Abstract
Setting
Detection of smear-positive pulmonary tuberculosis (PTB) cases is vital for tuberculosis control. Methods to augment sputum collection are available but their additional benefit is uncertain in resource-limited settings.
Objective
To compare the diagnostic yields using five methods to obtain sputum from adults diagnosed with smear-negative PTB in Malawi.
Design
Self-expectorated sputum was collected under supervision for microscopy and mycobacterial culture in the study laboratory. Confirmed smear-negative patients, provided physiotherapy-assisted sputum and induced sputum followed, the next morning, by gastric washing and bronchoalveolar-lavage samples.
Results
150 patients, diagnosed with smear-negative PTB by the hospital service, were screened. 39 (26%) were smear-positive from supervised self-expectorated sputum examined in the study laboratory. The remaining 111 confirmed smear-negative patients were enrolled; 89% were HIV positive. Seven additional smear-positive cases were diagnosed using the augmented sputum collection techniques. No differences were observed in the numbers of cases detected using the different methods. 44 (95.6%) of the 46 smear-positive cases could be detected from self-expectorated and physiotherapy-assisted samples
Conclusions
For countries like Malawi, the best use of limited resources to detect smear-positive PTB cases would be to improve the quality of self-expectorated sputum collection and microscopy. The additional diagnostic yield using bronchoalveolar-lavage after induced sputum is limited.
Keywords: Induced sputum, gastric washings, physiotherapy, BAL, HIV
INTRODUCTION
The HIV pandemic has caused an exponential increase in the number of patients diagnosed with smear-negative tuberculosis (TB) in Africa 1. In Malawi, patients are diagnosed with smear-negative pulmonary tuberculosis (PTB) on the basis of cough for more than three weeks, failure to respond to standard antibiotics, an abnormal chest X-ray (where available), and negative microscopy for acid-fast bacilli (AFB) from three self-expectorated sputum samples collected over at least two days. Smear-negative PTB patients have worse outcomes in Malawi than smear positive patients and this is partly due to patients being treated for TB who do not have PTB but another form of HIV-related lung disease 2.
Using sputum microscopy and mycobacterial culture, we evaluated potential improvements in diagnostic yield using augmented methods of sputum sample collection. We compared self-expectorated sputum with physiotherapy-assisted sputum, gastric washing (GW), induced sputum (IS) and bronchoalveolar-lavage (BAL). Physiotherapy-assisted sputum collection is a simple method requiring only staff training but no sophisticated equipment and could be readily adopted in resource-limited settings.
STUDY POPULATION AND METHODS
Subjects and setting
The study took place at Queen Elizabeth Central Hospital, Blantyre, Malawi from June 2005 until August 2006. Adult inpatients, clinically diagnosed with smear negative PTB but not yet on anti-TB treatment, and with three recent self-expectorated sputa reported as smear-negative by direct smear examination from the hospital laboratory, were invited to participate in the study. Potential study recruits were identified at the point of registering for smear-negative PTB treatment or after referral from other clinicians in the department. Patients with pleural effusions, ascites or asthma and patients with oxygen saturations of <94% on air were excluded. The study had ethical approval from the Research Ethics Committees of the College of Medicine, Malawi and the Liverpool School of Tropical Medicine, UK.
Sample collection and study design
A satisfactory self-expectorated sputum sample (mucoid and not salivary) was collected under direct observation from all subjects for examination in the research laboratory. If this sputum sample was reported AFB positive, the patient was excluded from further investigation. Confirmed smear-negative patients who gave informed consent were enrolled and physiotherapy-assisted sputum and IS samples were collected. Clinical data was collected including HIV status (if known) and past medical and current medical histories. For physiotherapy-assisted sputum collection, the patients were instructed to take deep breaths while a clinician clapped the chest wall with their cupped hands. This was followed by shaking of the patient's chest for approximately five minutes. Finally, the patient was asked to blow out against pursed lips. At any point during this process, if the patient started coughing and a satisfactory sample was produced, the procedure was stopped. IS was collected using standard techniques 3. Briefly, a Mistogen EN145 ultrasonic nebuliser was used containing 15-20ml of sterile 3% saline solution. The patient breathed through the nebuliser for 10-20 minutes or until a satisfactory sample was produced.
GW were collected the following morning before the patient ingested food or drink. A 14-gauge nasogastric tube was passed and gastric aspiration performed with or without a 20ml saline flush depending on the volume of aspirate collected. Immediately following this, bronchoscopy was performed as previously described 4. Briefly, topical lignocaine was used and BAL collected from the right middle lobe using 50ml aliquots of sterile normal saline repeated to a maximum volume of 200mls flush or until 50mls of fluid had been aspirated. Patients were reviewed once all the microscopy results were available and appropriate treatment was prescribed by the clinician responsible for their care. There was no further follow up within the study.
Mycobacterial microscopy and culture
BAL and GW samples were centrifuged at 3000 rpm for 15 minutes and the supernatant discarded. Sputum obtained by self-expectoration, physiotherapy-assistance and IS and the GW and BAL deposits were first decontaminated by an equal volume of 4% sodium hydroxide treatment. This mixture was vortex agitated and left at room temperature for 15 minutes (and also vortexed at 10 minutes). After 15 minutes, the mixture was vortexed then centrifuged at 3000 rpm for 15 minutes and the supernatant discarded. Slides were prepared from the deposits and stained using the Ziehl-Neelson method for microscopy. Two drops of deposit were inoculated on to Lowenstein-Jensen slopes, one acid-egg slope and one acid-egg + pyruvate slope for each specimen. These slopes were incubated in air at 37°C for up to eight weeks. The slopes were checked each week for signs of colony growth.
Statistical methods
We planned to recruit a total of 150 patients as previous studies showed that a third of smear-negative patients would be microbiologically confirmed as PTB 5. Fifty confirmed PTB patients (confidence interval 25-41) would allow a statistically significant difference between different diagnostic methods to be shown by a halving of the number of cases detected (25 cases; CI 11-23) using 95% confidence intervals calculated with Stata version 9 (StataCorp, TX, USA).
Quality control
External quality assurance of microscopy was performed at the Liverpool School of Tropical Medicine on 100 consecutive slides and all positive slides. The reader of the 100 consecutive slides was blinded to the results. Mycobacterial speciation was carried out on positive cultures transported to the UK.
RESULTS
One hundred and fifty-four patients, clinically diagnosed with smear-negative PTB, were screened for the study and four declined to take part. The remaining 150 patients provided an observed self-expectorated sputum sample. Thirty-nine (26%) of these patients were diagnosed with smear-positive PTB on this sample. The remaining 111 confirmed smear-negative patients consented to invasive techniques: mean age was 36 years (range 18-71), 58% were male and 84 (75.6%) patients knew their HIV status. Seventy-five (89%) were HIV positive and nearly all had stage 3 or 4 disease. All recruits had recent AFB negative self-expectorated sputum results from the hospital laboratory; median (range) time from negative result to recruitment was 4.5 (1-21) days.
Using the augmented sputum collection methods and mycobacterial culture, 18 of the 111 patients recruited had the diagnosis of smear-positive and/or culture-positive PTB confirmed. Clinical features, including age, past history of TB, HIV prevalence and symptoms were similar between these 18 patients and the 93 patients without a confirmed diagnosis of PTB. Patients reporting a household TB contact were more likely to have a confirmed diagnosis of PTB; odds ratio 3.4 (CI 1.16-10), p=0.017. Nine of the 18 diagnoses could be made by culture of the self-expectorated sputum sample. Inclusion of the augmented sputum collection methods yielded only an additional nine PTB diagnoses. Physiotherapy-assisted sputum detected 5/9 of the new cases, IS 4/9, GW 4/9 and BAL 2/9. Fifteen of the 18 (83.3%) smear and/or culture positive patients were diagnosed without the need for GW or BAL.
Many resource-limited countries rely only on sputum microscopy to diagnose PTB and mycobacterial culture is unavailable. Using the augmented collection techniques, seven additional smear-positive cases were diagnosed from the 111 confirmed smear-negative patients. Physiotherapy-assisted sputum detected 5/7 of these new smear-positive cases, IS 4/7, GW 3/7 and BAL 3/7. Two of the five new smear-positive cases detected from physiotherapy-assisted sputum were also culture-positive from self-expectorated sputum.
Diagnoses by sample collection method
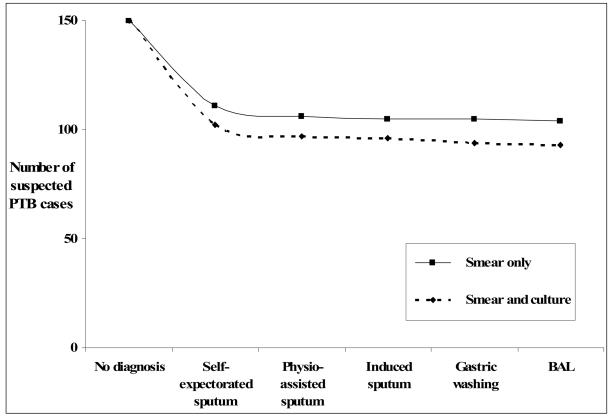
Figure 1 shows the additional diagnostic gain using samples obtained by the different collection methods used in order of increasingly invasiveness; first self-expectoration, then physiotherapy-assisted, IS, GW and finally BAL. Two lines are plotted, one showing only the smear results and the other the result when the smear and culture results were considered together. Table 1 shows the numbers of each procedure that were done on the 111 patients who underwent invasive sampling (having proved smear-negative on repeat self-expectorated sample) and the numbers of smear-positive and smear and/or culture positive results (with 95% CI) obtained using each method. We were unable to perform all the techniques in all 111 patients due to equipment and/or staff limitations (two physiotherapy-assisted, five GW, and 18 BAL), lack of consent (13 GW, 14 BAL) and poor patient health (three GW, eight BAL). There were no significant differences in the detections rates using the different methods, the 95% CI overlapped for each method. KS lesions were observed in the bronchial trees of six of the 71 (8.5%) patients who had bronchoscopy and none of these patients had positive TB results.
Figure 1.
Number of confirmed diagnoses of PTB by smear (——) and smear and culture (----) using samples are obtained by increasingly invasive techniques
TABLE 1.
Numbers of each procedure performed and of smear-positive and smear and/or culture-positive samples (with 95% CI) obtained from the 111 patients who proceeded to invasive sampling (39 patients found to be smear-positive on repeat self-expectorated sputum not included).
| Self- expectorated sputum |
Physiotherapy assisted |
Induced sputum |
Gastric washings |
BAL | |
|---|---|---|---|---|---|
|
| |||||
|
No. of Patients |
111 | 109 | 111 | 91 | 71 |
|
| |||||
|
No. (%) Smear- Positive |
0 | 5 (4.6%) | 4 (3.6%) | 3 (3.3%) | 3 (4.2%) |
| 95% CI | 1.5 - 10.4 | 0.9 - 9.0 | 0.6 - 9.3 | 0.9 - 11.9 | |
|
| |||||
|
No. (%) Smear and/or Culture- Positive |
9 (8.1%) | 10 (9.2%) | 13 (11.7%) | 10 (11%) | 6 (8.5%) |
| 95% CI | 3.8 - 14.8 | 4.4-16.2 | 6.4-19.1 | 5.4-19.3 | 3.2-17.5 |
All the slides reported as positive by the study microscopist were confirmed positive when re-examined in Liverpool and no new positive slides were identified. Mycobacterial cultures from 6 of the culture positive patients were sent for speciation. All 6 were confirmed Mycobacterium Tuberculosis Complex.
DISCUSSION
Case detection of tuberculosis patients by smear microscopy is a vital part of TB control strategy, especially for an HIV-endemic country like Malawi 6. Several methods are available to obtain samples in patients with suspected PTB though the availability of these varies between poorer countries and richer countries. We compared diagnostic yields from sputum obtained using increasingly invasive methods in patients diagnosed clinically with smear-negative PTB. We found no significant differences in the detections rates using the different methods.
Fifty-seven (38.7%) of the 150 patients diagnosed with smear-negative PTB by the hospital service had a final smear and/or culture confirmed diagnosis of PTB, and this figure is in keeping with previous studies from Africa 5. Simply by improving the quality of self-expectorated sputum collection and microscopy, we detected 39 (26%) smear-positive cases. An additional seven smear-positive cases were detected using the augmented sputum collection methods. In total, 46 smear-positive cases were found, and 44 (95.7%) of these could be detected using self-expectorated and physiotherapy-assisted samples only. This is encouraging for a setting such as Malawi as more invasive or sophisticated methods of sputum collection are rarely available.
Using mycobacterial culture and the augmented sputum collection methods, we diagnosed smear-positive and/or culture-positive PTB in 18 (16.2%) of the 111 confirmed smear-negative patients. 54/57 (93%) of all the confirmed smear-positive and/or culture-positive PTB cases were detected without the need for GW and BAL. Figure 1 illustrates that in this patient group, the additional diagnostic yield of confirmed PTB cases diminishes sharply as more invasive and resource demanding investigations are used.
For 93 patients we did not establish a confirmed final diagnosis for their chronic coughs. Other common causes in HIV positive adults in this setting include Pneumocystis jiroveci pneumonia (PcP), pulmonary Kaposi's sarcoma (KS), bacterial pneumonia and non-typhoidal salmonella infections. KS lesions were observed in the bronchial trees of six (8.5%) patients in the study who had bronchoscopy.
The study may have been underpowered to detect differences in case detection rates by the different methods of sputum collection and the number of confirmed PTB patients that we detected might have been higher had we performed IS more than once in each patient 7. In addition, BAL was performed only from the right middle lobe irrespective of the site of disease on CXR and this may have resulted in a lower yield.
Improving sputum quality has been shown previously to improve the yield of sputum microscopy. In a study from Pakistan, women given guidance on how to produce a good sputum sample prior to specimen submission were more likely to test smear positive than controls 8. In another study from Indonesia, patient instruction prior to sputum production resulted in a 15.1% higher detection rate compared to the control group 9. In addition to the quality of the sputum specimen obtained, other factors may have contributed to the increased diagnostic yield from the repeated self-expectorated samples. These include improved quality of microscopy and disease progression between the initial sputum sampling within the routine health system and the repeat sampling in the study. Sputum concentration using bleach and centrifugation prior to microscopy increases the sensitivity compared to direct smear microscopy 10; one study in an HIV positive cohort reporting an 11% increase in sensitivity 11. In this study, microscopy was performed in a research laboratory using concentrated smears, while the initial microscopy was performed using direct smears in a busy hospital laboratory where there a number of constraints which compromise the quality of smear microscopy 12. The median time from the negative sputum result from the hospital laboratory to recruitment was short, 4.5 days (range 1-21), so although disease progression would have been limited in most cases, this may have accounted for some of the additional smear-positive cases detected in the study.
Reports on the diagnostic yields by the different methods of sputum collection vary; one study from New Zealand showed that IS testing was more sensitive than BAL for detecting PTB 13, and another study found IS to be more sensitive than GW in young children in South Africa 14. IS has been shown previously to improve diagnosis of PTB in Malawian adults and children 3, 15. It has the advantage of not requiring hospital admission (as for GW) or sophisticated equipment and expertise (as for BAL) but does require equipment such as nebuliser that is not usually available. The IS technique usually includes chest physiotherapy and a study of South African children reported that chest physiotherapy alone was as effective as IS in diagnosing PTB (unpublished data, S Kara). Our results are consistent with a recent study which compared multiple IS and GW procedures and BAL for the diagnosis of PTB in 107 patients unable to self expectorate. The study took place in the UK and around 3.5% of the patients were HIV positive. The study concluded that the use of three IS samples was more sensitive than three GW samples in that patient group and that bronchoscopy and BAL did not increase diagnostic sensitivity 16.
Our data suggest that in countries such as Malawi that are endemic for TB/HIV, the best use of limited resources for detection of smear-positive PTB cases would be to improve the quality of self-expectorated sputum collection and microscopy. For countries like the UK, with mycobacterial culture facilities and ready access to IS or BAL, physicians should be aware of that the additional diagnostic yield using BAL after IS, in the investigation of suspected PTB, may be limited. In our study, simple chest physiotherapy techniques to assist sputum production resulted in 5 additional smear-positive diagnoses over and above those obtained by self-expectoration. Although this was not significantly more than the yield using the other methods (Table 1), physiotherapy requires no hypertonic saline or equipment and is non-invasive and further studies in sub-Saharan Africa to assess its possible role in an operational setting should be planned.
ACKNOWLEDGMENTS
Thanks to Shifa Kara, physiotherapist, for training staff in the techniques of chest physiotherapy and induced sputum. Thanks also to Richard Cooke, the study team Gersham Mwafulirwa, Rose Malamba, Neema Mthunkama, Marie Kunkeyani and George Musowa and the patients and staff at Queen Elizabeth Central Hospital, Malawi.
FUNDING
The study was funded by the Wellcome Trust (WT). DJB held a WT Training Fellowship in Clinical Tropical Medicine (no. 066681), SMG was a recipient of WT funding (core grant 074124/Z/04/Z), NF held a WT Career development Fellowship (no. 061230) and SBG held a WT Career development Fellowship (no. 061231). The Wellcome Trust had no role in the planning and execution of the study or in the data analysis and preparation of the manuscript.
Footnotes
CONFLICTS OF INTEREST STATEMENT
None of the authors declare any conflicts of interest.
References
- 1.Harries AD, Hargreaves NJ, Kemp J, et al. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet. 2001 May 12;357(9267):1519–23. doi: 10.1016/S0140-6736(00)04639-0. [DOI] [PubMed] [Google Scholar]
- 2.Hargreaves NJ, Kadzakumanja O, Whitty CJ, Salaniponi FM, Harries AD, Squire SB. ‘Smear-negative’ pulmonary tuberculosis in a DOTS programme: poor outcomes in an area of high HIV seroprevalence. Int J Tuberc Lung Dis. 2001 Sep;5(9):847–54. [PubMed] [Google Scholar]
- 3.Parry CM, Kamoto O, Harries AD, et al. The use of sputum induction for establishing a diagnosis in patients with suspected pulmonary tuberculosis in Malawi. Tuber Lung Dis. 1995 Feb;76(1):72–6. doi: 10.1016/0962-8479(95)90583-9. [DOI] [PubMed] [Google Scholar]
- 4.Gordon SB, Irving GR, Lawson RA, Lee ME, Read RC. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun. 2000 Apr;68(4):2286–93. doi: 10.1128/iai.68.4.2286-2293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hargreaves NJ, Kadzakumanja O, Phiri S, et al. What causes smear-negative pulmonary tuberculosis in Malawi, an area of high HIV seroprevalence? Int J Tuberc Lung Dis. 2001 Feb;5(2):113–22. [PubMed] [Google Scholar]
- 6.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006 Mar 18;367(9514):926–37. doi: 10.1016/S0140-6736(06)68383-9. [DOI] [PubMed] [Google Scholar]
- 7.Al Zahrani K, Al Jahdali H, Poirier L, Rene P, Menzies D. Yield of smear, culture and amplification tests from repeated sputum induction for the diagnosis of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2001 Sep;5(9):855–60. [PubMed] [Google Scholar]
- 8.Khan MS, Dar O, Sismanidis C, Shah K, Godfrey-Faussett P. Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: a pragmatic randomised controlled trial. Lancet. 2007 Jun 9;369(9577):1955–60. doi: 10.1016/S0140-6736(07)60916-7. [DOI] [PubMed] [Google Scholar]
- 9.Alisjahbana B, van CR, Danusantoso H, et al. Better patient instruction for sputum sampling can improve microscopic tuberculosis diagnosis. Int J Tuberc Lung Dis. 2005 Jul;9(7):814–7. [PubMed] [Google Scholar]
- 10.Steingart KR, Ng V, Henry M, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006 Oct;6(10):664–74. doi: 10.1016/S1473-3099(06)70602-8. [DOI] [PubMed] [Google Scholar]
- 11.Bruchfeld J, Aderaye G, Palme IB, Bjorvatn B, Kallenius G, Lindquist L. Sputum concentration improves diagnosis of tuberculosis in a setting with a high prevalence of HIV. Trans R Soc Trop Med Hyg. 2000 Nov;94(6):677–80. doi: 10.1016/s0035-9203(00)90230-x. [DOI] [PubMed] [Google Scholar]
- 12.Mundy CJ, Harries AD, Banerjee A, Salaniponi FM, Gilks CF, Squire SB. Quality assessment of sputum transportation, smear preparation and AFB microscopy in a rural district in Malawi. Int J Tuberc Lung Dis. 2002 Jan;6(1):47–54. [PubMed] [Google Scholar]
- 13.McWilliams T, Wells AU, Harrison AC, Lindstrom S, Cameron RJ, Foskin E. Induced sputum and bronchoscopy in the diagnosis of pulmonary tuberculosis. Thorax. 2002 Dec;57(12):1010–4. doi: 10.1136/thorax.57.12.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005 Jan 8;365(9454):130–4. doi: 10.1016/S0140-6736(05)17702-2. [DOI] [PubMed] [Google Scholar]
- 15.Shata AM, Coulter JB, Parry CM, Ching'ani G, Broadhead RL, Hart CA. Sputum induction for the diagnosis of tuberculosis. Arch Dis Child. 1996 Jun;74(6):535–7. doi: 10.1136/adc.74.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown M, Varia H, Bassett P, Davidson RN, Wall R, Pasvol G. Prospective study of sputum induction, gastric washing, and bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in patients who are unable to expectorate. Clin Infect Dis. 2007 Jun 1;44(11):1415–20. doi: 10.1086/516782. [DOI] [PubMed] [Google Scholar]