Abstract
The effectiveness of lung radiotherapy is limited by radiation tolerance of normal tissues and by the intrinsic radiosensitivity of lung cancer cells. The chemopreventive agent curcumin has known antioxidant and tumor cell radiosensitizing properties. Its usefulness in preventing radiation-induced pneumonopathy has not been tested previously. We evaluated dietary curcumin in radiation-induced pneumonopathy and lung tumor regression in a murine model. Mice were given 1%or 5%(w/w) dietary curcumin or control diet prior to irradiation and for the duration of the experiment. Lungs were evaluated at 3 weeks after irradiation for acute lung injury and inflammation by evaluating bronchoalveolar lavage (BAL) fluid content for proteins, neutrophils and at 4 months for pulmonary fibrosis. In a separate series of experiments, an orthotopic model of lung cancer using intravenously injected Lewis lung carcinoma (LLC) cells was used to exclude possible tumor radioprotection by dietary curcumin. In vitro, curcumin boosted antioxidant defenses by increasing heme oxygenase 1 (HO-1) levels in primary lung endothelial and fibroblast cells and blocked radiation-induced generation of reactive oxygen species (ROS). Dietary curcumin significantly increased HO-1 in lungs as early as after 1 week of feeding, coinciding with a steady-state level of curcumin in plasma. Although both 1% and 5% w/w dietary curcumin exerted physiological changes in lung tissues by significantly decreasing LPS-induced TNF-α production in lungs, only 5%dietary curcumin significantly improved survival of mice after irradiation and decreased radiation-induced lung fibrosis. Importantly, dietary curcumin did not protect LLC pulmonary metastases from radiation killing. Thus dietary curcumin ameliorates radiation-induced pulmonary fibrosis and increases mouse survival while not impairing tumor cell killing by radiation.
INTRODUCTION
The utility of thoracic radiotherapy is limited by the radiosensitivity of normal lung parenchyma (1). Clinically significant radiation lung injury occurs in up to 30% of patients irradiated for lung cancer (2) and in about 10–15% of other thoracic oncology patients (3). A far greater proportion of patients will have subclinical effects of radiation on the lung, identifiable by imaging and/or physiological testing (4).
Two phases of radiation lung injury have been described. Acute radiation pneumonopathy (pneumonitis) can occur from several weeks to 6 months postirradiation. If a large volume of lung has been affected, this phase can be life-threatening (5). In late radiation-induced lung injury, occurring months to years after irradiation, the number of inflammatory cells decreases and deposition of collagenous occurs, resulting in irreversible lung fibrosis. This late process has also been modeled in many different animal systems; C57/B6 mice appear to be especially susceptible to this fibrotic reaction (6).
Currently, the only way to decrease the chance of developing life-threatening radiation pneumonopathy is to minimize the amount of lung exposed to radiation. A safe and effective biological radioprotector would be extremely useful. Preclinical data suggest that antioxidant molecules and/or enzymes might protect the lung (7). Our group has recently shown that systemic administration of polyethylene glycol-conjugated antioxidant enzymes at the time of irradiation alters several early biomarkers of lung injury (Bax, p21 and TGF-β1), decreases apoptosis, and ameliorates late pulmonary fibrosis in a mouse model (8). A recent study by our group confirmed similar findings with dietary administration of flaxseed, a whole grain with potent anti-inflammatory and antioxidant properties (9).
Curcumin (diferuloylmethane) is a naturally occurring biphenolic compound contained in the spice tumeric. Curcumin has been found to have antioxidant, anti-inflammatory and anti-tumor activity in a variety of animal models of human diseases (10, 11). Madan et al. demonstrated that in systemic LPS-induced sepsis, curcumin inhibited the transmigration and infiltration of neutrophils from blood vessels to the underlying liver tissue, suppressing damage to the tissue (12), while other investigators reported that curcumin ameliorated ischemia-reperfusion renal injury (13) and alcohol-induced liver disease (14).
The mechanisms by which curcumin affects multiple biochemical and inflammatory conditions appear to be cell- and stimulus-specific and to involve effects on the cell’s transcriptional machinery (e.g., NF-κB, COX-2) and redox homeostasis. Curcumin has direct antioxidant activities (15), and it is a potent inhibitor of prostaglandin synthesis (16). It inhibits both activation of AP-1 and AP-1 binding (17) and blocks JNK activation (18). Other anti-inflammatory properties of curcumin may come from its ability to act as a potent inhibitor of NF-κB activation in some in vitro model systems (19, 20). Finally, curcumin boosts expression of cytoprotective enzymes such as HO-1 in renal epithelial cells, possibly via the induction of the transcription factor Nrf2 (nuclear factor-E2 p45-related factor transcription factor) (21, 22). These enzymes in turn catalyze reactions that produce antioxidant molecules. Nrf2 is the key transcription factor that activates the antioxidant promoter response element (ARE) in the 5′ regulatory region of genes encoding the Phase II enzymes and other antioxidant and protective enzymes such as HO-1 (23, 24). Little is known about the effect of curcumin on HO-1 expression and function in lung injury.
One goal of the present study was to determine whether administering curcumin in the diet would result in circulating blood levels that were biologically efficacious. Previous studies have been hampered by the very low solubility of curcumin, necessitating cumbersome daily gastric gavage for administration (12, 25, 26). An additional goal of this study was to investigate, for the first time, the effect of curcumin administered in the diet in both an acute and a long-term, clinically relevant, well-characterized model of radiation pneumonopathy.
MATERIALS AND METHODS
Animals
Except where noted, female C57BL/6 mice aged 6–10 weeks (Charles River Laboratories, Inc. Wilmington, MA) were used. All animals were housed at the Children’s Hospital of Philadelphia (CHOP) Animal Facility (Philadelphia, PA). All protocols were performed in accordance with NIH guidelines and with the approval of the CHOP and the University of Pennsylvania Animal Use Committees.
Curcumin
Curcumin (diferuloylmethane) was purchased from Sigma-Aldrich, Inc. (St Louis, MO, product no. C7727). Diets consisting of standard mouse chow (5015) with 1% or 5% curcumin weight/weight were made by Purina Mills TestDiet (Richmond, IN).
Analytical Evaluation of Curcumin Content in Murine Plasma Samples
Plasma samples were analyzed by liquid chromatography tandem mass spectrometry (LC/MS/MS) as described previously (27). The HPLC system used was model HP 1050 (Agilent, Inc.). Detection was achieved using a VG Quattro II (Micromass, Inc.) triple-quadrupole mass spectrometer operating in multiple reaction monitoring (MRM) mode.
Chromatography conditions
Separation of the curcuminoids was carried out using gradient elution. The mobile phase consisted of two eluents: A, 0.1% acetic acid in acetonitrile (Fisher Scientific, Pittsburgh, PA), and B, 0.1% acetic acid in HPLC-grade water (Fisher). The eluent composition was varied linearly from 50% to 100% A over the period 0 to 10 min with a plateau at 100% A from 10 to 11 min. The composition returned to 50% A over the period 11 to 12 min followed by a reconditioning at 50% A from 12 to 20 min. The flow rate was 0.5 ml/min, and the column was a Phenomenex Aqua (Phenomenex, Torrance, CA) endcapped C18 having dimensions 150 × 4.6 mm with a 5-μm particle size. The column was heated at 40°C, and the samples were stored at room temperature in the autosampler. The total analysis time was 20 min. A volume of 25 μl was injected. Retention times for the two monitored curcuminoid peaks were 6.4 and 9.6 min.
Mass spectrometry conditions
Curcuminoids were monitored by negative ion electrospray tandem mass spectrometry. The MRM transition monitored was (parent ion > daughter ion) 367 > 367. The capillary voltage, cone voltage and collision energy were optimized at 2.5 kV, 19 V and 8 V, respectively, with a collision cell gas pressure of 2.2 × 10−3 mBar.
Standards
Curcumin was obtained in 84% (98% curcuminoids) purity from Sigma. Standard solutions were prepared in methanol and stored at −20°C. EDTA-stabilized mouse plasma was purchased from Valley Biomedical (Winchester, VA) and stored at −20°C prior to use.
Extraction
All plasma samples were stored at −20°C prior to analysis. Plasma was allowed to come to room temperature before extraction. Standards were prepared by adding 20 μl of the curcuminoid standard in methanol to 150 μl of EDTA-stabilized mouse plasma. Standards and controls were vortexed to mix. Then 150 μl of each sample and 0.5 ml 134 U/ml β glucuronidase solution (Sigma) in sodium acetate buffer (pH 5.0) was added to the standards. All were vortexed, covered and incubated at 37 ± 2°C for 1 h. Buffered plasma was extracted two times with 2 ml ethyl acetate (Fisher) by vortex mixing for 2 min followed by centrifugation (2,000g) to separate layers. The combined organic layers were evaporated to dryness under nitrogen at 40°C. The extracts were reconstituted to 200 μl with 1:1 acetonitrile:water.
Cell Culture
For fibroblast isolation, mouse lungs were harvested, minced and incubated with dispase (2 mg/ml; Sigma-Aldrich) for 45 min. Pieces were plated, and fibroblasts were cultured as described previously (28) and used between passages 3 and 5. Identification of fibroblasts was based on the expression of vimentin, collagen and smooth muscle actin. Pulmonary microvascular endothelial cells (PMVEC) were isolated from mouse lungs as described previously (29). Briefly, freshly harvested mouse lungs were treated with collagenase followed by isolation of cells by adherence to magnetic beads coated with mAb to platelet endothelial cell adhesion molecule (PECAM).
Measurement of Reactive Oxygen Species (ROS) Induced by γ Radiation
Generation of ROS was detected by fluorescence microscopy using the ROS sensitive dye H2DCFDA, dichlorodihydrofluorescein diacetate (no. D-399, Invitrogen, Carlsbad, CA). A 5 mM stock solution of H2DCFDA was prepared in 100% ethanol, stored under N2 at −20°C in the dark, and added to confluent monolayers of PMVEC to make a final concentration of 5 μM (29). As control, we used 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate (carboxy-DCFDA), which is the non-oxidizable analog of H2DCFDA (no. C-369, Invitrogen). PMVEC were incubated with curcumin at 5, 10, 25 50 or 100 μM for 4 h prior to exposure to 2 Gy γ radiation (30) from a Mark 1 cesium irradiator (J. L. Shepherd, San Fernando, CA) at a dose rate of 1.7 Gy/min. H2DCFDA was immediately added to the monolayers and cells were imaged. H2DCFDA is a cell-permeant and non-fluorescent compound that internalizes into the cell, after which the acetate group is cleaved by the internal esterases with the formation of H2DCF, which acts as a substrate for intracellular ROS to generate the highly fluorescent DCF. We routinely used this dye (9, 31, 32) for lung and endothelial cell imaging. Although H2DCFDA was originally used to detect H2O2 (33), it is oxidized by other ROS as well and is thus a measure of ROS in general. For each condition, three or four random fields were selected for quantification of fluorescence.
Imaging and Quantification of Fluorescence
For each set of experiments, all images for control and treatment conditions were processed to the same scale, and the average intensity of each field (consisting of a large number of cells) was obtained. Metamorph Imaging Software (MDS, Toronto) was used for image display and processing as described previously (9, 29). Images were processed on a preset scale of arbitrary units ranging from 0 to 3500.
Analysis of Effects of Curcumin on Lung Tumors
To test the possibility of potential tumor protection by curcumin, a cohort of mice (n = 25 mice per diet group) was injected with 2 million murine LLC cells (34) and assayed 10–14 days after injection.
Tumor Morphometry
Quantitative morphometric analysis was performed on 5-μm serial lung sections stained with H&E using Image Pro-Plus image-analysis software (Spot 3.0 driver software: RTkE Diagnostic, Inc., Image-pro plus 4.0: Media Cybernetics, Silver Spring, MD) as described earlier (9). Images were obtained with an Olympus BX41 light microscope fitted with a high-resolution RTkE Diagnostic camera. Each image was captured under the same reproducible conditions and digitized. Values, originally expressed in pixels, were converted to square micrometers and were expressed as a percentage of the total area. Tumor multiplicity was determined as the number of nodules per section.
Western Blotting for HO-1
Mice were fed control or curcumin-supplemented diets for 7, 10 and 14 days and lung homogenates were evaluated for HO-1 protein expression by Western blotting (29) with primary antibodies to HO-1 (Stressgen, San Diego, CA). In other experiments, isolated pulmonary mouse fibroblasts, mouse embryonic fibroblasts (MEFs), and PMVEC were incubated with curcumin (0–50 μM) or vehicle (DMSO), and whole cell lysates were analyzed for HO-1 by Western blotting.
Induction of TNF-α in BAL Fluid by LPS Instillation in Lungs
Lipopolysacchride (LPS)-induced lung injury was induced via intratracheal (i.t.) injection of 0.5 μg of LPS (serotype 0111:B4, Sigma) in 100 μl of saline (35). Preliminary studies were conducted to map the time course of the peak pulmonary response to the LPS stimulus by evaluation of TNF-α levels in bronchoalveolar lavage fluid (BAL fluid) using a commercially available kit [BD OptEIA™ ELISA Set (mono/mono), BD Pharmingen] and read on a Bio-Rad model 550 microplate reader. Optical density at 450 nm was measured and compared to the standard curve generated with each assay.
Irradiation of Mice
The radiation source was a 250 kVp orthovoltage machine using a 2-mm copper filter and a tube current of 13 mA. A customized jig was created that allowed the irradiation of up to eight mice simultaneously and homogeneously; lead shielding was used over each animal’s head/neck and abdomen/pelvis so that only the thorax was irradiated. The midplane dose (not corrected for air/lung/tissue inhomogeneity) was 13.5 Gy (8-min irradiation session). Dosimetric analysis, including thermoluminescence dosimeters, was performed and confirmed that dose heterogeneity in the animals’ lungs was within acceptable levels (9). After the completion of irradiation, mice were given a subcutaneous injection of 1 ml normal saline to prevent postirradiation dehydration resulting from decreased feeding from possible acute radiation-induced esophagitis. Nonirradiated controls were also given the saline injection.
Mouse Survival, Tissue Harvesting and Evaluation of Lung Injury
Mouse survival was recorded twice a week after irradiation, and the experiment was terminated after 4 months, the earliest time at which radiation-induced fibrosis was detectable. Separate animals were used for short-term experiments. At 3 weeks after irradiation, mice were killed for evaluation of acute radiation pneumonitis. From our experience (8, 9, 36), 3 weeks after irradiation, mice exhibit reliable and consistent elevations in all BAL fluid markers of lung injury over those in unirradiated control mice. Lungs were harvested, inspected, photographed and lavaged for BAL protein and WBC and PMN determination or processed for histology.
Quantitative Assessment of Fibrosis-Quantification of Lung Hydroxyproline Content
Whole collagen content of mouse lung was evaluated by determining hydroxyproline content. Briefly, after recovery of the BAL fluid, all lung lobes were removed, weighed and cut into sections (1 mm thick). Dried lung samples were hydrolyzed with 2 ml of 6 N HCl at 120°C for 16 h in sealed glass tubes. Hydroxyproline in the hydrolysate was measured according to Woessner (37). Commercial hydroxyproline (hydroxy-L-proline, Sigma) was used to establish a standard curve. The data are expressed as (μg hydroxyproline/ml)/entire lung.
Clonogenic Survival
LLC cells or PMVEC were plated in 60-mm culture dishes at densities of 100, 300, 1000 or 10,000 cells per plate in triplicate. Curcumin was added for 4 h at indicated doses; then cells were irradiated with a Mark 1 cesium irradiator at a dose rate of 1.7 Gy/min. Curcumin was removed by exchange of fresh medium 1 h after radiation. Cells were incubated for 10 to 14 days after irradiation and then fixed with 10% methanol/10% acetic acid and stained with 0.4% crystal violet. Colonies containing more than 50 cells were counted. The plating efficiencies were determined for each treatment and normalized to unirradiated controls. The curves were fitted using a second-order polynomial function. The average normalized surviving fraction from three independent experiments and the standard errors of the mean (SEM) are reported.
Statistical Analysis
Results are expressed as means ± SEM. Differences among groups were determined using one-way analysis of variance (ANOVA). When statistically significant differences were found (P < 0.05), individual comparisons were made using the Bonferroni/Dunn test (Statview 4.0). Survival studies were assessed using Kaplan-Meier survival curves and analyzed with the Mantel-Cox log rank test.
RESULTS
Dietary Curcumin Supplementation Results in Stable Levels in the Plasma
A diet consisting of standard mouse chow with 5% curcumin by weight/weight was fed to test mice and was well tolerated by the mice during all experiments. Weight gain in the control and curcumin-fed groups was identical over a 4-week period of observation (results not shown).
We measured plasma levels of curcumin using LC/MS/MS to analyze plasma samples from mice fed a 5% diet (n = 5 mice/time). Mice fed 5% curcumin reached a steady-state plasma concentration of 20–25 μg/ml (equivalent to 54–68 μM) by day 7 on the 5% curcumin diet (Fig. 1A). No liver toxicity was detected by the plasma liver enzyme AST/ALT activity levels (Fig. 1B) or by histological evaluation of H&E-stained liver sections.
FIG. 1.
Evaluation of lung and liver enzyme kinetics and plasma curcumin levels after curcumin supplementation in mice. Panel A: Plasma levels of curcuminoids in mice fed a 5% curcumin diet. Plateau levels are reached by day 8. Panel B: Liver enzyme analysis from mouse serum collected from mice fed a 5% curcumin diet. No significant differences were noted between early and later times. Panel C: Total lung homogenates from mice fed control chow (Ctrl) or 5% curcumin were evaluated for expression levels of HO-1 using semi-quantitative immunoblotting. Panel D: Quantification of HO-1 expression from immunoblot shown in panel C, determined by densitometry. Data are means ± SEM. *P < 0.04 compared to basal diet control.
Increased levels of heme oxygenase 1 (HO-1) were seen in mice fed a 5% diet compared to those in mice fed the control diet (Fig. 1C). The levels of HO-1 increased by nearly 12-fold over baseline levels at 14 days (Fig. 1D).
Curcumin Up-regulates Heme Oxygenase 1 (HO-1) Protein Levels In Vitro
Previous studies have shown that curcumin up-regulates the cytoprotective enzyme HO-1 through activation of the transcription factor Nrf-2 in renal epithelial cells (35). To determine whether incubation with curcumin at doses achievable in vivo in this model system (0–100 μM) could also induce HO-1 expression in primary cells derived from mouse lungs, we evaluated HO-1 levels in isolated pulmonary microvascular endothelial cells (PMVEC) and primary fibroblasts. Curcumin induced a dose-dependent increase of HO-1 expression ranging from three- to 18-fold over baseline in both primary lung cell lines tested (endothelial cells and fibroblasts) (Fig. 2A–D). This increase in HO-1 expression was statistically significant at 25 μM for PMVEC and at 25–100 μM for fibroblasts. HO-1 induction in PMVEC followed a bell-shaped curve, which may be an indication of a regulatory feedback loop that is activated at higher doses. These results demonstrate that plasma curcumin levels achieved after administration of dietary curcumin can induce significant biological effects in vitro.
FIG. 2.
Dose response of curcumin on HO-1 expression in primary lung cells. Representative blots of three experiments for HO-1 expression in primary lung cells. Panel A: Pulmonary artery microvascular cells (PMVEC) were isolated from mouse lungs and exposed to curcumin for 4 h at different concentrations (0–100 μM), and HO-1 expression was determined by Western blotting. Panel C: Primary cultures of mouse lung fibroblasts were established from naïve mice and cells incubated with curcumin as in panel A. Panels B, D: Densitometry was performed on Western blots from panels A and C. Data are means ± SEM. *P < 0.05 compared to non-treated control cells.
Curcumin Supplementation Reduces ROS Generation by Pulmonary Endothelial Cells after γ Irradiation
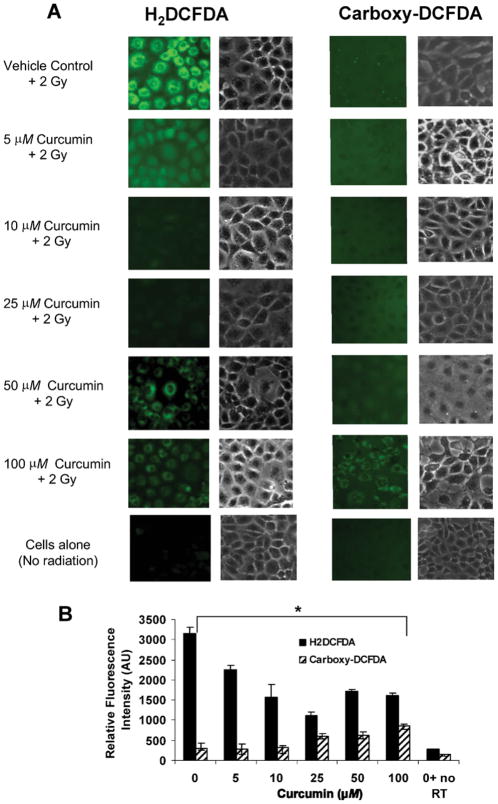
To evaluate the antioxidant properties of curcumin in relation to radiation-generated oxidative stress, curcumin-treated PMVEC were exposed to γ radiation and ROS was assayed from fluorescent images of cells using H2DCFDA (Fig. 3A). ROS generation was significantly lower in curcumin-treated irradiated cells, in a dose-dependent manner, compared to non-curcumin-treated, irradiated controls (Fig. 3B). Control experiments using carboxy-DCFDA, a non-oxidizable analog of H2DCFDA, confirmed that fluorescence did not increase with either radiation or addition of curcumin. A slight increase in fluorescence was observed with both dyes at higher curcumin doses, which may be due to autofluorescence of curcumin.
FIG. 3.
Curcumin effects on radiation-induced ROS generation. Panel A: Mouse pulmonary microvascular endothelial cells (PMVEC) were preincubated with curcumin for 4 h, exposed to 2 Gy γ radiation (RT), loaded with 5 μM H2DCFDA (left panels) or the non-oxidizable analog carboxy-DCFDA (right panels), and imaged to monitor ROS generation. Panel B: For each condition, three or four random fields were used for quantification of fluorescence. Black bars: H2DCFDA; hatched bars: carboxy-DFCDA. Values are means ± SD. *P < 0.0001 by ANOVA for all curcumin values compared to irradiated vehicle control.
Kinetics of Curcumin Effects in Lung Tissues and Dosing of Curcumin in the Diet
To determine the lowest dose of dietary curcumin needed to exert significant physiological effects in lung tissues, we used intratracheal (i.t.) LPS to activate alveolar macrophages and increase TNF-α release through a Toll-like receptor/NF-κ B-dependent mechanism. The peak concentration of TNF-α in BAL fluid occurred at 4 h after LPS instillation (Fig. 4A). We fed mice control diet or diets containing 1% and 5% curcumin for 10 and 14 days and subjected mice to i.t. LPS challenge to assess the effects of curcumin diet on TNF-α release. Figure 4B shows the amount of TNF-α in BAL fluid from control animals compared to those given a curcumin-supplemented diet at 4 h after LPS administration. one percent curcumin significantly (P < 0.05) decreased TNF-α in the BAL fluid; the decrease was more pronounced when curcumin was given at a dose of 5%.
FIG. 4.
Effect of curcumin on TNF-α in BAL fluid (BALF) from LPS-challenged mice. Panel A: Mice received intratracheal LPS (0.5 μg in 100 μl) and were assayed at indicated times (n = 4 mice per time). Panel B: Mice on the 1% or 5% curcumin diet for 10 or 14 days were challenged with intratracheal LPS (0.5 μg). Four hours after administration of LPS (the time of peak TNF-α release), bronchoalveolar lavage was performed and TNF-α levels were measured (n = 5 mice per group). Data are means ± SEM. (*P < 0.05 for 10 days compared to 14 days).
Dietary Curcumin Supplementation does not Ameliorate Markers of Acute Radiation Pneumonitis
Our C57/BL6 mice develop radiation pneumonitis as early as 3 weeks after a single fraction of radiation (13.5 Gy) as shown by BAL fluid measures of lung inflammation and injury (8). Compared to mice fed the control diet, mice fed 5% curcumin for 2 weeks prior to irradiation did not exhibit any significant differences in BAL fluid measures of inflammatory cell accumulation (macrophages or neutrophils) or alveolar damage (BAL proteins): 245 ± 45 compared to 251 ± 33 × 106 BAL WBCs, 24 ± 4% compared to 31 ± 4% BAL neutrophils, and 0.25 ± 0.01 compared to 0.26 ± 0.02 mg/ml BAL proteins in control diet compared to 5% curcumin-fed mice, respectively. All values are mean ± SEM for n = 11–12 mice per group.
Dietary Curcumin Supplementation Inhibits Pulmonary Fibrosis and Improves Long-Term Survival after Thoracic Irradiation
We tested whether curcumin feeding could affect long-term survival and the degree of fibrotic lung disease 4 months after a single dose of thoracic radiation. With a 1% curcumin-supplemented diet given 14 days prior to irradiation and for the entire duration of the study, there was no statistically significant benefit for either lung fibrosis (Fig. 5A) as judged by hydroxyproline content in lungs (8, 37) or overall survival (Fig. 5B). However, hydroxyproline levels in irradiated lungs were significantly lower in mice fed the 5% curcumin diet (Fig. 5C). Specifically, irradiated lungs from mice fed 5% curcumin had a 45% increase in hydroxyproline content after irradiation, while irradiated lungs from mice fed the control diet had a 112% increase from nonirradiated controls (P = 0.05) (Fig. 5C). Kaplan-Meier survival analysis showed survival rates in 5% curcumin-fed compared to control-diet fed mice to be 45% compared to 23%, respectively (Fig. 5D), a statistically significant improvement (P < 0.0001).
FIG. 5.
Effects of curcumin on lung fibrosis and survival 4 months after irradiation. Lung fibrosis (panels A, C; hydroxyproline content) and mouse survival (panels B, D) were evaluated 4 months postirradiation (single fraction, 13.5 Gy). Mice were given a control or curcumin-supplemented diet at 1% or 5% content for at least 2 weeks prior to irradiation and were kept on the diet for the duration of the experiment. Bars are means ± SEM with n = 10 mice per group for irradiated mice and n = 5 for nonirradiated groups. P = 0.05 for irradiated curcumin-supplemented compared to irradiated, control diet-fed mice.
Curcumin Supplementation does not Affect Tumor Response to Radiation
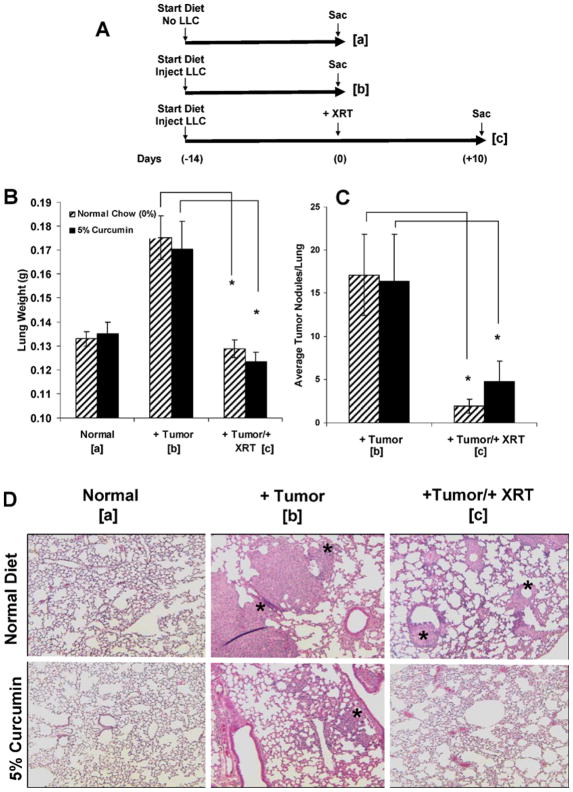
To determine whether curcumin feeding alters lung tumor response to single-fraction thoracic irradiation (13.5 Gy), mice were injected i.v. with 1 × 106 LLC cells and started on either a control or 5% curcumin diet according to the scheme in Fig. 6A (n = 25 mice per diet group; 50 mice for all three cohorts used in two repeat experiments). Cohort a was not injected with tumor cells and was killed at the time of peak tumor growth in cohorts b and c to determine the basal lung weight. Cohorts that were injected with LLC cells were killed either on the day of irradiation (cohort b) at peak tumor growth to determine maximal tumor burden or 10 days postirradiation (cohort c) to determine whether diet affected radiation killing of tumor cells.
FIG. 6.
Effects of curcumin on lung tumor growth after irradiation. Panel A: Experimental plan. Panel B: Lung weights (mg). Panel C: Nodule counts. Results are findings from two experiments. Panel D: Representative histological profiles of paraffin-embedded lung sections stained with H&E in each cohort. Original magnification 1003. Asterisks represent tumor nodules. *P < 0.02 (panel B) and *P < 0.05 (panel C) for mice in cohort b compared to cohort c (i.e., irradiated compared to nonirradiated) for each diet (0% or 5% curcumin).
We used lung weight (Fig. 6B), the number of tumor nodules per lung (Fig. 6C), and the tumor area per lung (see below) to determine the overall tumor burden in lungs. No statistically significant difference in lung weight was observed between unirradiated mice on the control diet and on the 5% curcumin diet (cohort b). Similarly, no significant difference was observed between irradiated mice on the control diet and on the 5% curcumin diet (cohort c). However, mice in both diet groups displayed significantly reduced lung weight (i.e., tumor burden decrease) after irradiation (see differences between cohorts b and c, Fig. 6B). The number of tumor nodules was significantly lower after irradiation than without irradiation for mice on both diets (Fig. 6C and D).
Tumor morphometry was performed to quantify the percentage lung area occupied by tumor before and after irradiation for each diet. For mice on the control diet, 11.1 ± 3.0% of the lung was occupied by tumor before and 2.6 ± 0.6% after irradiation compared to 9.6 ± 1.9% before and 4.0 ± 1.0% after for the mice on 5% curcumin. There was a significant decrease in the percentage tumor area after irradiation in mice fed both the control diet (P < 0.01) and 5% curcumin (P < 0.02) compared to their nonirradiated counterparts. Importantly, there were no significant differences between the irradiated control and curcumin groups.
Curcumin Radiosensitizes LLC Cells but not Endothelial Cells In Vitro
We treated LLC cells or PMVEC with 10 or 25 μM curcumin or DMSO (vehicle control) for 4 h followed by graded doses of γ radiation. Clonogenic survival was measured. Curcumin significantly increased radiation killing of the LLC cells in a dose-dependent manner (Fig. 7A) but did not increase the killing of PMVEC cells (Fig. 7B).
FIG. 7.
Curcumin sensitizes carcinoma cells but not endothelial cells to ionizing radiation. LLC cells (panel A) and PMVEC (panel B) were treated with DMSO or curcumin for 4 h before irradiation. Control cells were mock-irradiated. Clonogenic assays were used to assay cell survival. Points are averages of three independent experiments (± SEM). *P < 0.05.
DISCUSSION
In the current study, we demonstrated for the first time that feeding a diet consisting of 5% curcumin (w/w) can be used to (1) achieve μM steady-state plasma levels, (2) exert local effects in the lung as seen in the up-regulation of a marker cytoprotective enzyme (HO-1), and (3) ameliorate lung fibrosis while improving mouse survival in an experimental model of radiation-induced lung fibrosis. Importantly, lung protection from adverse effects of radiation was not achieved at the cost of decreasing radiation killing of lung tumor metastases. Our findings suggest that dietary administration of curcumin may represent an effective therapeutic adjuvant for the treatment of certain types of chronic, fibrotic lung disease.
We used a single large fraction of radiation given to the entire thorax. One reason for selecting a single dose of 13.5 Gy is logistical, including less stress on the animals. Nonetheless, we believe that a single large fraction is well justified scientifically for our preclinical study based on other successful preclinical investigations (38, 39). Lung tissue is highly sensitive to injury from large fractions of radiation; radiobiological models predict that a single dose of 13.5 Gy to lung tissue is approximately the equivalent of a fractionated radiotherapy dose of at least 45 Gy, which is a clinically relevant dose (40). We hypothesize that if curcumin treatment can protect animals from radiation pneumonopathy after a single large dose of radiation, it is reasonable to expect that it could protect against fractionated radiotherapy. This has been demonstrated for the prototype radioprotector Amifostine (41).
One focus of our group is to explore the use of natural dietary supplements such as curcumin as a means of ameliorating acute and chronic lung diseases that implicate oxidative stress such as that observed with radiation pneumonopathy. In analogous experiments we explored the use of dietary wholegrain flaxseed, which has been shown to ameliorate acute oxidative lung damage by decreasing lipid peroxidation in irradiated lungs (27, 29) as well as radiation-induced lung inflammation and fibrosis related to late-phase pneumonitis at 4 months postirradiation (9). We showed that dietary flaxseed is a potent inducer of Nrf2-regulated enzymes in lung tissues (29). Further studies need to be performed to analyze the role of these antioxidant enzymes in lung protection from radiation damage. Interestingly, flaxseed, as with curcumin in the current study, did not abrogate early lung inflammation seen 3 weeks postirradiation.
Gamma radiation is known to induce oxidative stress in cells via the generation of ROS (9, 42). Pretreating primary lung endothelial cells with curcumin for as little as 4 h prior to radiation significantly inhibited radiation-induced ROS generation in a dose-dependent manner. This is the first report that curcumin directly inhibits radiation-induced ROS in non-malignant primary cells derived from lung tissues. Previous studies have shown that curcumin can reduce radiation-induced oxidative stress as seen in decreased lipid peroxidation in normal cells such as mouse splenic lymphocytes (43). However, the focus has been on the pro-oxidant properties of curcumin in malignant cells, thus promoting radio-sensitization (42). This interesting dichotomy may explain our findings that curcumin protected normal lung tissue from injury from radiation exposure while appearing to radiosensitize lung tumor cells in vitro.
Zampetaki et al. showed that transgenic mice engineered to express high levels of a human HO-1 transgene in the lung epithelium had significantly lower levels of inflammatory cytokines in BAL fluid compared to controls after challenge with either hyperoxia or LPS (44). We found increased levels of HO-1 enzyme in the lungs of animals fed curcumin. Up-regulation of HO-1 in radiation-induced oxidative stress has been found to be protective in lung (45). Therefore, curcumin-induced up-regulation of HO-1 at the time of irradiation may explain the protection seen in this model. Our study, however, can only confirm a temporal association of HO-1 expression in lung tissues with dietary curcumin administration and cannot claim a definitive role on protection until further studies are performed. Since HO-1 is an Nrf2-regulated gene, future studies using Nrf2 null mice could help establish whether this up-regulation occurs in association with activation of Nrf2, i.e., confirm a cause-effect relationship between the two.
As summarized in a recent review, curcumin has been studied extensively as an anticarcinogenic agent and has been shown to interfere with multiple cell signaling pathways, including the cell cycle (cyclin D1 and cyclin E), apoptosis (activation of caspases and down-regulation of anti-apoptosis gene products), proliferation (HER-2, EGFR and AP-1), survival (PI3K/AKT pathway), invasion (MMP-9 and adhesion molecules), angiogenesis (VEGF) and metastasis (CXCR-4) (46). Although our study was not aimed at investigating the anticarcinogenic effects of curcumin, it confirmed that curcumin does not protect tumor cells in vitro or in vivo from radiation killing. We also found that curcumin radiosensitized tumor cells in a dose-dependent manner while sparing primary non-tumor lung cells from radiation killing. The dual action of curcumin in both radioprotection and radiosensitization, i.e., enhancing the effect of radiation on tumor cells and simultaneously protecting normal cells against radiation, has been investigated in several studies and has been reviewed by Garg (47) and Jagetia (48). Javvadi et al. showed that curcumin radiosensitizes squamous cell carcinoma cells in vitro (42). It has been proposed that the antioxidant properties of curcumin may be responsible for radio-protection, whether by direct free radical scavenging or detoxification via enhanced antioxidant enzyme expression. In contrast, curcumin’s radiosensitizing activity may be due to enhanced pro-oxidant activities and persistent activation of ERK1 and 2 in malignant cells (42).
In summary, this study evaluated curcumin as a potential dietary supplement in the setting of thoracic radiotherapy. Potential mechanisms of the effects of prolonged curcumin feeding include altering immediate radiation-induced markers of lung damage, creating a baseline radioprotective state prior to irradiation by inducing protective gene expression as well as having potent direct antioxidant scavenging activity. Our long-term goal is to be able to provide adequate radio-protection not only to prevent the side effects of radiation on normal lung parenchyma but also to allow for greater doses of radiation to affect further clinical response and cure against thoracic malignancies.
Acknowledgments
This study was funded in part by NIH-R01 CA133470-01A1 (MCS), NIH-R21 CA-118111-01 (MCS), American Institute for Cancer Research no. AICR-03B024 (MCS), the University of Pennsylvania Research Foundation (MCS), pilot project support from 1P30 ES013508-02 awarded to MCS, and NIH-5R01CA104922 (CK). The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
References
- 1.Wang JY, Chen KY, Wang JT, Chen JH, Lin JW, Wang HC, Lee LN, Yang PC. Outcome and prognostic factors for patients with non-small-cell lung cancer and severe radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2002;54:735–741. doi: 10.1016/s0360-3016(02)02994-2. [DOI] [PubMed] [Google Scholar]
- 2.Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:89–94. doi: 10.1016/s0360-3016(00)00648-9. [DOI] [PubMed] [Google Scholar]
- 3.Hughes-Davies L, Tarbell NJ, Coleman CN, Silver B, Shulman LN, Linggood R, Canellos GP, Mauch PM. Stage IA–IIB Hodgkin’s disease: management and outcome of extensive thoracic involvement. Int J Radiat Oncol Biol Phys. 1997;39:361–369. doi: 10.1016/s0360-3016(97)00085-0. [DOI] [PubMed] [Google Scholar]
- 4.Marks LB, Fan M, Clough R, Munley M, Bentel G, Coleman RE, Jaszczak R, Hollis D, Anscher M. Radiation-induced pulmonary injury: symptomatic versus subclinical endpoints. Int J Radiat Biol. 2000;76:469–475. doi: 10.1080/095530000138466. [DOI] [PubMed] [Google Scholar]
- 5.Gross NJ. The pathogenesis of radiation-induced lung damage. Lung. 1981;159:115–125. doi: 10.1007/BF02713907. [DOI] [PubMed] [Google Scholar]
- 6.Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the early phase. Radiat Res. 1989;119:1–14. [PubMed] [Google Scholar]
- 7.Zhang X, Epperly MW, Kay MA, Chen ZY, Dixon T, Franicola D, Greenberger BA, Komanduri P, Greenberger JS. Radioprotection in vitro and in vivo by minicircle plasmid carrying the human manganese superoxide dismutase transgene. Hum Gene Ther. 2008;19:820–826. doi: 10.1089/hum.2007.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machtay M, Scherpereel A, Santiago J, Lee J, McDonough J, Kinniry P, Arguiri E, Shuvaev VV, Sun J, Christofidou-Solomidou M. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol. 2006;81:196–205. doi: 10.1016/j.radonc.2006.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, Vachani A, Solomides CC, Cengel KA, Christofidou-Solomidou M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther. 2009;8:47–53. doi: 10.4161/cbt.8.1.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madan B, Ghosh B. Diferuloylmethane inhibits neutrophil infiltration and improves survival of mice in high-dose endotoxin shock. Shock. 2003;19:91–96. doi: 10.1097/00024382-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Shoskes DA. Effect of bioflavonoids quercetin and curcumin on ischemic renal injury. Transplantation. 1998;66:147–152. doi: 10.1097/00007890-199807270-00001. [DOI] [PubMed] [Google Scholar]
- 14.Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Thomas P, Dannenberg AJ. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G321–327. doi: 10.1152/ajpgi.00230.2002. [DOI] [PubMed] [Google Scholar]
- 15.Das KC, Das CK. Curcumin (diferuloylmethane), a singlet oxygen (1O2) quencher. Biochem Biophys Res Commun. 2002;295:62–66. doi: 10.1016/s0006-291x(02)00633-2. [DOI] [PubMed] [Google Scholar]
- 16.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–819. [PubMed] [Google Scholar]
- 17.Bierhaus A, Zhang Y, Quehenberger P, Luther T, Haase M, Muller M, Mackman N, Ziegler R, Nawroth PP. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost. 1997;77:772–782. [PubMed] [Google Scholar]
- 18.Chen YR, Tan TH. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene. 1998;17:173–178. doi: 10.1038/sj.onc.1201941. [DOI] [PubMed] [Google Scholar]
- 19.Kang BY, Chung SW, Chung WJ, Im SY, Hwang SY, Kim TS. Inhibition of interleukin-12 production in lipopolysaccharide-activated macrophages by curcumin. Eur J Pharmacol. 1999;384:191–195. doi: 10.1016/s0014-2999(99)00690-1. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Dhawan S, Hardegen NJ, Aggarwal BB. Curcumin (diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem Pharmacol. 1998;55:775–783. doi: 10.1016/s0006-2952(97)00557-1. [DOI] [PubMed] [Google Scholar]
- 21.Baboolal HA, Ichinose F, Ullrich R, Kawai N, Bloch KD, Zapol WM. Reactive oxygen species scavengers attenuate endotoxin-induced impairment of hypoxic pulmonary vasoconstriction in mice. Anesthesiology. 2002;97:1227–1233. doi: 10.1097/00000542-200211000-00028. [DOI] [PubMed] [Google Scholar]
- 22.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 24.Otterbein LE, Choi AM. The saga of leucine zippers continues: in response to oxidative stress. Am J Respir Cell Mol Biol. 2002;26:161–163. doi: 10.1165/ajrcmb.26.2.f226. [DOI] [PubMed] [Google Scholar]
- 25.Venkatesan N, Punithavathi V, Chandrakasan G. Curcumin protects bleomycin-induced lung injury in rats. Life Sci. 1997;61:PL51–58. doi: 10.1016/s0024-3205(97)00443-8. [DOI] [PubMed] [Google Scholar]
- 26.Venkatesan N. Pulmonary protective effects of curcumin against paraquat toxicity. Life Sci. 2000;66:PL21–PL28. doi: 10.1016/s0024-3205(99)00576-7. [DOI] [PubMed] [Google Scholar]
- 27.Kinniry P, Amrani Y, Vachani A, Solomides CC, Arguiri E, Workman A, Carter J, Christofidou-Solomidou M. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J Nutr. 2006;136:1545–1551. doi: 10.1093/jn/136.6.1545. [DOI] [PubMed] [Google Scholar]
- 28.Eickelberg O, Kohler E, Reichenberger F, Bertschin S, Woodtli T, Erne P, Perruchoud AP, Roth M. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-beta1 and TGF-beta3. Am J Physiol. 1999;276:L814–L824. doi: 10.1152/ajplung.1999.276.5.L814. [DOI] [PubMed] [Google Scholar]
- 29.Lee JC, Bhora F, Sun J, Cheng G, Arguiri E, Solomides CC, Chatterjee S, Christofidou-Solomidou M. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2008;294:L255–265. doi: 10.1152/ajplung.00138.2007. [DOI] [PubMed] [Google Scholar]
- 30.Cengel KA, Voong KR, Chandrasekaran S, Maggiorella L, Brunner TB, Stanbridge E, Kao GD, McKenna WG, Bernhard EJ. Oncogenic K-Ras signals through epidermal growth factor receptor and wild-type H-Ras to promote radiation survival in pancreatic and colorectal carcinoma cells. Neoplasia. 2007;9:341–348. doi: 10.1593/neo.06823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chatterjee S, Levitan I, Wei Z, Fisher AB. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Micro-circulation. 2006;13:633–644. doi: 10.1080/10739680600930255. [DOI] [PubMed] [Google Scholar]
- 32.Milovanova T, Chatterjee S, Hawkins BJ, Hong N, Sorokina EM, Debolt K, Moore JS, Madesh M, Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta. 2008;1783:1866–1875. doi: 10.1016/j.bbamcr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keston AS, Brandt R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem. 1965;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- 34.Bertram JS, Janik P. Establishment of a cloned line of Lewis lung carcinoma cells adapted to cell culture. Cancer Lett. 1980;11:63–73. doi: 10.1016/0304-3835(80)90130-5. [DOI] [PubMed] [Google Scholar]
- 35.Albelda SM, Lau KC, Chien P, Huang ZY, Arguiris E, Bohen A, Sun J, Billet JA, Christofidou-Solomidou M, Schreiber AD. Role for platelet-endothelial cell adhesion molecule-1 in macrophage Fcgamma receptor function. Am J Respir Cell Mol Biol. 2004;31:246–255. doi: 10.1165/rcmb.2003-0404OC. [DOI] [PubMed] [Google Scholar]
- 36.Christofidou-Solomidou M, Scherpereel A, Solomides CC, Muzykantov VR, Machtay M, Albelda SM, DiNubile MJ. Changes in plasma gelsolin concentration during acute oxidant lung injury in mice. Lung. 2002;180:91–104. doi: 10.1007/s004080000084. [DOI] [PubMed] [Google Scholar]
- 37.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 38.Travis EL, Parkins CS, Holmes SJ, Down JD, Fowler JF. WR-2721 protection of pneumonitis and fibrosis in mouse lung after single doses of x rays. Int J Radiat Oncol Biol Phys. 1984;10:243–251. doi: 10.1016/0360-3016(84)90010-5. [DOI] [PubMed] [Google Scholar]
- 39.Vansteenkiste JF, Vandebroek JE, Nackaerts KL, Weynants P, Valcke YJ, Verresen DA, Devogelaere RC, Marien SA, Humblet YP, Dams NL. Clinical-benefit response in advanced non-small-cell lung cancer: A multicentre prospective randomised phase III study of single agent gemcitabine versus cisplatin-vindesine. Ann Oncol. 2001;12:1221–1230. doi: 10.1023/a:1012208711013. [DOI] [PubMed] [Google Scholar]
- 40.Sharma RA, Vallis KA, MWG . Basics of radiation therapy. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG, editors. Abeloff Clinical Oncology, 4th Edition – Expert Consult Premium Edition: Enhanced Online Features and Print. Vol. 2592. Elsevier Health Sciences; London: 2008. [Google Scholar]
- 41.Vujaskovic Z, Feng QF, Rabbani ZN, Samulski TV, Anscher MS, Brizel DM. Assessment of the protective effect of amifostine on radiation-induced pulmonary toxicity. Exp Lung Res. 2002;28:577–590. doi: 10.1080/01902140290096791. [DOI] [PubMed] [Google Scholar]
- 42.Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73:1491–1501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunwar A, Narang H, Priyadarsini KI, Krishna M, Pandey R, Sainis KB. Delayed activation of PKCdelta and NFkappaB and higher radioprotection in splenic lymphocytes by copper (II)-Curcumin (1:1) complex as compared to curcumin. J Cell Biochem. 2007;102:1214–1224. doi: 10.1002/jcb.21348. [DOI] [PubMed] [Google Scholar]
- 44.Zampetaki A, Minamino T, Mitsialis SA, Kourembanas S. Effect of heme oxygenase-1 overexpression in two models of lung inflammation. Exp Biol Med. 2003;228:442–446. doi: 10.1177/15353702-0322805-02. [DOI] [PubMed] [Google Scholar]
- 45.Risom L, Moller P, Vogel U, Kristjansen PE, Loft S. X-ray-induced oxidative stress: DNA damage and gene expression of HO-1, ERCC1 and OGG1 in mouse lung. Free Radic Res. 2003;37:957–966. doi: 10.1080/1071576031000150788. [DOI] [PubMed] [Google Scholar]
- 46.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 47.Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–1647. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 48.Jagetia GC. Radioprotection and radiosensitization by curcumin. Adv Exp Med Biol. 2007;595:301–320. doi: 10.1007/978-0-387-46401-5_13. [DOI] [PubMed] [Google Scholar]