Abstract
Studies using PPARγ agonists in mouse skin have suggested that peroxisome proliferator-activated receptor gamma (PPARγ) is irrelevant to cutaneous photobiology. However, in several epithelial cell lines, ultraviolet B (UVB) has been shown to induce the nonenzymatic production of oxidized phospholipids that act as PPARγ agonists. UVB is also a potent inducer of prostaglandin E2 (PGE2) production and COX-2 expression in keratinocytes and PPARγ is coupled to increased PGE2 production in other cell lines. In this current study, we demonstrate that PPARγ agonists, but not PPARα or PPARβ/δ agonists, induce PGE2 production and COX-2 expression in primary human keratinocytes (PHKs). Importantly, PPARγ agonist-induced COX-2 expression and PGE2 production were partially inhibited by the PPARγ antagonist, GW9662, indicating that both PPARγ-dependent and -independent pathways are likely involved. GW9662 also suppressed UVB and tert-butylhydroperoxide- (TBH-) induced PGE2 production in PHKs and intact human epidermis and partially inhibited UVB-induced COX-2 expression in PHKs. These findings provide evidence that PPARγ is relevant to cutaneous photobiology in human epidermis.
1. Introduction
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated nuclear transcription factors that were initially identified as being crucial for regulating the formation of intracellular organelles, called peroxisomes, that are involved in lipid metabolism (reviewed in [1]). Three different PPARs subtypes have been cloned (α, β/δ, and γ) that differ in ligand specificity, tissue expression, and transcriptional targets. All three PPARs require heterodimerization with the retinoid X receptors (RXRs) for transcriptional activity. PPAR:RXR heterodimers induce target gene transcription by binding to specific peroxisome proliferators response elements (PPREs) in the promoter region of target genes [1]. Importantly, PPARα, PPARβ/δ, and PPARγ are all expressed in keratinocytes and in human and rodent epidermis [1–3]. We have recently demonstrated that PPARγ is expressed in adult primary human keratinocytes and three different immortalized or malignant human keratinocyte cell lines (A431, HaCaT, and KB cells) [4, 5]. In KB epidermoid carcinoma cells and SZ95 sebocytes cells, we have also demonstrated that oxidative stress, including ultraviolet B irradiation, results in the production of oxidized lipid species with potent PPARγ ligand activity [4, 5].
Natural PPARγ ligands include metabolites of both the cyclooxygenase (COX) and lipoxygenase pathways, including the cyclopentanone prostaglandin, 15-deoxy-Δ12,14-prostaglandin J2, and 13-hydroxyoctadecadienoic acid (13-HODE) (reviewed in [2]). However, these compounds are relatively low-affinity ligands that also exhibit PPARγ-independent functions [6]. The most potent natural ligands for PPARγ have been shown to be oxidized alkyl phospholipids. This includes 1-hexadecyl 2-azelaoyl phosphatidylcholine (azPC), a nonenzymatically oxidized alkyl glycerophosphocholine first discovered associated with oxidized low-density lipoprotein [7]. Importantly, azPC has been shown to be produced following UVB irradiation [4]. In addition, the thiazolidinedione (TZD) compounds, troglitazone, ciglitazone, rosiglitazone, and pioglitazone, are synthetic PPARγ agonists that are widely used in the treatment of type II diabetes. However, synthetic TZD PPARγ agonists have also been shown to exhibit PPARγ-independent effects [8–10]. This underscores the importance of using appropriate controls, such as the PPARγ-selective antagonist, GW9662, to verify PPARγ-specific results when using these agonists [11].
While it is clear that UVB and other oxidative stressors can result in PPARγ ligand production, a previous study using exogenous PPARγ agonists failed to demonstrate any effect on either chemical carcinogenesis or UVB-induced skin cancer formation [12]. These negative findings raise doubts concerning the relevance of PPARγ to cutaneous photobiology. Yet, it should be noted that mice with heterozygous germline deletion of PPARγ or mice with epidermal-specific loss of PPARγ exhibit an increase in chemical carcinogen-induced skin tumors [13, 14]. This suggests the possibility that loss of function models, such as the use of PPARγ antagonists rather than agonists, might be more informative for studies designed to examine the role of PPARγ in photobiology. Given that we have already demonstrated that UVB induces PPARγ ligand formation, we hypothesized that the lack of effect of exogenous PPARγ ligands on photocarcinogenesis could be explained by the fact that PPARγ is already engaged by UVB-induced ligand production. Inasmuch as our previous studies using cell lines indicate that PPARγ is coupled to epithelial COX-2 expression and PGE2 production, we therefore utilized the PPARγ antagonist, GW9662, to determine whether PPARγ is involved in regulating UVB-induced COX-2 expression and PGE2 production in primary human keratinocytes and intact human epidermal explants. The results of the present studies indicate that PPARγ is functionally coupled to a readily measured photobiological response in human primary epidermal keratinocytes and is therefore relevant to cutaneous photobiology.
2. Materials and Methods
2.1. Materials
Ciglitazone, GW501516, and WY-14,643 were obtained from Alexis Biochemicals (San Diego, CA). AzPC (1-O-Hexadecyl-2-Azelaoyl-sn-Glycero-3-Phosphocholine) was purchased from Avanti Polar Lipids (Alabaster, AL). The specific PPARγ antagonist, GW9662, was obtained from Cayman Chemical (Ann Arbor, MI). The selective COX-2 inhibitor, NS398, was obtained from Sigma-Aldrich (St. Louis, MO). All other reagents were obtained from Sigma-Aldrich unless otherwise noted.
2.2. Cell Culture
Adult primary human keratinocytes (PHKs) were prepared from discarded epidermis that was obtained from reductive mammoplasties and panniculectomies as previously described [15]. Telomerase-immortalized primary human keratinocytes (N/TERT-1) were obtained from Dr. Rheinwald (Department of Medicine and Harvard Skin Disease Research Center, Brigham, and Women's Hospital, Boston, MA) [16]. PHKs and N/TERT-1 cells were cultured on tissue culture plastic or wells that were precoated with type I collagen. PHKs and N/TERT-1 cells were grown in serum-free media (Keratinocyte serum-free media, K-SFM; Gibco Invitrogen, Carlsbad, CA). Media were supplemented with 40 IU per mL penicillin, 40 μg per mL streptomycin, and 0.1 μg per mL amphotericin B. The cells were cultured in 95% air and 5% CO2 at 37°C. All studies were done using PHKs or N/TERT-1 cells that were plated at sufficient density to achieve approximately 70%–80% confluence prior to experimental manipulation. All studies with human skin have been approved by the Indiana University-Purdue University at Indianapolis Institutional Review Boards using the Declaration of Helsinki Principles.
2.3. Immunoblotting
Immunoblotting for PPARγ was done using mouse monoclonal anti-PPARγ antibody (clone E8; Santa Cruz Biotechnology, Santa Cruz, CA), essentially as described in [4]. The specificity of this antibody for PPARγ has previously been demonstrated in wild-type versus tissue-specific PPARγ knockout mice [17].
2.4. Ultraviolet B Irradiation of Cultured Keratinocytes
For UVB irradiation studies, a Philips F20T12/UV-B lamp (270–390 nm), containing 2.6% UVC, 43.6% UVB, and 53.8% UVA, was utilized. The UVB dose was measured using an IL1700 radiometer and a SED240 UVB detector (International Light, Newburyport, MA). All irradiations were performed at a distance of 8 cm from the UVB light source. All irradiations were done on PHKs grown in 24-well plates. For GW9662- and NS398-treated cells, media containing vehicle, 1 μM GW9662, or 10 μM NS398 were added either 1 hour (GW9662) or 30 minutes (NS398) prior to UVB irradiation. The cells were then washed twice with PBS, and then the cells were irradiated with 600 J/m2 UVB. DMEM containing 10% FBS was then added containing vehicle, GW9662, or NS398. After eight hours at 37°C, the culture supernatants were removed for PGE2 quantitation, the cell monolayer was trypsinized, and the cells were counted. PGE2 was then normalized to cell count.
2.5. Tert-Butylhydroperoxide (TBH) Studies in PHKs In Vitro
For TBH studies, cells were plated onto 24-well plates in high-calcium DMEM containing 10% fetal bovine serum. The cells were then pretreated with GW9662 or NS398 as detailed in the previous section prior to TBH addition. Culture supernatants were then collected after an eight-hour incubation at 37°C.
2.6. PPAR Agonist Studies
For PPAR agonist studies, agonists were added in low-calcium K-SFM media or in K-SFM media supplemented with 1 mM calcium and incubated for 24 hours at 37°C. To assess PPARγ-dependent induction of PGE2, GW9662 (1 μM) was added 1 hour prior to agonist addition. PGE2 was then quantitated in tissue culture media by EIA and normalized to either cell count or total cellular protein (BCA assay; Pierce Biotechnology, Rockford, IL).
2.7. RNA Isolation and COX-2 Quantitative RT-PCR
PHKs were treated with vehicle and ciglitazone (with and without GW9662) as detailed in the previous section. At 2, 4, 8, and 24 hours after the addition of reagents, the cell monolayers were processed for RNA isolation using an RNeasy kit (Qiagen) according to the manufacturer's protocol. Following first-strand DNA synthesis, quantitative RT-PCR (qRT-PCR) was performed using primers specific to human COX-2 and 18S rRNA with a Cepheid Smart Cycler real-time PCR instrument (Fisher Scientific, Pittsburgh, PA). COX-2 and 18S qRT-PCR were performed as previously described [5]. COX-2 results were then normalized to 18S using the ΔΔCt method [18].
2.8. Human Epidermal Explant Preparation and UVB Irradiation
Adult human epidermis obtained from panniculectomies was obtained postoperatively. Subdermal fat and a portion of the lower dermis were immediately removed using a scalpel blade. The epidermis was then cut into small (approx. 8 × 8 mm) sections, weighed, and then cultured in 12-well plates submerged in Keratinocyte-SFM media for 48–72 hours. The explants were then pretreated with vehicle, 1 μM GW9662 (1 hour), or 10 μM NS398 (30 minutes) prior to UV treatment. After pretreatment, the explants were washed twice with PBS and a minimal amount of PBS was added to cover the explants to maintain hydration. The explants were then irradiated with 1,800 J/m2 of UVB light. Control cells received no UVB light. The explants were then submerged in serum-free DMEM containing vehicle, 1 μM GW9662, or 10 μM NS398 and cultured for 8 hours at 37°C. At this time the media were removed for PGE2 quantitation. All PGE2 levels were then normalized to tissue weight.
2.9. COX-2 Immunoblot
Second-passage PHKs grown on 6-well plates to near confluence were treated with 1 μM GW9662 or vehicle 1 hour prior to washing the wells with HBSS, then irradiating the cells with 300 J/m2 of UVB. The HBSS was then replaced with media containing vehicle or 1 μM GW9662, and the cells were processed 20 hours later in RIPA buffer supplemented 1 : 100 with a protease inhibitor cocktail (Sigma-Aldrich). Protein was quantitated using a DC protein assay, and equal amounts of total protein (50 μg/lane) were then separated on 7.5% SDS-PAGE gels. Rabbit polyclonal anti-COX-2 (Cayman Chemical) diluted 1 : 1000 in TBS with 0.1% Tween 20 and 2% Blotto was added and incubated overnight at 4°C in 2% Blotto. Goat anti-rabbit HRP conjugate (1 : 10,000; Source) in 5% Blotto was applied and the immunoreactive bands were detected enhanced chemiluminescence. The exposed films were developed and scanned and band intensity was determined using NIH Image J software. The band intensity of the COX-2 band at 72 kDa was normalized to that of an invariant non-specific high-molecular-weight band (>180 kDa) observed in all lanes.
2.10. PGE2 Quantitation
PGE2 was quantitated as previously described in culture media using a commercial PGE2 EIA kit (Cayman Chemical, Ann Arbor, MI) [4].
3. Results and Discussion
3.1. Primary Human Keratinocytes Express PPARγ
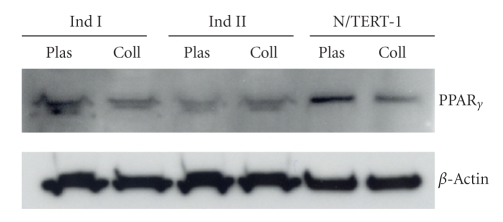
Studies in mouse skin have shown that PPARγ is expressed at low levels and may be functionally irrelevant in mouse keratinocytes [8, 12]. We therefore first verified that PPARγ is expressed in the PHKs from several different individuals under several different culture conditions (culture on plastic or type I collagen) (Figure 1). To exclude the possibility that the PPARγ seen in these primary cultures was derived from other cell types, immunoblotting for PPARγ was also done on telomerase-immortalized human keratinocytes (N/TERT-1) [16]. This data supports previous reports that PHKs express mRNA and protein for PPARγ [19–21].
Figure 1.
PPARγ expression by immunoblot in PHKs. 40 μg cellular protein isolated from PHKs from two different individuals was grown on tissue culture plastic coated with (Coll) and without (Plas) type I rat tail collagen. Protein from telomerase-immortalized primary human keratinocytes (N/TERT-1) was also utilized. In all cases, the proteins were separated on a 10% SDS-PAGE and PPARγ protein was detected using a monoclonal anti-PPARγ antibody. Loading was verified by stripping the blots and performing an immunoblot for β-Actin.
3.2. PPARγ Agonists, but not PPARα and PPARβ/δ Agonists, Stimulate PGE2 Formation in PHKs through Both PPARγ-Dependent and -Independent Mechanisms
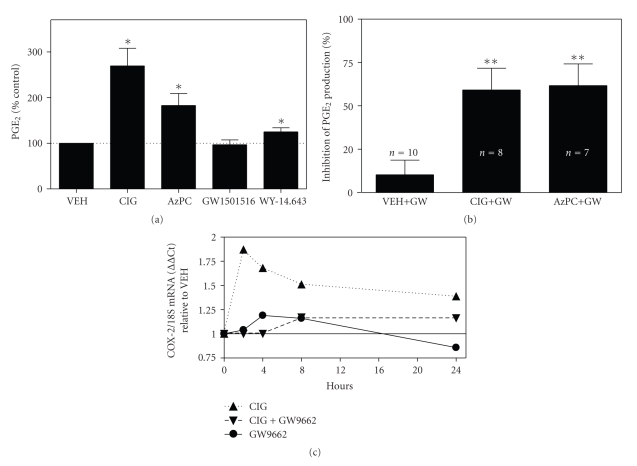
In SZ95 sebocytes, we have previously demonstrated that GW9662 was effective in blocking PGE2 production elicited by the synthetic PPARγ agonist ciglitazone [5]. We therefore examined whether ciglitazone could also induce PGE2 formation in PHKs. Given that a TZD type of PPARγ agonist has been reported to induce COX-2 expression independent of PPARγ in a mouse keratinocyte cell line [22], we also examined whether the endogenous PPARγ ligand, azPC, could also induce PGE2 production in PHKs. Finally, the ability of PPARα or PPARβ/δ to regulate PGE2 production in PHKs was also examined. In Figure 2(a), we demonstrate that treatment of PHKs with the PPARγ agonists, ciglitazone, and azPC results in a significant increase in PGE2 production. The PPARα agonist, WY-14,643, resulted in only a slight, but reproducible, increase in PGE2 production. Treatment with a PPARβ/δ agonist (GW501516, 500 nM) did not alter PGE2 production. It should be noted that NS398 significantly blocked both basal and PPARγ agonist-induced changes in PGE2 production. As a percent of the vehicle control-treated cells, PGE2 levels were 8.1 ± 7.4% (n = 4), 11.2 ± 12.5% (n = 4), and 12.2 ± 8.8% (n = 3) for NS398 treatment alone, ciglitazone + NS398, and azPC + NS398-treated cells, respectively.
Figure 2.
PPARγ agonists, but not PPARα or β/δ agonists, induce PGE2 production in PHKs; PPARγ agonist-induced PGE2 production and COX-2 mRNA expression are inhibited by GW9662. (a) PPARγ agonists, but not PPARα or β/δ agonists, induce PGE2 production in PHKs. PHKs were treated for 24 hours with vehicle (CONT), 5 μM of the PPARγ agonist, ciglitazone (CIG), 1 μM of the endogenous PPARγ agonist, azPC, a PPARα agonist (WY-14,643, 1 μM), or a PPARβ/δ agonist (GW501516, 500 nM). The media were then removed and PGE2 was quantitated in the culture media using a commercial PGE2 EIA kit. Values represent the mean ± SEM of PGE2 levels as a percent of control levels (N = 5 experiments done in triplicate). *P < .05, one sample t-test. (b) GW9662 inhibits PPARγ agonist-induced PGE2 production in PHKs but has no significant effect on vehicle-treated cells. PHKs were pretreated for 1 hour with 1 μM GW9662 prior to addition of vehicle (VEH), ciglitazone (5 μM), or azPC (1 μM) for 24 hours. PGE2 was then quantitated in tissue culture supernatants. The results shown represent the percent inhibition of vehicle or agonist-induced PGE2 formation by GW9662. Mean and SEM. **P < .01, one-sample t-test. (c) GW9662 treatment inhibits ciglitazone-induced COX-2 mRNA expression. PHKs were treated with vehicle and ciglitazone, with and without GW9662, as detailed in Figure 2(b) above. At 2, 4, 8, and 24 hours, total RNA was isolated and quantitative RT-PCR was performed for COX-2 mRNA and 18S rRNA expression. The results are shown as COX-2 expression normalized to 18S and expressed as a fold change relative to VEH control (assigned a value of 1 for all time points). When data for all time points was analyzed, only ciglitazone-treated cells exhibited a significant induction in COX-2 expression (one-sample t-test, P = .0101).
The above studies suggest that PPARγ, but not PPARα or PPARβ/δ, is coupled to PGE2 production in PHKs. To determine whether ciglitazone- and azPC-dependent PGE2 production was specific to PPARγ, we next examined the ability of GW9662 to block PPARγ agonist-induced PGE2 production. As shown in Figure 2(b), pretreatment of PHKs with 1 μM GW9662 resulted in a significant inhibition of both ciglitazone- and azPC-induced PGE2 production. However, this inhibition was not complete: GW9662 inhibited ciglitazone- and azPC-induced PGE2 production by 59% and 62%, respectively. In contrast, GW9662 had no significant effect on vehicle-treated keratinocytes. Inasmuch as this same dose of GW9662 completely inhibited ciglitazone-induced PGE2 formation in SZ95 sebocytes [5], this data suggests that PGE2 production induced by azPC and ciglitazone occurs through both PPARγ-dependent and -independent mechanisms in PHKs.
As noted above, the ability of NS398 to inhibit basal and PPARγ agonist-induced PGE2 production suggests that PPARγ agonists induce expression of COX-2. We have previously demonstrated that PPARγ is coupled to UVB- and tert-butylhydroperoxide-mediated COX-2 expression in SZ95 sebocytes. This idea is supported by studies in SZ95 sebocytes in which the PPARγ antagonist GW9662 completely blocks PGE2 formation induced by ciglitazone, UVB, and TBH [5]. Moreover, the COX-2 promoter is known to contain putative peroxisome proliferators response elements (PPREs) [23]. Thus, we next examined the ability of ciglitazone to induce COX-2 expression in PHKs. In Figure 2(c), we show that COX-2 mRNA expression is induced from 2 to 24 hours after ciglitizone addition. Moreover, GW9662 treatment suppressed COX-2 induction.
3.3. PPARγ Mediates UVB and Oxidative Stressor-Induced PGE2 Production
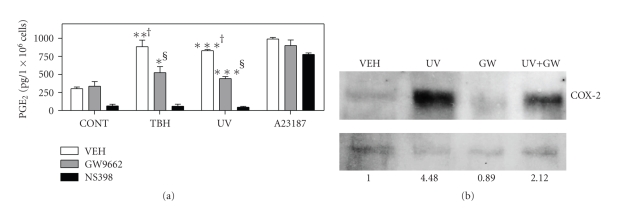
We have previously demonstrated that UVB exposure activates PPARγ via the production of oxidized lipid species in KB epidermal carcinoma cells and SZ95 sebocytes [4, 5]. Moreover, UVB-induced PPARγ activation is necessary for optimal UVB-mediated COX-2 expression or PGE2 production in both KB cells and SZ95 sebocytes [4, 5]. Finally, the lipid soluble oxidant, TBH, also induces COX-2 and PGE2 production in SZ95 sebocytes through a PPARγ-dependent mechanism [5]. We therefore sought to establish the importance of PPARγ in photobiology by demonstrating that PPARγ antagonism could alter UVB-mediated induction of PGE2 production in PHKs. As shown in Figure 3, both UVB and the lipid soluble oxidant TBH were capable of inducing a marked increase in PGE2 production in PHKs. Importantly, pretreatment with the PPARγ-specific antagonist, GW9662, blocked 68% of the TBH and 80% of the UVB-mediated increases in PGE2. The ability of the selective COX-2 inhibitor, NS398, to inhibit PGE2 formation was utilized as a negative control. As expected, NS398 markedly suppressed basal and UVB-induced PGE2 production. The calcium ionophore, A23187, which is a potent activator of both COX-2 and upstream phospholipases, was utilized as an irrelevant positive control. As expected, GW9662 had no affect on ionophore-induced PGE2.
Figure 3.
The specific PPARγ antagonist GW9662 blocks UVB- and TBH-induced PGE2 production and UVB-induced COX-2 protein expression. (a) GW9662 inhibits UVB- and TBH-induced PGE2 production in PHKs. PHKs were pretreated with vehicle control (VEH), GW9662 (1 μM) for 1 hour, or the COX-2 inhibitor NS398 (10 μM) for 30 minutes, then treated with 10 μM tert-butylhydroperoxide (TBH), 500 nM calcium ionophore A23187, or exposed to 600 J/m2 UVB irradiation (UV), respectively. PGE2 production was assayed in the tissue culture supernatants after 8 hours and was normalized to tissue weight. The values of PGE2 expression shown are mean ± SEM and are representative of three experiments (*P < .05, **P < .01, ***P < .001 by t-test compared with nonirradiated vehicle control †, or from the respective UV- or TBH-treated cells without GW9662 pretreatment §). (b) GW9662 inhibits UVB-induced COX-2 expression by immunoblot. PHKs were pretreated with vehicle (VEH) or 1 μM GW9662 (GW) 1 hour prior to irradiation with 300 J/m2 UVB (UV). After 20 hours, total cellular protein was isolated and immunoblots were performed for COX-2 expression (a) as detailed in the methods section. (b) shows a high MW nonspecific invariant band used to normalize for COX-2 expression. After film exposure, band intensities were assessed using NIH Image J. Normalized band intensities are shown at the bottom as a relative increase from VEH control cells.
Finally, we examined the ability of GW9662 to inhibit UVB-induced COX-2 expression. In Figure 3(b), we show a representative immunoblot demonstrating that GW9662 strongly inhibits UVB-induced COX-2 expression (mean suppression of 61.5% in two separate experiments).
3.4. UVB-Induced PGE2 Production Occurs via PPARγ in Human Epidermal Explants
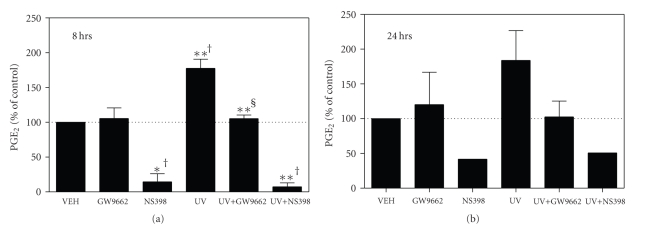
The above studies indicate that PPARγ antagonism suppresses UVB- and TBH-induced PGE2 production and COX-2 expression in cultured PHKs in vitro. We next sought to determine whether PPARγ antagonism could alter UVB-induced PGE2 formation in intact human skin ex vivo. In Figure 4(a), we show that skin explants exposed to 1,800 J/m2 of UVB exhibited a significant increase in PGE2 production at 8 hours. Importantly, UVB-induced PGE2 production was nearly completely blocked by pretreatment with 1 μM GW9662. A similar induction of PGE2 production was observed 24 hours after UVB irradiation, which was also blocked by pretreatment with GW9662 (Figure 4(b)). Finally, as a negative control, we show that the COX-2 inhibitor, NS398, suppresses both control and irradiated PGE2 levels. These studies show that GW9662 is effective topically and also verifies the importance of PPARγ in mediating a photobiological response in intact human skin.
Figure 4.
PPARγ activation is a potent inducer of PGE2 production in human epidermal explants. Human epidermal explants were prepared as described in the methods section and placed into K-SFM media (Invitrogen). Prior to UVB (UV) irradiation, the explants were pretreated with 1 μM GW9662 for 1 hour or 10 μM NS398 for 30 minutes. The explants were then washed once in PBS and irradiated with 1800 J/m2 UVB. Media containing vehicle (CONT), 1 μM GW9662, or 10 μM NS398 were then added back and the explants were cultured for 8 hours (a) or 24 hours (b). Tissue culture supernatants were then removed for PGE2 quantitation by EIA. PGE2 results were normalized to tissue weight. Results are presented as a percent change from control PGE2 levels. Mean and SEM of 2–4 experiments were done in triplicate. (*P < .05, one-sample t-test compared with nonirradiated vehicle control †, or from UV-treated skin without GW9662 pretreatment §).
4. Conclusions
Inasmuch as the epidermis is constantly exposed to ultraviolet light and other oxidative stressors, it is not surprising that epidermal cells would have developed intracellular mechanisms to detect and respond to these stresses. Among the many cellular signaling pathways that are induced by UV light, COX-2 induction and PGE2 production have been well described [5, 23, 24]. We have recently demonstrated that UVB induces PGE2 synthesis via the production of oxidative-stress-induced PPARγ-specific ligands in other cell types [4, 5]. Thus, PPARγ may serve as a cellular “signal transducer” that converts oxidative stress into cellular responses. In this study, we demonstrate that PPARγ agonists, including ciglitazone and azPC, are capable of inducing PGE2 production in cultured PHKs. We then demonstrate that UVB- and TBH-induced PGE2 production in human epidermis ex vivo and cultured PHKs in vitro occurs via a PPARγ-dependent mechanism. The role of COX-2 in PGE2 production in PHKs was verified by demonstrating that GW9662 inhibits ciglitazone-induced COX-2 mRNA expression as well as UVB-induced COX-2 protein expression. Collectively, these findings strongly suggest that PPARγ acts as a photosensor (and likely an oxidative stress sensor) that acts to translate the insult into a biochemical signaling cascade.
Previous work has generated conflicting data regarding the functional relevance of PPARγ in epidermal and keratinocyte biology. In mice, one group failed to observe PPARγ expression in cultured SKH-1 hairless mouse keratinocytes by RT-PCR [8]. In yet another study, PPARγ was observed to be expressed at the mRNA level in human primary keratinocytes, yet functional PPARγ activity was not observed using a PPRE-luciferase reporter assay [19]. However, another group demonstrated that PPARγ was expressed in keratinocytes isolated from SENCAR mice using immunoblot analysis [25]. Yet another study using PPARγ conditional knockout mice demonstrated that PPARγ is involved in epidermal differentiation [26]. In our studies, we demonstrate that PPARγ is expressed at the protein level in primary human keratinocytes and that PPARγ is coupled to UVB- and TBH-induced PGE2 production. Our findings that PPARγ mediates UVB- and TBH-induced PGE2 production provide additional evidence that PPARγ is functionally relevant to human cutaneous biology and, in particular, is relevant to human keratinocyte photobiology. This is particularly important given previous work by our group and others demonstrating that endogenous PPARγ ligand formation is induced by oxidative stress, including UVB [4, 5, 27, 28].
While our studies indicate a clear role for PPARγ in regulating UVB- and TBH-induced PGE2 production in PHKs, previous studies have shown that PPARα and PPARβ/δ are also expressed in human epidermis and cultured keratinocytes [1, 20]. Though a PPARα agonist induced a small amount of PGE2 formation in PHKs (Figure 2(a)), our data does not support a role for either PPARα or PPARβ/δ as major mediators of increased PGE2 production in PHKs.
Recent studies have demonstrated that mice with hemizygous germline loss of PPARγ and epidermal-specific loss of PPARγ exhibit increased susceptibility to cutaneous chemical carcinogenesis [13, 14]. This data indicates a possible role for PPARγ as a tumor-suppressing agent in skin. Studies are currently underway to examine the potential role of PPARγ in cutaneous photocarcinogenesis. It should be noted that recent studies by He et al. (2005) indicate that topical or systemic administration of TZD-type PPARγ agonists have no significant effect on chemical (DMBA/phorbol ester) or UVB-mediated carcinogenesis [12]. There could be several explanations for this discrepancy. First, we and others have previously shown that UVB or other oxidative stressors induce PPARγ ligand production from cellular glycerophosphocholines [4, 7]. Moreover, phorbol esters act to induce oxidative stress, including the production of oxidized lipids in epidermis [29, 30]. Thus, it is quite possible that PPARγ receptors are already engaged by endogenous PPARγ ligands produced through phospholipid oxidation, thus mitigating the effects of exogenous ligand. Alternatively, a followup study by He et al. (2006) indicated that TZD-type PPARγ agonists induce COX-2 expression in a keratinocyte cell line lacking PPARγ [22]. The authors speculate that this PPARγ-independent production of protumorigenic COX-2 may counteract any tumor-suppressing function of PPARγ. This PPARγ-independent induction of COX-2 would also explain the results seen in Figures 2 and 3, in which GW9662 was unable to completely abolish PPARγ agonist or UVB-induced PGE2 production and COX-2 expression. Finally, the differences could potentially be explained by variations in the murine genetic background or carcinogenesis protocol.
In addition to a potential role for PPARγ in cutaneous photocarcinogenesis, independent lines of evidence suggest that PPARγ should be a focus for future studies examining its potential role as a mediator of phototherapeutic responses. Recently, PPARγ agonists have been shown to be effective antipsoriatic agents by reducing the keratinocyte hyperplasia associated with psoriasis [31]. This is significant as UVB exposure is a well-known treatment option for patients with psoriasis. Since psoriasis is thought to occur as a result of deregulated T-cell function [32, 33], it is interesting that both PPARγ [34–36] and PGE2 [37–39] act as T-cell and dendritic cell immunosuppressants and that topical application of PGE2 has been shown to result in clinical improvement of psoriatic lesions [40]. Given our findings that UVB activates keratinocyte PPARγ, it is possible that some of the therapeutic benefits of UVB treatment may be due to PPARγ activation.
In conclusion, we provide evidence that PPARγ stimulates PGE2 formation in PHKs. Moreover, PPARγ mediates UVB- and TBH-induced PGE2 production in human epidermis and primary human keratinocytes. This suggests that PPARγ may play an important role in UVB- and oxidative-stress-induced changes in epidermal biology.
Acknowledgments
The authors gratefully acknowledge Ms. Jennifer Snider for her help in obtaining the human epidermis used in these studies. This work was partially supported by NIH K08 AR02150 (Raymond L. Konger), R03 AR053710 (Raymond L. Konger), HL062996 (Raymond L. Konger, Jeffrey B. Travers), and U19 A1070448 (Jeffrey B. Travers) Grants. Raymond L. Konger is also supported by an Indiana University-Purdue University Indianapolis Research Support Fund Grant, a Clarian Values Fund for Research Grant, and a Prevent Cancer Foundation Grant. Jeffrey B. Travers is also supported by a VA Merit Award Grant.
References
- 1.Kuenzli S, Saurat J-H. Peroxisome proliferator-activated receptors in cutaneous biology. British Journal of Dermatology. 2003;149(2):229–236. doi: 10.1046/j.1365-2133.2003.05532.x. [DOI] [PubMed] [Google Scholar]
- 2.Friedmann PS, Cooper HL, Healy E. Peroxisome proliferator-activated receptors and their relevance to dermatology. Acta Dermato-Venereologica. 2005;85(3):194–202. doi: 10.1080/00015550510030104. [DOI] [PubMed] [Google Scholar]
- 3.Michalik L, Wahli W. Peroxisome proliferator-activated receptors (PPARs) in skin health, repair and disease. Biochimica et Biophysica Acta. 2007;1771(8):991–998. doi: 10.1016/j.bbalip.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Southall MD, Mezsick SM, et al. Epidermal peroxisome proliferator-activated receptor γ as a target for ultraviolet B radiation. The Journal of Biological Chemistry. 2005;280(1):73–79. doi: 10.1074/jbc.M409795200. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Seltmann H, Zouboulis CC, Konger RL. Involvement of PPARγ in oxidative stress-mediated prostaglandin E2 production in SZ95 human sebaceous gland cells. Journal of Investigative Dermatology. 2006;126(1):42–48. doi: 10.1038/sj.jid.5700028. [DOI] [PubMed] [Google Scholar]
- 6.Hsi LC, Wilson LC, Eling TE. Opposing effects of 15-lipoxygenase-1 and -2 metabolites on MAPK signaling in prostate: alteration in peroxisome proliferator-activated receptor γ . The Journal of Biological Chemistry. 2002;277(43):40549–40556. doi: 10.1074/jbc.M203522200. [DOI] [PubMed] [Google Scholar]
- 7.Davies SS, Pontsler AV, Marathe GK, et al. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor γ ligands and agonists. The Journal of Biological Chemistry. 2001;276(19):16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 8.He G, Thuillier P, Fischer SM. Troglitazone inhibits cyclin D1 expression and cell cycling independently of PPARγ in normal mouse skin keratinocytes. Journal of Investigative Dermatology. 2004;123(6):1110–1119. doi: 10.1111/j.0022-202X.2004.23465.x. [DOI] [PubMed] [Google Scholar]
- 9.Bae M-A, Rhee H, Song BJ. Troglitazone but not rosiglitazone induces G1 cell cycle arrest and apoptosis in human and rat hepatoma cell lines. Toxicology Letters. 2003;139(1):67–75. doi: 10.1016/s0378-4274(02)00468-x. [DOI] [PubMed] [Google Scholar]
- 10.Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) ligand, selectively induces the early growth response-1 gene independently of PPARγ: a novel mechanism for its anti-tumorigenic activity. The Journal of Biological Chemistry. 2003;278(8):5845–5853. doi: 10.1074/jbc.M208394200. [DOI] [PubMed] [Google Scholar]
- 11.Leesnitzer LM, Parks DJ, Bledsoe RK, et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41(21):6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 12.He G, Muga S, Thuillier P, Lubet RA, Fischer SM. The effect of PPARγ ligands on UV- or chemically-induced carcinogenesis in mouse skin. Molecular Carcinogenesis. 2005;43(4):198–206. doi: 10.1002/mc.20111. [DOI] [PubMed] [Google Scholar]
- 13.Nicol CJ, Yoon M, Ward JM, et al. PPARγ influences susceptibility to DMBA-induced mammary, ovarian and skin carcinogenesis. Carcinogenesis. 2004;25(9):1747–1755. doi: 10.1093/carcin/bgh160. [DOI] [PubMed] [Google Scholar]
- 14.Indra AK, Castaneda E, Antal MC, et al. Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor α in epidermal keratinocytes. Journal of Investigative Dermatology. 2007;127(5):1250–1260. doi: 10.1038/sj.jid.5700672. [DOI] [PubMed] [Google Scholar]
- 15.Konger RL, Malaviya R, Pentland AP. Growth regulation of primary human keratinocytes by prostaglandin E receptor EP2 and EP3 subtypes. Biochimica et Biophysica Acta. 1998;1401(2):221–234. doi: 10.1016/s0167-4889(97)00114-6. [DOI] [PubMed] [Google Scholar]
- 16.Dickson MA, Hahn WC, Ino Y, et al. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Molecular and Cellular Biology. 2000;20(4):1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specffic knockout and agonist of peroxisome proliferator-activated receptor-γ both induce cardiac hypertrophy in mice. Circulation Research. 2005;97(4):372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 18.ABI. Relative quantitation of gene expression. User Bulletin #2: ABI Prism 7700 Sequence Detection System, Applied Biosciences, 1997.
- 19.Rivier M, Safonova I, Lebrun P, Griffiths CEM, Ailhaud G, Michel S. Differential expression of peroxisome proliferator-activated receptor subtypes during the differentiation of human keratinocytes. Journal of Investigative Dermatology. 1998;111(6):1116–1121. doi: 10.1046/j.1523-1747.1998.00439.x. [DOI] [PubMed] [Google Scholar]
- 20.Westergaard M, Henningsen J, Svendsen ML, et al. Modulation of keratinocyte gene expression and differentiation by PPAR-selective ligands and tetradecylthioacetic acid. Journal of Investigative Dermatology. 2001;116(5):702–712. doi: 10.1046/j.1523-1747.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- 21.Jiang YJ, Kim P, Elias PM, Feingold KR. LXR and PPAR activators stimulate cholesterol sulfotransferase type 2 isoform 1b in human keratinocytes. Journal of Lipid Research. 2005;46(12):2657–2666. doi: 10.1194/jlr.M500235-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.He G, Sung YM, Fischer SM. Troglitazone induction of COX-2 expression is dependent on ERK activation in keratinocytes. Prostaglandins Leukotrienes and Essential Fatty Acids. 2006;74(3):193–197. doi: 10.1016/j.plefa.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Pontsler AV, St Hilaire A, Marathe GK, Zimmerman GA, McIntyre TM. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor γ and oxidized alkyl phospholipids from oxidized low density lipoprotein. The Journal of Biological Chemistry. 2002;277(15):13029–13036. doi: 10.1074/jbc.M109546200. [DOI] [PubMed] [Google Scholar]
- 24.Ashida M, Bito T, Budiyanto A, Ichihashi M, Ueda M. Involvement of EGF receptor activation in the induction of cyclooxygenase-2 in HaCaT keratinocytes after UVB. Experimental Dermatology. 2003;12(4):445–452. doi: 10.1034/j.1600-0625.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 25.Thuillier P, Anchiraico GJ, Nickel KP, et al. Activators of peroxisome proliferator-activated receptor-α partially inhibit mouse skin tumor promotion. Molecular Carcinogenesis. 2000;29(3):134–142. doi: 10.1002/1098-2744(200011)29:3<134::aid-mc2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Mao-Qiang M, Fowler AJ, Schmuth M, et al. Peroxisome-proliferator-activated receptor (PPAR)-γ activation stimulates keratinocyte differentiation. Journal of Investigative Dermatology. 2004;123(2):305–312. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- 27.Konger RL, Marathe GK, Yao Y, Zhang Q, Travers JB. Oxidized glycerophosphocholines as biologically active mediators for ultraviolet radiation-mediated effects. Prostaglandins and Other Lipid Mediators. 2008;87(1–4):1–8. doi: 10.1016/j.prostaglandins.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies SS, Pontsler AV, Marathe GK, et al. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor γ ligands and agonists. The Journal of Biological Chemistry. 2001;276(19):16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura Y, Kozuka M, Naniwa K, et al. Arachidonic acid cascade inhibitors modulate phorbol ester-induced oxidative stress in female ICR mouse skin: differential roles of 5-lipoxygenase and cyclooxygenase-2 in leukocyte infiltration and activation. Free Radical Biology and Medicine. 2003;35(9):997–1007. doi: 10.1016/s0891-5849(03)00440-4. [DOI] [PubMed] [Google Scholar]
- 30.Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004;25(4):549–557. doi: 10.1093/carcin/bgh034. [DOI] [PubMed] [Google Scholar]
- 31.Kuenzli S, Saurat J-H. Peroxisome proliferator-activated receptors as new molecular targets in psoriasis. Current Drug Targets: Inflammation & Allergy. 2004;3(2):205–211. doi: 10.2174/1568010043343976. [DOI] [PubMed] [Google Scholar]
- 32.Bachelez H. Immunopathogenesis of psoriasis: recent insights on the role of adaptive and innate immunity. Journal of Autoimmunity. 2005;25(supplement):69–73. doi: 10.1016/j.jaut.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 33.Rotsztejn H, Zalewska A, Trznadel-Budzko E, et al. Influence of systemic photochemotherapy on regulatory T cells and selected cytokine production in psoriatic patients: a pilot study. Medical Science Monitor. 2005;11(12):CR594–CR598. [PubMed] [Google Scholar]
- 34.Genolet R, Wahli W, Michalik L. PPARs as drug targets to modulate inflammatory responses? Current Drug Targets: Inflammation & Allergy. 2004;3(4):361–375. doi: 10.2174/1568010042634578. [DOI] [PubMed] [Google Scholar]
- 35.Klotz L, Dani I, Edenhofer F, et al. Peroxisome proliferator-activated receptor γ control of dendritic cell function contributes to development of CD4+ T cell anergy. Journal of Immunology. 2007;178(4):2122–2131. doi: 10.4049/jimmunol.178.4.2122. [DOI] [PubMed] [Google Scholar]
- 36.Bright JJ, Natarajan C, Muthian G, Barak Y, Evans RM. Peroxisome proliferator-activated receptor-γ-deficient heterozygous mice develop an exacerbated neural antigen-induced Th1 response and experimental allergic encephalomyelitis. Journal of Immunology. 2003;171(11):5743–5750. doi: 10.4049/jimmunol.171.11.5743. [DOI] [PubMed] [Google Scholar]
- 37.Fujimoto Y, Iwagaki H, Ozaki M, et al. Involvement of prostaglandin receptors (EPR2-4) in in vivo immunosuppression of PGE2 in rat skin transplant model. International Immunopharmacology. 2005;5(7-8):1131–1139. doi: 10.1016/j.intimp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Gorman S, Tan JW-Y, Thomas JA, et al. Primary defect in UVB-induced systemic immunomodulation does not relate to immature or functionally impaired APCs in regional lymph nodes. Journal of Immunology. 2005;174(11):6677–6685. doi: 10.4049/jimmunol.174.11.6677. [DOI] [PubMed] [Google Scholar]
- 39.Pockaj BA, Basu GD, Pathangey LB, et al. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Annals of Surgical Oncology. 2004;11(3):328–339. doi: 10.1245/aso.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 40.Remy W, Sigl I, Leipold B. Prostaglandin E2 gel improvement of psoriatic lesions. International Journal of Dermatology. 1986;25(4):266–268. doi: 10.1111/j.1365-4362.1986.tb02240.x. [DOI] [PubMed] [Google Scholar]