colorectal liver metastases.
Methods:
From January 1994 to December 2008, 720 patients underwent liver resections at two institutions for colorectal liver metastases. The feasibility and outcomes of two-staged hepatectomies were evaluated.
Results:
Forty-five patients were eligible for the two-stage approach and both stages were completed in 35 patients (78%). Reasons for failure included disease progression (n= 7), poor performance status (n= 1) and death after the first stage (n= 2). Patients who completed both stages had significantly fewer lesions than patients who failed to complete the second stage (5 vs. 8; P= 0.02). No differences between the two groups were observed with regard to lesion size, receipt of radiofrequency ablation (RFA) or presence of extrahepatic disease. Post-operative morbidity (24% vs. 26%; P= 0.9) and mortality (4% vs. 5%; P= 0.8) was similar between the first and second stages. Median overall survival was 16 months. Three-year survival was significantly worse for patients failing to complete both stages (18%) compared with patients completing both stages (58%) (P < 0.001). Similar survival rates were observed between patients who completed two-stage vs. patients treated with a planned single-stage hepatectomy (58% vs. 53%; P= 0.34).
Conclusion:
The two-stage strategy for colorectal liver metastases can be performed with acceptable morbidity and mortality. The second stage will not be feasible in 20–25% of patients.
Patients who are able to complete the two-stage approach, however, may have long-term survival comparable to patients treated with a planned single-stage hepatectomy.
Keywords: colorectal, liver metastases, resection, two-stage, outcome
Introduction
Colorectal carcinoma is the second leading cause of cancer-related deaths both in the United States and Europe,1 with more than 50% of patients developing liver metastasis during the course of their illness.2–4 Median survival for patients treated with chemotherapy alone usually does not exceed 18 months. When possible, hepatic resection is therefore the treatment of choice for patients with colorectal liver metastasis. In fact, resection of colorectal liver metastasis is the only potentially curative treatment option, and has been associated with 5-year survival rates ranging from 50% to 58%.5–7 Unfortunately, up to 75% to 85% of patients with colorectal metastasis are not candidates for surgical resection on presentation.8–10 In particular, patients with multiple liver metastases in both hemi-livers are less likely to be candidates for surgery. Traditionally, the number of intrahepatic metastases was considered a major adverse prognostic factor,11,12 but more recently complete resection of multiple hepatic lesions has been associated with a 5-year survival up to 50%.13 Improvements in chemotherapeutic agents and surgical technique have expanded the number of patients with multiple metastases who may be candidates for surgical resection. However, despite the use of pre-operative chemotherapy,14 portal vein embolization (PVE),15 and the combination of resection and ablation,16 some patients with bilateral, diffuse colorectal liver metastases remain ineligible for curative resection as a result of the lack of a sufficient future liver remnant (FLR).
In patients with multiple liver metastases in whom complete extirpation of the metastases is not feasible by a single hepatectomy, a sequential – or multiple hepatectomies – has been advocated. In this approach, an initial operation removes a portion of the metastatic disease, which is then followed by a period of time to allow hypertrophy of the remaining liver. Then a second, curative-intent operation is performed to extirpate all remaining disease. Such planned staged approaches are distinguished from unplanned repeat hepatectomies for recurrent disease.17 To date, most data on two-stage hepatectomy have been limited. Most series are from single institutions and have included few patients.18–21
In the present study, we sought to examine the role of two-stage hepatectomy in the treatment of initially unresectable patients with diffuse liver metastases from colorectal cancer. Specifically, we analyse not only the feasibility, but also the peri-operative and long-term outcomes after two-stage hepatectomy. In addition, we identify those factors associated with outcome after two-stage hepatectomy in an international dual-centre cohort of patients.
Patients and methods
Between January 1994 and December 2008, 720 patients underwent 853 liver resections for colorectal liver metastases at two major hepatobiliary centres [Hepato-Biliary-Pancreatic and Transplantation Centre (HBPTC), Curry Cabral Hospital, Lisbon, Portugal (n= 308 patients, 372 resections) and Department of Surgery, The Johns Hopkins University (JHU) School of Medicine, Baltimore, Maryland, Unites States (n= 412 patients, 481 resections)]. The study was approved by the Institutional Review Boards of the respective institutions. Only patients with colorectal liver metastases who were operated on with curative intent were included in the study. Curative intent surgery was defined as planned complete extirpation of all known intrahepatic disease. Patients were deemed to have disease amenable to a single curative hepatectomy if it was anticipated that the metastases could be completely resected, at least two adjacent liver segments could be spared, vascular inflow and outflow could be preserved and the volume of the liver remaining after resection would be adequate.22,23 If these criteria were not satisfied, the patient was considered for a two-stage hepatectomy.
Therapeutic approach
Initially in our experience we employed an operative approach that involved initial resection of the major disease at the first operation followed by removal of the minor disease at the second surgery. Over time, we adopted a sequentially more aggressive approach, in which the minor hepatectomy was performed as the first stage followed by the major hepatectomy as the second stage. At the discretion of the treating surgeon, portal vein ligation (PVL) or embolization (PVE) was performed if the FLR volume was 20% or less of the estimated total liver volume. The timing of the second-stage procedure was determined by the adequacy of the FLR and tumour response to chemotherapy. In the absence of any significant tumour progression, a major hepatectomy was later performed as the second stage. Peri-operative chemotherapy was administered at the discretion of the treating surgeon and medical oncologist.
Data collection
Standard demographic and clinicopathologic data were collected on each patient such as gender, age, carcinoembryonic antigen (CEA) level, as well as treatment-related variables including history of chemotherapy. Data were also collected on tumour characteristics. Specifically, data were collected on primary tumour location, American Joint Commission on Cancer (AJCC)/International Union against Cancer (UICC) stage (T, N, M) and presentation (synchronous vs. metachronous). The number, size and distribution of the hepatic metastases were noted. Resection was classified as less than a hemihepatectomy (e.g. segmentectomy or subsegmentectomy), hemihepatectomy or extended hepatectomy (≥5 liver segments).24 Operative details, including the type of resection performed and whether radiofrequency ablation (RFA) was utilized at each stage were recorded. Peri-operative morbidity was noted and complications were scored according to the Clavien grading system.25 Operative mortality was defined as death during the same hospitalization or within 90 days of surgery. Date of last follow-up, recurrence and vital status were recorded.
Statistical analyses
Variables of interest were compared using Student's t-test, Pearson's χ2-test, or Fisher's exact test as appropriate. Cut-off values for continuous variables were obtained using receiver-operating curves (ROC). Survival time was estimated using the non-parametric product limit method (Kaplan–Meier). Differences in survival were examined using the log-rank test. Factors associated with recurrence and survival were examined using univariate and multivariate analyses. A P-value less than 0.05 was considered significant. All statistical analyses were performed using SPSS Version 16.0 (Chicago, IL, USA).
Results
Patient and tumour characteristics
Table 1 shows the clinicopathological features of the 45 patients in the study. The median patient age was 58 years (range, 27–67 years) and the majority of patients were male (n= 35; 78%). Most patients had a primary colon tumour (n= 30; 66%). The majority of the primary tumours were advanced, with stage T3/T4 lesions (n= 39; 86%) and had associated lymph node metastasis (n= 25; 55%). A significant portion of patients had synchronous presentation of the liver metastases (n= 34; 75%). All patients had multiple bilateral hepatic metastases. The median number of metastases was five (range 2–13) and mean dimension of the largest lesion was 4.0 cm (range, 1–25). Overall, 35 (78%) of patients underwent PVL or PVE prior to the second hepatectomy. Specifically, 32 (71%) patients underwent PVL (n= 5, PVL only vs. n= 28, PVL plus distal injection of an alcohol sclerosant).
Table 1.
Comparison of patients who completed and failed the two-stage approach
| Variable | Completed two-stage approach (n= 35) | Failed two-stage approach (n= 10) | P |
|---|---|---|---|
| Patient characteristics | |||
| Age (mean ± SD), y | 56.7 ± 9.6 | 60.6 ± 6.2 | 0.23 |
| Gender, n (%) | |||
| Male | 26 (74.3) | 9 (90.0) | 0.29 |
| Female | 9 (25.7) | 1 (10.0) | |
| Primary tumour site | |||
| Primary tumour location, n (%) | |||
| Colon | 23 (65.7) | 7 (70.0) | 0.80 |
| Rectum | 12 (34.3) | 3 (30.0) | |
| AJCC T category, % | |||
| 1/2 | 3.2 | 0.0 | 0.58 |
| 3/4 | 96.8 | 100.0 | |
| AJCC N category, % | |||
| 0 | 33.3 | 37.5 | 0.83 |
| 1/2 | 66.7 | 62.5 | |
| Liver metastases | |||
| Presentation, n (%) | |||
| Synchronous | 27 (77.1) | 7 (70.0) | 0.64 |
| Metachronous | 8 (22.9) | 3 (30.0) | |
| Number of metastases (mean) | 5 | 8 | 0.02 |
| Size of largest metastases (mean), cm | 4.9 | 3.0 | 0.14 |
| Pre-operative level of CEA (mean), ng/mL | 24.4 | 35.6 | 0.71 |
| Presence of concomitant extrahepatic disease, n (%) | 2 (5.7) | 1 (10.0) | 0.55 |
| Portal vein ligated or embolized, n (%) | 28 (80.0) | 7 (70.0) | 0.66 |
| Chemotherapy | |||
| Before first stage, n (%) | 22 (62.9) | 10 (10.0) | 0.02 |
| Before second stage, n (%) | 24 (68.6) | 4 (40.0) | 0.10 |
| After second stage, n (%) | 20 (57.1) | – |
AJCC, American Joint Commission on Cancer; CEA, carcinoembryonic antigen.
Extrahepatic metastases
Six (13%) patients in the series had or developed extrahepatic disease. Three patients had extrahepatic metastases prior to the first hepatectomy. Of these three patients, two patients had the extrahepatic disease resected at the time of the second hepatectomy. The other patient developed disease progression after the first hepatectomy and did not complete the second hepatectomy. Three additional patients developed extrahepatic metastases between the first and second hepatectomies. All patients with extrahepatic disease had received chemotherapy prior to the first hepatectomy. Two patients received chemotherapy between the first resection and the anticipated second hepatectomy. In total, five patients underwent a second planned hepatectomy with extrahepatic disease which was resected during the second operation.
Feasibility
Of the 45 patients in whom a two-stage hepatectomy strategy was planned, 35 patients actually underwent the second hepatectomy (78%). Ten patients failed to complete the second procedure after the first hepatectomy. Reasons for failure included disease progression (n= 7), poor performance status (n= 1) or death after the first-stage (n= 2). Of the patients who did not undergo the planned second stage, two were alive with disease and five were dead as a result of disease progression at last follow-up. The median time to death from the date of the first hepatectomy was 8 months.
Patients with a completed two-stage hepatectomy
No statistically significant differences were observed between patients who did or did not complete the two-stage hepatectomy in terms of age, gender or location of the primary. Compared with those who did not complete the two-stage hepatectomy approach, patients who succeeded had similar mean tumour size (4.9 vs. 3.0 cm, P= 0.14), rate of synchronous primaries (n= 27 vs. n= 7, P= 0.64) and CEA levels (24.4 ng/mL vs. 35.6 ng/mL, P= 0.71). Peri-operative chemotherapy was administrated in 32 patients (91%), of which 22 (63%) had pre-operative chemotherapy. Of the 27 patients who presented with synchronous liver metastases, 19 (70%) received pre-operative chemotherapy prior to a synchronous resection of the primary and first hepatectomy. Overall, 24 (69%) patients had interval chemotherapy and 20 (57%) had adjuvant chemotherapy after both operations. In 19 patients (54%) a partial response was noted whereas in 4 (11%) a stabilization occurred. On statistical analysis, the only factors associated with an increased success of completing the second stage hepatectomy were a lower mean number of metastases (5 vs. 8, P= 0.02) and less exposure to chemotherapy prior to the first hepatectomy (63% vs. 100%, P= 0.02).
Surgery
Seventeen patients (49%) underwent simultaneous resection of the primary tumour at the time of the first surgery. First hepatectomies were minor resections (<3 segments) in the majority of patients (n= 34, 75%). In addition, eight (15%) patients underwent combined resection with RFA and four (8%) patients underwent open RFA alone. In addition to resection, ethanol ablation of liver metastases was performed in one patient. PVL with or without alcohol sclerosant was performed in a total of 33 (73%) patients at the time of the first operation. Two (4%) additional patients underwent PVE after the first operation. The majority of patients underwent the second operation within 6 months (n= 26, 58%) with a median interval between first and second stage operations of 4.5 months (range, 2–22).
At the time of the second operation, the majority of resections required major anatomic resections (n= 28, 80%). In addition, 6 (17%) patients also received concomitant RFA. One patient was explored and found to have an unresectable lesion and subsequently underwent RFA alone. Four patients had extrahepatic disease and underwent concomitant resections with curative intent of lung, diaphragm or localized peritoneal disease. One patient had extrahepatic disease involving the pancreas that was also resected.
Surgical complications and mortality
The morbidity and mortality associated with the first and second stage operations are summarized in Table 2. No difference was observed in postoperative morbidity between the first and second hepatectomies (n= 9 vs. n= 9, P= 0.9). Infectious complications were the most common morbidity after both the first and second procedures (n= 3 vs. n= 3). Other complications included cardiovascular (n= 2), pulmonary (n= 2) and gastrointestinal (n= 2). Reoperation was needed in two patients in the first operation and one patient following the second. Percutaneous drainage was required in one patient after the first hepatectomy and four patients after the second. There was a tendency towards more severe complications after the second operation (Clavien grades III and IV), with severe complications accounting for 33% of complications after the first hepatectomy compared with 71% after the second hepatectomy (P= 0.05).
Table 2.
Comparison of operative data between the first and second stage (n= 35)
| Variable | First stage of two-stage approach | Second stage of two-stage approach | P |
|---|---|---|---|
| Type of liver-directed therapy, n (%) | |||
| Resection only | 23 (65.7) | 28 (80.0) | 0.28 |
| Non-resection only | 4 (11.4) | 1 (2.9) | |
| Both | 8 (22.9) | 6 (17.1) | |
| Type of resection, n (%) | |||
| Anatomical | 9 (29.0) | 24 (70.6) | 0.001 |
| Non-anatomical | 8 (25.8) | 1 (2.9) | |
| Both | 14 (45.2) | 9 (26.5) | |
| Major hepatectomy (≥3 segments), n (%) | 7 (20.0) | 28 (80.0) | <0.001 |
| Post-operative morbidity, n (%) | 9 (25.7) | 9 (25.7) | 0.88 |
| Type of Complications, n (%) | |||
| Infectious | 3 (33.3) | 3 (33.3) | |
| Other | 3 (33.3) | 1 (11.1) | |
| Gastrointestinal | 1 (11.1) | 2 (22.2) | |
| Cardiovascular | 2 (22.2) | 0 | |
| Pulmonary | 0 | 2 (22.2) | |
| Renal | 0 | 1 (11.1) |
The overall postoperative mortality rate was 8.8%. No difference was observed in postoperative mortality between the first and second hepatectomies (n= 2; 4% vs. n= 2; 5%, P= 0.8). Causes of mortality after the first operation were due to post-operative hepatic insufficiency (n= 1) and pulmonary embolism (n= 1). Post-operative hepatic insufficiency was the sole cause of mortality after the second operation. Two of the three patients with hepatic insufficiency had prior PVL or PVE.
Outcome
Disease recurrence was diagnosed in 62% of patient who had successfully completed both stages. Specifically, of the 35 patients who completed the two-stage approach, 14 patients (40%) had died of disease, eight (23%) patients were alive with disease and 13 (37%) were disease free. Overall disease-free survival was comparable between patients who completed the two-stage approach and those who underwent a planned single-stage hepatectomy (P= 0.44) (Fig. 1). On an intention-to-treat basis, overall 3-year survival was 26% for all patients. The overall median survival was 16 months. Three-year survival for patients completing two-stage hepatectomy was 58% compared with 18% for patients who failed to complete both stages (P= 0.02) (Fig. 2). Of note, patients who completed the two-stage approach had a similar overall survival as patients who were able to be treated with a planned single-stage hepatectomy (58% vs. 53%; P= 0.34) (Fig. 3).
Figure 1.
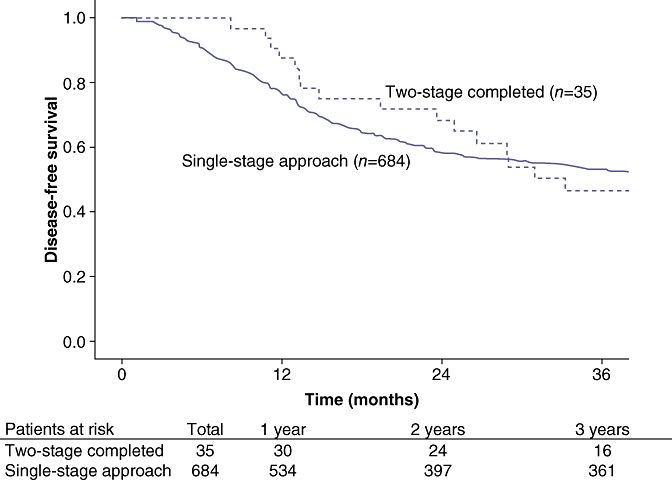
Overall disease-free survival was comparable between patients who completed the two-stage approach and those who underwent a planned single-stage hepatectomy
Figure 2.
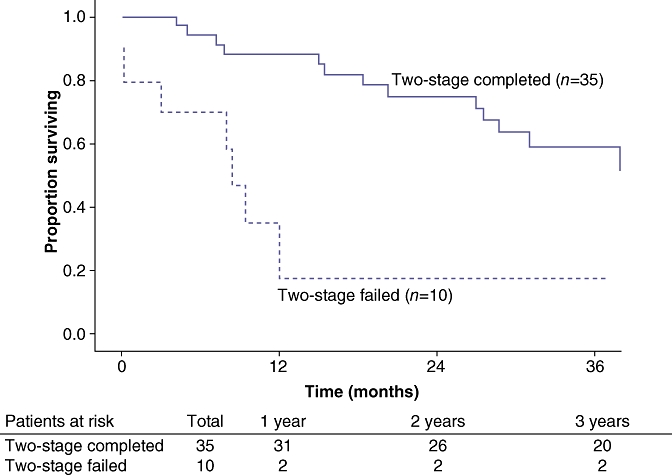
Three-year survival for patients completing a two-stage hepatectomy was 58% compared with only 18% for patients who failed to complete both stages
Figure 3.
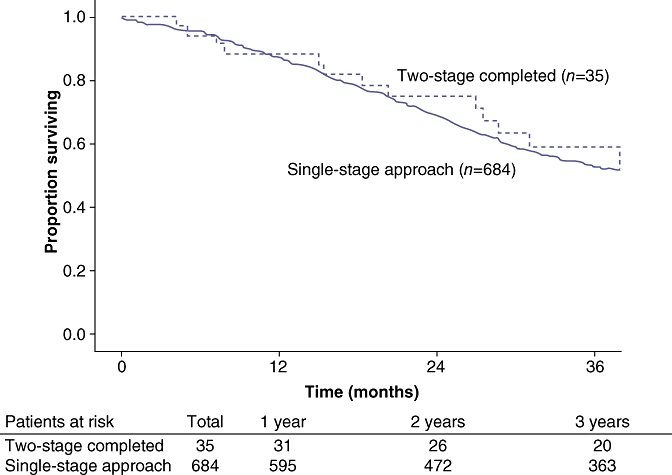
Patients who completed the two-stage approach had a similar overall survival as patients who were able to be treated with a planned single-stage hepatectomy
Discussion
In the past decade, advances in chemotherapeutic efficacy and surgical techniques have allowed surgeons to become more aggressive in the management of bilateral, multifocal colorectal metastases to the liver. The two-stage hepatectomy was initially developed as a strategy that takes advantage of the liver's regenerative capabilities to allow for radical extirpation of widespread disease. Several series in the literature have reported varying success in selecting and successfully completing both stages of the two-stage approach. Previous data on two-stage hepatectomy, however, have been limited with most series being from single institutions that included few patients.18–21 We herein report a dual-centre international study, which is one of the largest series to investigate the two-stage strategy for colorectal liver metastases. We report that a two-stage strategy for colorectal liver metastases can be performed safely with acceptable morbidity and mortality. Importantly, the second stage was not feasible in 20–25% of patients in whom it was initially planned. However, for those patients who were able to complete the two-stage approach, long-term survival was possible and was comparable with patients treated with a planned single-stage hepatectomy.
The feasibility of being able to complete both stages of the two-stage approach has always been of some concern. Several small series had previously reported that the second stage was only feasible in 60–81% of patients with bilateral multifocal colorectal liver metastases.18–21,26 Historically, resection of patients with multiple lesions – especially those with four or more lesions – was controversial with reported poor disease-free and overall survival for this group of patients.11,12 More recently, in the era of more effective cytotoxic chemotherapy, Pawlik et al. has reported a 5-year survival of over 50% for patients with four or more metastases treated with hepatic resection and systemic chemotherapy.13 In addition, a recent meta-analysis examining the outcomes of patients with more than four colorectal liver metastases demonstrated a benefit of resection.26 Given the improvement in overall survival after hepatic resection in patients with larger burdens of intrahepatic disease, as well as the improved morbidity and mortality rates associated with hepatectomy, some have advocated for a more expanded criteria of resectability for colorectal liver metastasis.22 Specifically, if all disease within the liver can be removed while leaving an adequate FLR then resection may be considered. As such, the two-stage approach may help expand the pool of potentially resectable patients to include those with multiple bilateral hepatic metastases who otherwise would not be considered candidates for complete surgical extirpation of all metastatic disease.
We noted that patients who failed to complete the two-stage approach were more likely to have received chemotherapy prior to the first resection. To some degree, receipt of chemotherapy was likely a surrogate for a perceived more aggressive initial clinical presentation. Perhaps, more importantly, was the fact that of the 10 patients who failed to undergo the second stage, seven patients had progressive disease on chemotherapy. In contrast, most patients who completed the two-stage approach demonstrated stable disease or a partial response to chemotherapy. Wichert et al. similarly reported that patients who failed to complete the two-stage approach were more likely to have experienced multiple lines of chemotherapy.27 Tumour progression while receiving systemic chemotherapy has been shown to be a powerful poor prognostic indicator in patients with initially resectable metastases.28,29 Patients being considered for a two-stage hepatectomy probably represent a cohort of patients with even more advanced disease, and the relationship between chemotherapeutic responsiveness and outcome needs to be strongly considered when managing these patients.
The potential surgical morbidity in managing patients with multiple bilateral colorectal metastases has also been an area of ongoing concern. The two-stage approach involves the resection of a considerable amount of hepatic parenchyma and therefore may increase the risk of postoperative hepatic insufficiency. Initial experiences with the two-stage approach that did not incorporate PVL or PVE were associated with a high incidence of hepatic insufficiency and high mortality rates (9–15%).21,30 More recently, the addition of PVL or PVE has been incorporated into the two-stage strategy and has resulted in a reduction in the incidence of postoperative hepatic insufficiency and death.18–20,27 In our series, the majority of patients initially lacked a sufficient FLR and 78% of patients underwent either a PVL at the time of the first operation or PVE between the first and second stages. In addition to the volume of the FLR, underlying hepatic parenchymal injury may also contribute to risk of post-operative liver dysfunction. This is particularly important in patients being considered for a two-stage approach, as these patients all have extensive disease and have often received extensive preoperative chemotherapy. Previous studies have noted that prolonged courses of oxaliplatin and irinotecan-based chemotherapies were associated with hepatic injury and perhaps an increase in peri-operative mortality.31,32 In one series of two-stage hepatectomy patients, up to 64% were found to have macrosteatosis or steatofibrosis by liver biopsy at the time of the first-stage hepatectomy.18 As such, coordination between the medical oncologist and hepatobiliary surgeon is vital, to optimize the duration of chemotherapy so as to minimize the risk of prolonged chemotherapy.
Although early reports of survival after two-stage hepatectomy were initially modest, more recent series have reported 3-year overall survival rates ranging from 54% to 60% and a single series has reported a 5-year survival rate of 42%.18,27,33 Similarly, we observed a 3-year survival rate of 58% for patients completing the two-stage approach. Importantly, despite an initial presentation with bilateral, multifocal liver metastases, patients in the current series who successfully completed the two-stage approach had an overall survival that was comparable to patients who underwent a planned single-staged hepatectomy. These data strongly suggest that even in the setting of extensive metastatic disease, appropriately selected patients may derive a substantial survival benefit from two-stage hepatectomy when such an approach is performed with curative intent and complete extirpation of all disease. Our data similarly show that the two-stage approach can be done with reasonable safety that is comparable to population-based estimates of peri-operative morbidity and mortality for other hepatectomy patients.33,34 A multidisciplinary approach is paramount, however, in the selection and management of patients being considered for the two-stage approach in order to maximize the therapeutic options and clinical outcomes.
Conclusion
For patients with bilateral multifocal colorectal liver metastases, a two-stage approach can be performed with acceptable morbidity and mortality. The addition of PVE or PVL can decrease the incidence of postoperative hepatic insufficiency. With careful patient selection and management, patients managed with a two-stage approach can achieve long-term survival that is comparable to patients treated with a planned single-stage hepatectomy.
Conflict of interest
None declared.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Adson MA, van Heerden JA, Adson MH, Wagner JS, Ilstrup DM. Resection of hepatic metastases from colorectal cancer. Arch Surg. 1984;119:647–651. doi: 10.1001/archsurg.1984.01390180015003. [DOI] [PubMed] [Google Scholar]
- 3.Berney T, Mentha G, Roth AD, Morel P. Results of surgical resection of liver metastases from non-colorectal primaries. Br J Surg. 1998;85:1423–1427. doi: 10.1046/j.1365-2168.1998.00856.x. [DOI] [PubMed] [Google Scholar]
- 4.Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520. doi: 10.1097/00000658-199610000-00009. discussion 20–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. discussion 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 25–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Gramont A, Vignoud J, Tournigand C, Louvet C, Andre T, Varette C, et al. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer. 1997;33:214–219. doi: 10.1016/s0959-8049(96)00370-x. [DOI] [PubMed] [Google Scholar]
- 9.Rougier P, Sahmoud T, Nitti D, Curran D, Doci R, De Waele B, et al. Adjuvant portal-vein infusion of fluorouracil and heparin in colorectal cancer: a randomised trial. European Organisation for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group, the Gruppo Interdisciplinare Valutazione Interventi in Oncologia, and the Japanese Foundation for Cancer Research. Lancet. 1998;351:1677–1681. doi: 10.1016/s0140-6736(97)08169-5. [DOI] [PubMed] [Google Scholar]
- 10.Levi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997;350:681–686. doi: 10.1016/s0140-6736(97)03358-8. [DOI] [PubMed] [Google Scholar]
- 11.Hughes KS, Simon R, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. [PubMed] [Google Scholar]
- 12.Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hagerstrand I, Ranstam J, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. doi: 10.1002/bjs.1800730917. [DOI] [PubMed] [Google Scholar]
- 13.Pawlik TM, Abdalla EK, Ellis LM, Vauthey JN, Curley SA. Debunking dogma: surgery for four or more colorectal liver metastases is justified. J Gastrointest Surg. 2006;10:240–248. doi: 10.1016/j.gassur.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 57–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–175. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- 16.Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10:1059–1069. doi: 10.1245/ASO.2003.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 18.Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049. doi: 10.1097/01.sla.0000145965.86383.89. discussion 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Togo S, Nagano Y, Masui H, Tanaka K, Miura Y, Morioka D, et al. Two-stage hepatectomy for multiple bilobular liver metastases from colorectal cancer. Hepato-gastroenterology. 2005;52:913–919. [PubMed] [Google Scholar]
- 20.Shimada H, Tanaka K, Masui H, Nagano Y, Matsuo K, Kijima M, et al. Results of surgical treatment for multiple (> or = 5 nodules) bi-lobar hepatic metastases from colorectal cancer. Langenbeck's Archives of Surgery/Deutsche Gesellschaft fur Chirurgie. 2004;389:114–121. doi: 10.1007/s00423-003-0447-6. [DOI] [PubMed] [Google Scholar]
- 21.Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 23.Clavien PA, Emond J, Vauthey JN, Belghiti J, Chari RS, Strasberg SM. Protection of the liver during hepatic surgery. J Gastrointest Surg. 2004;8:313–327. doi: 10.1016/j.gassur.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepato-Biliary-Pancreatic Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 25.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith MD, McCall JL. Systematic review of tumour number and outcome after radical treatment of colorectal liver metastases. Br J Surg. 2009;96:1101–1113. doi: 10.1002/bjs.6735. [DOI] [PubMed] [Google Scholar]
- 27.Wicherts DA, Miller R, de Haas RJ, Bitsakou G, Vibert E, Veilhan LA, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
- 28.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. discussion 61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen PJ, Kemeny N, Jarnagin W, DeMatteo R, Blumgart L, Fong Y. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7:109–115. doi: 10.1016/S1091-255X(02)00121-X. discussion 16–17. [DOI] [PubMed] [Google Scholar]
- 30.Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000;231:743–751. doi: 10.1097/00000658-200005000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham SC, Choti MA, Pawlik TM. Two stage hepatectomy for colorectal hepatic metastases. Curr Colorectal Cancer Rep. 2008;4:93–99. [Google Scholar]
- 34.Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–851. doi: 10.1007/s11605-008-0494-y. [DOI] [PubMed] [Google Scholar]


