Abstract
Background:
The complete resection of liver metastases from colorectal cancer is the major determinant of longterm survival. The effectiveness of current chemotherapy regimens has made treatment algorithms more flexible and resulted in many different options. Recently, the pathological response to chemotherapy has emerged as another important prognostic marker. Different systems have been used to grade the pathological response in these patients.
Methods:
This study prospectively evaluates the prognostic value of the pathological response grade (PRG) in liver metastases treated with neoadjuvant chemotherapy.
Results:
Between 2002 and 2006, 50 patients were treated with a sandwich chemotherapy regimen and underwent liver resection. Complete resection was achieved in 45 patients (90%). A strong pathological response to chemotherapy (<10% viable tumour cells in all lesions) was seen in 17 patients (34%). It was associated with a statistically significant longer overall survival (P= 0.019) and was also identified on multivariate analysis as an independent predictor of survival (odds ratio = 243).
Conclusions:
This pilot study demonstrates the prognostic potential of the PRG, which could be used clinically to select patients for an aggressive multimodal adjuvant algorithm. Larger multicentre studies are required to validate this particular grading system. The keys to longterm survival are resectability and chemo-responsiveness.
Keywords: hepatectomy, neoadjuvant chemotherapy, neoplasm metastasis, survival, recurrence
Introduction
At present, liver resection is the only treatment for colorectal liver metastases (CRLM) that can achieve cure. In the most recently published series, the 5-year survival rate after resection has reached 60%.1–4 Progress can be attributed to improvements in surgical techniques, perioperative care and the emergence of more effective chemotherapy regimens.
The use of neoadjuvant chemotherapy (NAC) first came to prominence because it allows for the downstaging of initially unresectable disease and enables complete resection.5–10 Its role in the management of resectable disease, however, has been less clear. Nevertheless, an increase in the use of NAC has been reported11 and is in general probably the worldwide experience. One large randomized trial has shown a significant benefit of NAC to progression-free survival compared with surgery alone and the results of other multicentre trials are awaited.12 Other retrospective series have shown a benefit of NAC in specific CRLM subgroups, such as in patients with five or more lesions13 or in the setting of delayed liver resection of aggressive synchronous metastases.14 The response to neoadjuvant treatment in synchronous metastases also has prognostic value.15
Different prognostic markers and scoring systems2,11,16,17 have been promoted for CRLM, incorporating a combination of baseline preoperative and intraoperative characteristics. The most common is the ‘clinical risk score’ (CRS) developed by the Memorial Sloan-Kettering Cancer Centre.18,19 A clinical response to NAC, as measured by preoperative computed tomography (CT) scans, has also been shown to have prognostic value in the resection of liver metastases.20,21 Recently, the pathological response of a tumour to chemotherapy has been shown to be an important prognostic factor in the treatment of CRLM.22–24 Different pathological grading systems have been proposed,25 but the common element has been the proportion of viable tumour cells that remain after chemotherapy.
At the McGill University hospitals in Montreal, patients with resectable CRLM were treated with perioperative chemotherapy sandwiched around the liver resection(s). This algorithm was initially used to target patients with advanced metastatic disease who would require multiple interventions to achieve disease-free status, such as patients who needed portal vein embolization or the use of a staged approach, and patients with a synchronous presentation of liver metastases or concurrent resectable lung metastases. The risk of disease progression during the extended length of time required for complete resection of all sites was of particular concern. Neoadjuvant chemotherapy theoretically represents the earliest systemic treatment for all macroscopic and subclinical metastatic deposits. The aim of this study was to correlate longterm clinical outcomes with the pathological response grade (PRG) after NAC in patients with resectable CRLM who were treated with a sandwich chemotherapy regimen.
Materials and methods
Fifty patients were assessed and treated with NAC for advanced resectable CRLM at the McGill University Health Centre (MUHC) and Jewish General Hospital, Montreal, from April 2002 until February 2006. Data were collected from the prospective database for hepato-pancreatico-biliary disease and verified by a review of medical records. The study was approved by the MUHC Institutional Review Board (A12-M114).
The treatment algorithm for advanced CRLM at the McGill University hospitals was as follows: after consultation with the hepatobiliary service and discussion at the multidisciplinary tumour board, patients with resectable liver metastases were offered NAC. The first-line regimen for CRLM was 5-fluorouracil, leucovorin and irinotecan (FOLFIRI). The target length of treatment prior to surgery was six courses. Clinical progress was monitored with triphasic liver protocol CT scans every 3 months. If adverse events prevented the completion of all cycles despite dose adjustments or if there was clinical evidence of disease progression, the second-line regimen, FOLFOX6 (oxaliplatin-based), was used. In addition, if lesions disappeared after treatment with NAC, the initially planned anatomic resection was performed based on the lesions' locations on the initial CT scans, without the placement of any type of markers. The response rate was assessed according to the response evaluation criteria in solid tumours (RECIST) score.26
All liver resections were performed at the MUHC. In patients with a synchronous metastatic presentation, the clinical practice during this study was to perform the intestinal resection first, followed by systemic chemotherapy and then liver resection. An interval of 4–6 weeks was required between the last course of chemotherapy and liver resection. At liver resection, exploratory laparotomy and intraoperative ultrasound were performed to rule out peritoneal spread and occult liver lesions. Parenchymal transection was performed most commonly with the aid of a Hydrojet® instrument (ERBE Elekromedizin GmbH, Tübingen, Germany). Extrahepatic control of the hepatic pedicles was used only for hilar lesions and all pedicles were divided in the parenchyma with vascular staplers (AutoSuture International, Inc., Norwalk, CT, USA). All major anatomic resections of three or more segments were performed according to the Brisbane classification system.27 Non-anatomic, wedge resections were used as required, especially in situations in which the preservation of liver parenchyma was critical. In patients for whom staged resections were planned, two of six courses of chemotherapy were given prior to the initial resection. When required, portal vein embolization was performed 3–4 weeks prior to resection. The right portal vein was embolized in two stages, starting with the posterior branch and followed by the anterior branch 2–3 weeks later. Concurrent lung metastases were referred for assessment by a thoracic surgeon prior to starting any therapy. If the involvement was judged to be resectable, liver resection and perioperative chemotherapy preceded the lung resection.
The pathological specimens were examined prospectively to assess and quantify the histological components of the liver metastases after treatment with NAC. The findings were independently verified by a second gastrointestinal pathologist. All resected lesions were scored for the percentage of histological components, including: fibrosis; mucin; necrosis, and viable tumour cells. Two sections were scored per centimetre diameter of the lesion. Sections were preferentially selected in areas judged to include the highest yield of viable tumour. The PRG system was proposed. According to this system, a complete response was indicated by the absence of any viable tumour cells in all metastatic lesions (PRG3) or a scant presence of viable tumour cells (<10%) in all metastatic lesions (PRG2); a weak response included >10% viable tumour cells in at least one lesion (PRG1). The strong PRG group included all patients with PRG3 or PRG2. For example, a patient with a complete resection of four lesions, of which one lesion had 25% viable tumour cell content and the remaining three were free of viable tumour cells, was included in the PRG1 group. The presence of steatosis and steatohepatitis were graded according to Kleiner et al.28
Postoperatively, all patients received adjuvant chemotherapy. In the case of a complete resection, six cycles of the same neoadjuvant regimen were given to complete 12 courses in total. In the event of toxicity that persisted despite dosing changes, or tumour progression, the second-line or an alternative regimen was used as per tumour board recommendations. Postoperative surveillance consisted of clinical examination and routine blood work every 3 months for 2 years, and serum CEA and CT scans of the chest, abdomen and pelvis every 6 months. Since 2005, a CT/PET (positron emission tomography) scan has been incorporated into the investigations for complex metastatic, recurrent or progressive disease. All recurrences were considered for further treatment with chemotherapy followed by possible resection. If unresectable and accessible, percutaneous radiofrequency ablation was performed. If the liver involvement was diffuse, bland angio-embolization, chemo-embolization or radio-bead embolization were considered. Isolated hepatic arterial chemotherapy was considered if toxicity prevented further use of systemic chemotherapy.
Statistical analysis was performed with spss Version 10.0 (SPSS, Inc., Chicago, IL, USA). The chi-squared test and Mann–Whitney U-test were used for categorical and continuous data analyses. The log-rank test using Kaplan–Meier estimates was used for survival analysis. Multivariate analysis was performed using a forward stepwise Cox regression analysis for an outcome of overall survival. A P-value of <0.05 was considered statistically significant.
Results
Pathological analysis of liver metastases after neoadjuvant chemotherapy
Fifty patients completed the NAC and underwent subsequent hepatic resection. A complete pathological response of the liver metastasis (PRG3) was seen in six patients (12%) and scant evidence (≤10%) of viable tumour cells (PRG2) was seen in a further 11 cases (22%). The remaining patients (66%) had >10% of viable tumour cells in at least one lesion (PRG1) (Fig. 1). The major non-cellular histological components of the lesions were fibrosis and necrosis. Neither had any statistical association with survival or recurrence (data not shown). The pathological examination of the background non-neoplastic liver found steatosis in 33 patients (66%) and was moderate to severe in 13 (26%). Six patients had signs of mild steatohepatitis (more than one foci of lobular inflammation/HPF). The presence of fatty liver disease was not statistically associated with increased morbidity or a worse survival (data not shown).
Figure 1.
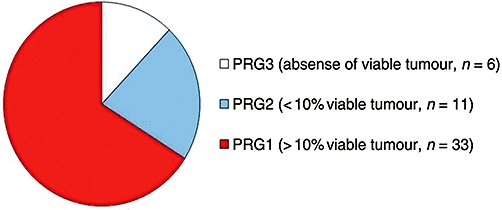
Pathological response grade (PRG)
Baseline clinical characteristics
The characteristics of the patients, primary colorectal cancer and liver metastases at initial presentation are shown in Table 1. The majority of cases (74%) were classified as ‘advanced’ as a result of synchronous presentation of liver metastases in 64%, planned staged resections in 14%, application of portal vein embolization in 10% and concurrent resectable lung metastases in 8%. The CRS18 was considered high (≥3) in 72%. The strong PRG group was associated with a significantly higher percentage of multiple lesions and bilobar disease. In addition, the strong PRG group included a greater proportion of patients with a high-risk CRS that approached statistical significance.
Table 1.
Demographics and characteristics of the metastatic disease at initial presentation by pathological response grade
| Group | Total | Strong | Weak | P-value |
|---|---|---|---|---|
| n | 50 | 17 | 33 | |
| Mean age, years | 56.0 | 56.2 | 56.8 | 0.680 |
| Male gender, n | 34 | 10 | 24 | 0.629 |
| Primary rectal cancer, n | 18 | 5 | 13 | 0.486 |
| Lymph node positive primary, n | 36 | 12 | 24 | 0.873 |
| Synchronous liver metastases, n | 32 | 13 | 19 | 0.187 |
| Synchronous lung metastases, n | 4 | 3 | 1 | 0.218 |
| Multiple liver lesions, n | 37 | 16 | 21 | 0.020 |
| Mean size of largest lesion, cm | 3.7 | 3.4 | 3.8 | 0.686 |
| Bilobar lesions, n | 26 | 13 | 13 | 0.013 |
| Clinical risk score 18≥3, n | 36 | 15 | 21 | 0.066 |
Neoadjuvant chemotherapy
The type and details of the neoadjuvant regimens are shown in Table 2. The majority (60%) of patients received a multi-drug regimen including irinotecan, after which 20% received oxaliplatin. The median number of courses given was eight. A clinical response according to the RECIST criteria (complete response/partial response) was seen in 48% of patients. Only one patient had a complete radiological response after receiving a neoadjuvant FOLFIRI regimen. There was a progression of disease (PD) that required a change in regimen in seven patients, each of whom had received between 14 and 35 cycles in the neoadjuvant setting. Two of these patients were initially given FOLFOX and then switched to FOLFIRI, whereas two were treated with the converse regimen. The other three patients progressed after receiving 5-FU/leucovorin; one of these subsequently received FOLFIRI and two proceeded directly to resection. No patients with PD had a strong PRG and there was a significant correlation of PRG and RECIST scores (P= 0.046). Portal vein embolization was performed in five patients (10%) during the neoadjuvant period.
Table 2.
Details of neoadjuvant chemotherapy regimen (n= 50)
| Group | Total | Strong | Weak | P-value |
|---|---|---|---|---|
| Neoadjuvant regimen | ||||
| FOLFIRI | 30 | 12 | 18 | 0.271 |
| FOLFOX | 10 | 3 | 7 | |
| Other | 10 | 2 | 8 | |
| Mean number of courses, n | 8 | 8 | 8 | 0.875 |
| Response rate by RECIST26 | 0.046 | |||
| Complete response | 1 | 1 | 0 | |
| Partial response | 23 | 11 | 12 | |
| Stable disease | 19 | 5 | 14 | |
| Progressive disease | 7 | 0 | 7 | |
| Portal vein embolization, n | 5 | 2 | 3 | 0.765 |
Operative characteristics and perioperative complications
A total of 43 cases were considered resectable in a single operation, whereas seven were considered to require staged resections. In total, 58 liver resections were performed. The operative details are outlined in Table 3. A wedge resection was completed in three of four patients who had a synchronous solitary lung lesion. A complete resection (R0) of all metastatic involvement, including liver and lung, was achieved in 47 patients (94%). A two-stage liver resection was required in four patients and a three-stage resection in one. The failure to complete the planned staged resections reflected an insufficient remnant liver in two patients and a progression of lung disease after liver resection in one patient.
Table 3.
Operative characteristics and postoperative course (n= 50)
| Group | Total | Strong | Weak | P-value |
|---|---|---|---|---|
| Liver resection, n | 58 | 21 | 37 | 0.520 |
| Major hepatectomy | 38 | 12 | 26 | |
| Bisegmentectomy/wedge | 20 | 9 | 11 | |
| Lung resection, n | 3 | 2 | 1 | 0.218 |
| Staged resection, planned | 7 | 5 | 2 | 0.858 |
| Completed | 5 | 3 | 2 | |
| Postoperative morbidity* | 15 | 4 | 11 | 0.474 |
| Perihepatic collection | 3 | 2 | 1 | |
| Intestinal haemorrhage | 1 | 0 | 1 | |
| Acute coronary syndrome | 1 | 0 | 1 | |
| Sepsis | 1 | 0 | 1 | |
| Deep vein thrombosis | 1 | 0 | 0 | |
| Pulmonary embolus | 1 | 0 | 1 | |
| Colitis, ischaemic | 1 | 1 | 1 | |
| Urinary infection | 5 | 1 | 4 | |
| Surgical site infection | 8 | 0 | 8 | |
| Perioperative blood transfusion | 12 | 1 | 11 | 0.031 |
Common terminology criteria of adverse events (CTCAE v3.0)
There were no perioperative mortalities within 90 days of liver resection. Serious or life-threatening (grade 3 or 4, CTC-AE v3.0; National Cancer Institute, Bethesda, MD, USA) complications were seen in 10% of patients. The overall morbidity rate was 30%, as shown in Table 3. Multiple complications occurred in 12% of patients. All perihepatic collections were amenable to percutaneous drainage. Sepsis occurred in one patient secondary to pneumonia and was associated with a bleeding duodenal ulcer, confusion and urinary tract infection necessitating re-admission to the intensive care unit. A perioperative blood transfusion was required in 12 patients (24%) and was significantly associated with a weak PRG. There were no significant differences in the percentages of major liver resection, successful staged resection, complete resection or morbidity between the two PRG groups.
Adjuvant therapies
All patients in this study received adjuvant chemotherapy, except one who developed bone metastases within 3 months of resection. The median total number of cycles received after the first hepatectomy was 12 (range three to 35 courses). The most common adjuvant chemotherapy regimen was FOLFIRI. Two patients received hepatic arterial chemotherapy via infusion pumps. Other adjuvant treatments for recurrence or residual disease included radiofrequency ablation, external beam radiation, hepatic arterial chemotherapy, radio-bead embolization and angio-embolization (Table 4).
Table 4.
Outcomes after neoadjuvant chemotherapy and liver resection (n= 50)
| Overall | Strong | Weak | P-value | |
|---|---|---|---|---|
| Outcomes | ||||
| Recurrence, n | 36 | 9 | 27 | 0.031 |
| Death, n | 16 | 2 | 14 | 0.029 |
| Site of recurrent disease | ||||
| Liver | 20 | 4 | 16 | |
| Lung | 15 | 5 | 10 | |
| Peritoneal/pelvic/retroperitoneal | 10 | 2 | 8 | |
| Skin/abdominal wall | 3 | 1 | 2 | |
| Bone | 5 | 0 | 5 | |
| Brain | 3 | 0 | 3 | |
| Adjuvant chemotherapy | ||||
| FOLFIRI | 24 | 10 | 14 | |
| FOLFOX | 15 | 5 | 10 | |
| 5FU/capecitabine | 12 | 4 | 8 | |
| Other | 5 | 2 | 3 | |
| Received multiple regimens, n | 23 | 12 | 11 | |
| Total number of cycles, mean | 12 | 13 | 12 | |
| Adjuvant therapies | ||||
| Radiofrequency ablation | 3 | 2 | 1 | |
| Hepatic arterial embolization | 4 | 2 | 2 | |
| Hepatic artery pump | 2 | 1 | 1 | |
| Radio-bead embolization | 3 | 1 | 2 | |
| External beam radiation | 1 | 0 | 1 | |
| Complete resection of recurrence | 6 | 1 | 5 |
Longterm outcomes
At last follow-up, we noted 16 mortalities, all of which resulted from metastatic disease. The median time of follow-up was 29.0 months. The estimated 1-year, 3-year and 5-year overall survivals were 86%, 70% and 61%, respectively. There have been 36 recurrences in patients who underwent complete resection. The median time to recurrence was 17.7 months. After complete resection, the estimated 1-year, 3-year and 5-year recurrence-free survival rates were 61%, 24% and 21%, respectively. Patients with a strong PRG (including PRG2 and PRG3) had a statistically significant longer overall survival by log-rank analysis (P= 0.019) compared with the weak PRG group (PRG1). (Fig. 2A) The estimated 5-year overall survival rates were 80% and 51%, respectively, for the strong and weak PRG groups. There was a near statistically significant difference in recurrence-free survival (P= 0.051). The estimated 5-year recurrence-free survivals were 42% and 10%, respectively. (Fig. 2B)
Figure 2.
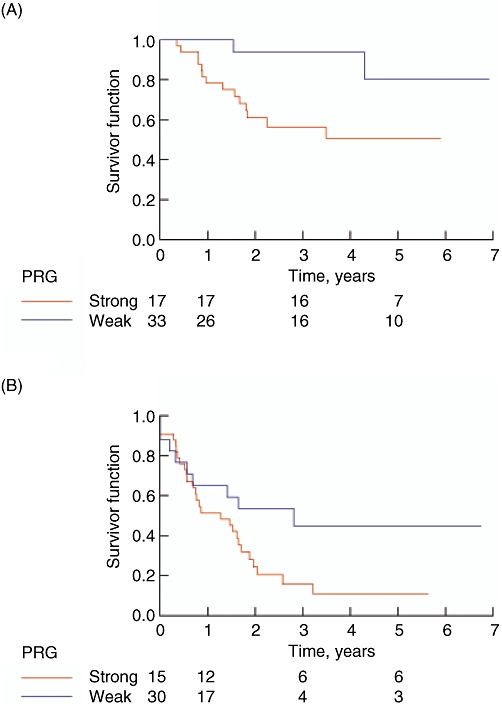
Estimated (A) overall and (B) recurrence-free survival rates in patients with strong and weak pathological responses (PRG)
The liver was the most common site of oncological failure, accounting for 56% of recurrences. The lung was the second most common site and was involved in 42% of patients with recurrent disease. Recurrences were observed in multiple sites in 15 patients. Six recurrences were completely resected by a wedge resection of the liver (n= 2), resection of a retroperitoneal mass (n= 1) and wedge excision of the lung (n= 3). One liver recurrence that was positive on CT/PET scan had a complete clinical response to systemic chemotherapy and at present shows no evidence of active disease in follow-up.
Univariate analysis for predictors of longterm survival identified a strong PRG (PRG3 + PRG2), a complete pathological response (PRG3), receipt of a second-line NAC regimen and a largest lesion >5 cm in diameter as statistically significant. The variables that did not reach significance included: gender; rectal cancer (vs. colon cancer); lymph node positive primary; synchronous metastases (vs. metachronous); bilobar involvement; multiple lesions; incomplete resection (R2); RECIST (CR/PR); CRS ≥ 3; need for perioperative transfusion, and the occurrence of a perioperative complication. When the variables with a significant trend were put into a multivariate model along with the continuous variables of age, number of lesions and number of NAC cycles, a lack of strong PRG, increase in number of CRLM and advanced age were found to be independent predictors of death (Table 5).
Table 5.
(A) Univariate analysis: 95% confidence intervals of mean overall survival time (days) for those binary variables for which there was a significant trend. (B) Independent predictors of overall survival: results of forward stepwise Cox multivariate regression analysis at the last step
| (A) Variable | P-valuea |
95% CI for mean survival |
||
|---|---|---|---|---|
| No | Yes | |||
| Strong PRG | 0.02 | 1105–1751 | 2038–2747 | |
| Received a second-line neoadjuvant chemotherapy regimen | 0.02 | 1730–2340 | 418–1525 | |
| Complete pathological response | 0.14 | 1276–1833 | 1925–2892 | |
| Largest lesion >5 cm diameter | 0.15 | 1713–2374 | 769–1482 | |
| (B) Variable | P-value | OR |
95% CI for ORb |
|
| Lower | Upper | |||
| No strong PRG | <0.001 | 243.2 | 11.4 | 5196.2 |
| Number of lesions | <0.001 | 2.8 | 1.7 | 4.6 |
| Age | 0.003 | 1.1 | 1.0 | 1.2 |
P-value for the log-rank test
The odds ratio is the odds that a patient with the parameter will reach the endpoint (death) before a patient without. Three variables with a significant trend from univariate analysis (complete pathological response was excluded) and the continuous variables age, number of lesions, size of largest liver lesion and number of neoadjuvant cycles were considered for the model. The odds ratio of continuous variables is expressed per unit (i.e. per unit increase in lesion number or per year, respectively)
95% CI, 95% confidence interval; PRG, pathological response grade; OR, odds ratio
Discussion
This study describes a system of grading the pathological response to chemotherapy for liver metastases of colorectal cancer. A strong PRG is indicated by the presence of <10% of viable tumour cells in all resected lesions and represents a vigorous response to the NAC. Patients with a strong PRG, demonstrated in 34% of this cohort, had a significantly better survival and approached a significantly longer recurrence-free survival. In addition, a strong PRG was also found to be an independent predictor of survival, with an odds ratio of 243. The other independent variables included advanced age and number of liver lesions, both of which have been previously identified in prognostic scoring systems. The overall 5-year survival rate of 61% observed in the entire study population is comparable with rates reported in recently published series for liver resection.1–4
The prognostic value of a pathological response to chemotherapy has been previously established in treatment of carcinomas of the breast,29 oesophagus30 and rectum.31,32 Two reports have also introduced different grading systems in CRLM. Rubbia-Brandt et al. presented the tumour regression grade (TRG), which is based on the amount of fibrosis and residual cancer cells, in a series of 112 patients. The major response group comprised tumours with rare or an absence of cancer cells and was associated a higher 3-year disease-free survival.23 Furthermore, the absence of a histological response was associated with a worse overall survival at 5 years. By comparison, the stratification PRG groups presented here are defined by one criterion, the percentage of viable tumour cells, although the strong PRG group would be closely analogous to the TRG major response group. Our analyses did not find any correlation of outcomes with percentages of mucin, fibrosis or necrosis, although the cohort size may have been too small to detect such differences. Practically, the use of a single component in the grading scale should be more widely and easily applicable to clinical practice.
A second study of 305 patients reported by Blazer et al. showed that overall survival was significantly different when stratified between complete response, a major response (1–49% residual cancer cells) and a minor response (≥50%).24 A multivariate analysis found that pathological response and margin status were the only independent predictors of survival after NAC and liver resection. Similarly, in our series the PRG was identified as an independent predictor of survival, but the complete resection of all metastatic deposits was not. No patient had a positive margin (R1), yet several had unresected lesions (R2) after failing to complete all the planned staged resections although this was probably not significant as a result of a type II error. In comparison with the PRG, we feel that the strong or major response subgroup is better defined by the presence of a lower percentage of viable tumour cells. The limit of 10% for the PRG evolved through the quantification of lesions that were previously described as showing ‘scant evidence of tumour cells in the remnant lesion’ in our published experience with NAC.33 Although a statistical difference may exist between the response groups, the cut-off score of 50% viable tumour cells, similar to a system used in oesophageal cancer, may be less specific as a marker of a biological response to chemotherapy as a certain degree of tumour necrosis or fibrosis exists in tumours even without chemotherapy.23 Further prospective studies will require larger cohorts to accurately define and validate the prognostic limits of a strong PRG. Other pathological findings, such as the histological pattern of residual disease, may also prove to have prognostic value34,35 and could be incorporated into future grading systems.
A complete pathological response has received by far the most attention and has been associated with superior overall and disease-free survival rates.22 A complete pathological response was seen in 4% and 9%, respectively, of these study populations.23,24 Another smaller series reported a 24% complete pathological response to FOLFOX-4.25 In our cohort, the rate of complete pathological response was similar, at 12%. Conceptually, the sub-stratification of a complete response group need not be the focus of a grading system. The system should be designed to identify a strong response to chemotherapy, in which the complete response belongs. The difference between a complete response (PRG3) and tumours with scant evidence of viable cells (PRG2) may largely be a product of the sensitivity of pathological processing and sampling error. A complete pathological response cannot be considered to represent a cure22 and should not be individually stratified.
This pilot study, although much smaller than those described in the previous two reports, is a prospective study that represents the use of a standardized protocol for pathological tissue handling and evaluation. Retrospective studies may be limited by a higher variance in the amount of tumour sampling and each lesion within a resected specimen may not have been sampled in a consistent fashion. Furthermore, it may be difficult in retrospect to accurately determine the sampling technique. In future studies, it will be critical to ensure the standardization of pathological protocols and to determine the reliability and interobserver variability of any pathological grading system. The future success and strength of this, or other, pathological grading systems will be integral to establishing a standard of care in the processing and analysis of resected liver metastases.
The major shortcomings of this study relate to its small cohort size and its use of a selected CRLM population. As stated previously, a larger series will be required to prospectively validate this grading system in a standardized treatment algorithm for CRLM, which should, we hope, diminish confounding factors such as the difference in transfusion rates found in this study. Furthermore, the exceedingly high odds ratio may be a product of the small population size of this study and local practice in our institution, in which patients with recurrent disease, who were previously identified as chemoresponsive, were treated aggressively with numerous successive regimens of chemotherapy and other adjuvant therapies. A difficult aspect of conducting such a multi-institutional study in the future will concern the standardization of the treatment of recurrent or residual disease when patients return to their primary hospitals. In such future studies, NAC will have to be approved and used to treat study patients, although it has yet to be considered a standard of care for resectable CRLM given its potential risk for perioperative complications and unknown benefits. Even in our institution at the time of this study, a significant percentage of patients with resectable liver metastases did not receive NAC. It is unclear whether any benefit observed in this study extends to patients with uncomplicated resectable CRLM.
Consideration must also be given to medical advancements such as the use of biological treatments, such as bevacizumab and cetuximab, and small molecule inhibitors, which any grading system must adjust for. In particular, these two drugs have pushed clinical response rates beyond those for the traditional FOLFOX and FOLFIRI regimens used in this study. The effects of these biologics and new therapies on the prognostic strength of the PRG remain to be established.
Conclusions
This study presents preliminary evidence that a strong pathological response to NAC can be found in a significant proportion of patients with liver metastases deriving from colorectal cancer and is an independent predictor of survival. A prospective trial with a broader population will be required to confirm the prognostic strength of the PRG and its impact on overall survival and on treatment algorithms for resectable and downstaged disease. In the future, the PRG could be used as an objective biological measure of the effectiveness of the chemotherapy. It may allow for the identification of patients in whom an aggressive multidisciplinary strategy can transform recurrent or residual cancer from a short-term prognosis into a chronic disease.
Conflicts of interest
None declared.
References
- 1.Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. discussion 447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in longterm survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikfarjam M, Shereef S, Kimchi ET, Gusani NJ, Jiang Y, Avella DM, et al. Survival outcomes of patients with colorectal liver metastases following hepatic resection or ablation in the era of effective chemotherapy. Ann Surg Oncol. 2009;16:1860–1867. doi: 10.1245/s10434-008-0225-3. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict longterm survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 657–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of non-resectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520. doi: 10.1097/00000658-199610000-00009. discussion 520–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelek L, Bugat R, Cherqui D, Ganem G, Valleur P, Guimbaud R, et al. Multimodal therapy with intravenous biweekly leucovorin, 5-fluorouracil and irinotecan combined with hepatic arterial infusion pirarubicin in non-resectable hepatic metastases from colorectal cancer (a European Association for Research in Oncology trial) Ann Oncol. 2003;14:1537–1542. doi: 10.1093/annonc/mdg404. [DOI] [PubMed] [Google Scholar]
- 8.Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 9.Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 10.Benoist S, Nordlinger B. Neoadjuvant treatment before resection of liver metastases. Eur J Surg Oncol. 2007;33(Suppl 2):35–41. doi: 10.1016/j.ejso.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Andres A, Majno PE, Morel P, Rubbia-Brandt L, Giostra E, Gervaz P, et al. Improved longterm outcome of surgery for advanced colorectal liver metastases: reasons and implications for management on the basis of a severity score. Ann Surg Oncol. 2008;15:134–143. doi: 10.1245/s10434-007-9607-1. [DOI] [PubMed] [Google Scholar]
- 12.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomized controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka K, Adam R, Shimada H, Azoulay D, Levi F, Bismuth H. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg. 2003;90:963–969. doi: 10.1002/bjs.4160. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero A, Vigano L, Polastri R, Muratore A, Eminefendic H, Regge D, et al. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643–1651. doi: 10.1007/s00268-007-9123-2. [DOI] [PubMed] [Google Scholar]
- 15.Allen PJ, Kemeny N, Jarnagin W, DeMatteo R, Blumgart L, Fong Y. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7:109–115. doi: 10.1016/S1091-255X(02)00121-X. discussion 116–117. [DOI] [PubMed] [Google Scholar]
- 16.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of longterm survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 18.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168–1172. doi: 10.1001/archsurg.139.11.1168. [DOI] [PubMed] [Google Scholar]
- 20.Gruenberger B, Scheithauer W, Punzengruber R, Zielinski C, Tamandl D, Gruenberger T. Importance of response to neoadjuvant chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer. 2008;8:120. doi: 10.1186/1471-2407-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumour progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. discussion 1061–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adam R, Wicherts DA, de Haas RJ, Aloia T, Levi F, Paule B, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008;26:1635–1641. doi: 10.1200/JCO.2007.13.7471. [DOI] [PubMed] [Google Scholar]
- 23.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumour response assessment in predicting the outcome in patients with colorectal liver metastases treated with neoadjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 24.Blazer DG, III, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome endpoint after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 25.Aloysius MM, Zaitoun AM, Beckingham IJ, Neal KR, Aithal GP, Bessell EM, et al. The pathological response to neoadjuvant chemotherapy with FOLFOX-4 for colorectal liver metastases: a comparative study. Virchows Arch. 2007;451:943–948. doi: 10.1007/s00428-007-0497-1. [DOI] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumours. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, Makuuchi M, Strong RW. The Brisbane 2000 Terminology of Liver Anatomy and Resections Terminology Committee of the International Hepato-Pancreato-Biliary Association: Chairman, SM Strasberg (USA) HPB. 2000;2:333–339. [Google Scholar]
- 28.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for non-alcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 29.Schott AF, Roubidoux MA, Helvie MA, Hayes DF, Kleer CG, Newman LA, et al. Clinical and radiologic assessments to predict breast cancer pathologic complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2005;92:231–238. doi: 10.1007/s10549-005-2510-1. [DOI] [PubMed] [Google Scholar]
- 30.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumour regression after preoperative chemoradiotherapy of oesophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 31.Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumour regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94:1121–1130. [PubMed] [Google Scholar]
- 32.Rullier A, Laurent C, Vendrely V, Le Bail B, Bioulac-Sage P, Rullier E. Impact of colloid response on survival after preoperative radiotherapy in locally advanced rectal carcinoma. Am J Surg Pathol. 2005;29:602–606. doi: 10.1097/01.pas.0000153120.80385.29. [DOI] [PubMed] [Google Scholar]
- 33.Znajda TL, Hayashi S, Horton PJ, Martinie JB, Chaudhury P, Marcus VA, et al. Postchemotherapy characteristics of hepatic colorectal metastases: remnants of uncertain malignant potential. J Gastrointest Surg. 2006;10:483–489. doi: 10.1016/j.gassur.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Ng JK, Urbanski SJ, Mangat N, McKay A, Sutherland FR, Dixon E, et al. Colorectal liver metastases contract centripetally with a response to chemotherapy: a histomorphologic study. Cancer. 2008;112:362–371. doi: 10.1002/cncr.23184. [DOI] [PubMed] [Google Scholar]
- 35.Mentha G, Terraz S, Morel P, Andres A, Giostra E, Roth A, et al. Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br J Surg. 2009;96:95–103. doi: 10.1002/bjs.6436. [DOI] [PubMed] [Google Scholar]


