Abstract
Aim
To examine whether withdrawal after abstinence and cue-elicited craving were associated with polymorphisms within two genes involved in regulating the endocannabinoid system, cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH). Two single nucleotide polymorphisms (SNPs) in the CNR1 (rs2023239) and FAAH (rs324420) genes, associated previously with substance abuse and functional changes in cannabinoid regulation, were examined in a sample of daily marijuana smokers.
Participants
Participants were 105 students at the University of Colorado, Boulder between the ages of 18 and 25 years who reported smoking marijuana daily.
Measurements
Participants were assessed once at baseline and again after 5 days of abstinence, during which they were exposed to a cue-elicited craving paradigm. Outcome measures were withdrawal and craving collected using self-reported questionnaires. In addition, urine samples were collected at baseline and on day 5 for the purposes of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC–COOH) metabolite analysis.
Findings
Between the two sessions, THC–COOH metabolite levels decreased significantly, while measures of withdrawal and craving increased significantly. The CNR1 SNP displayed a significant abstinence × genotype interaction on withdrawal, as well as a main effect on overall levels of craving, while the FAAH SNP displayed a significant abstinence × genotype interaction on craving.
Conclusions
These genetic findings may have both etiological and treatment implications. However, longitudinal studies will be needed to clarify whether these genetic variations influence the trajectory of marijuana use/dependence. The identification of underlying genetic differences in phenotypes such as craving and withdrawal may aid genetically targeted approaches to the treatment of cannabis dependence.
Keywords: Cannabis, CNR1, craving, cues, FAAH, genes, marijuana, withdrawal
Introduction
The investigation of genetic factors that influence the development and expression of drug-related problems is one of the most promising areas of research on addiction. With respect to the heritability of illicit drug use, and in particular cannabis use, recent studies have documented that genetic factors account for a significant portion of variance in use, abuse and dependence [1–7]. In an effort to identify genetic factors and the mechanisms by which they influence addiction, the utilization of an intermediate phenotype or ‘endophenotype’ approach may be useful. The ideal intermediate phenotype, or ‘endophenotype,’ would be one that is narrowly defined, associated with the clinical manifestation of addiction, and related to an underlying biological mechanism [8]. Importantly, the use of narrowly defined phenotypes increases the statistical power available to detect significant associations with particular candidate genes and facilitates the interpretation of the findings. While recent work on alcohol and drug abuse has moved in this direction, cannabis research has yet to follow because of the lack of cannabis dependence endophenotypes.
Craving and loss of control over drug use behavior are constructs that are central to the addiction process [9–14]. Craving has been linked to the action of drugs on the mesocorticolimbic dopamine pathways in the brain and the repeated activation of these pathways is thought to be an important factor in the etiology of addiction [15–17]. The study of cue-elicited craving for alcohol, tobacco and other drugs has been proven to be a useful approach to understanding basic mechanisms related to addiction. Numerous studies have indicated that exposure to smoking cues (e.g. the sight and smell of a lit cigarette) markedly increases craving for tobacco [17–20] and that exposure to alcohol cues increases craving for alcohol [21–24].
With respect to the cannabis literature, cue-elicited craving for cannabis represents a potentially powerful, and untapped, endophenotype for research on genetic factors related to cannabis abuse. To date, there is only one published study of cue-elicited craving for cannabis. In this report, scripts describing an individual experiencing cannabis craving were used to manipulate craving [25]. The results of this study demonstrated the reliability and validity of a marijuana craving measure (the Marijuana Craving Questionnaire) and demonstrated that this measure was sensitive to script condition (no urge, low urge and high urge), demonstrating that imagery scripts can be used to elicit craving for cannabis.
With respect to marijuana withdrawal, a growing number of controlled studies suggest the presence of a reliable and empirically valid withdrawal syndrome for cannabis users [26–31]. Withdrawal symptoms observed include anger, aggression, irritability, restlessness, shakiness, sleep disturbance, decreased appetite and decreased weight [26]. The time–course of withdrawal symptoms is similar to the time–course of symptoms associated with other drugs such as tobacco. Onset begins within 1–3 days of abstinence, with the peak effects occurring on or before day 6, persisting for as long as 10–14 days [26,30]. Reports suggests that as many as 67% of cannabis-dependent adolescents reported problems with withdrawal [32], while 16% of individuals who smoked at least 21 days per year reported withdrawal [33].
While cannabis withdrawal may not include some of the serious medical problems observed with alcohol and opioid withdrawal, it is likely that the symptoms associated with cannabis withdrawal (e.g. negative affect, appetite and sleep disturbance) contribute to the development and intractability of cannabis dependence. In this sense, cannabis withdrawal may be analogous to other, better-understood withdrawal syndromes (e.g. tobacco withdrawal, alcohol withdrawal) that have been the target of intervention efforts. Furthermore, cannabis withdrawal has been described increasingly in terms of the physiological sequelae that coincide with this syndrome, including alterations in dopamine neurotransmission [34], as well as alterations in other systems [35].
The first candidate gene we examined was the cannabinoid receptor 1 gene (CNR1). A report by Zhang and colleagues [36] suggests that an intronic single nucleotide polymorphism (SNP) (rs2023239) may create an alternative CNR1 transcript in the brain and suggests that this SNP and two others are associated with substance abuse in general. However, a recent study conducted by Herman and colleagues [37] failed to replicate these findings in both European American and African American populations with a primary diagnosis of alcohol dependence. The second candidate gene chosen for investigation was the fatty acid amide hydrolase gene (FAAH). Previous studies have demonstrated that the fatty acid amide hydrolase (FAAH), the enzyme that metabolizes Δ9-tetrahydrocannabinol (THC) in the brain, is a critical temporal regulator of endocannabinoid signaling [38–40]. One SNP in FAAH of interest (rs324420) involves a non-synonomous 385C to A substitution that converts a conserved proline residue to threonine. A previous study found an association between this SNP and substance abuse [38]. Although the functional significance of this non-synonomous SNP is not understood fully, the 385A variant encodes for a mutant FAAH enzyme characterized by reduced cellular stability compared to the wild-type [41]. Thus, the A variant may lead to lower FAAH enzyme levels, resulting in greater endocannabinoid activity via less efficient degradation of endocannabinoids and in turn may have an impact on measures of withdrawal, craving and mood.
The objectives of the present study were to examine craving and withdrawal after 5 days of marijuana abstinence in a sample of daily marijuana smokers. In addition, the present study was designed to test whether putative functional SNPs within the CNR1 and FAAH genes were associated with changes in craving and withdrawal after abstinence.
Methods
Participants
All participants gave their written, informed consent before participating in this study. Participants were students at the University of Colorado, Boulder between the ages of 18 and 25 years. Individuals were excluded from the study if they were taking any psychotropic medications, currently using any recreational drugs other than marijuana or attempting to quit smoking marijuana. In addition, participants had to be daily marijuana smokers during the past year (smoke marijuana at least once a day, 5–7 days per week). Participants received $50 for participating in this study. The University of Colorado Human Research Committee approved all procedures.
Procedure
Session 1
After completing a telephone screen, which included questions concerning the quantity and frequency of marijuana use, use of other recreational drugs and use of psychotropic medications, participants were invited to the laboratory for the first session of the study. Participants were instructed not to drink any alcohol 24 hours prior to, or smoke any marijuana 6 hours prior to, their session. Upon arrival to the laboratory, participants read and signed an informed consent form and provided a cheek cell and saliva sample for DNA analysis. Individuals were also breathalyzed to ensure that they had not recently used alcohol. In addition, they gave a urine sample in order to obtain a baseline THC level. Finally, participants completed a series of questionnaires pertaining to their drug use patterns, personality and mood. After this session was completed, they were scheduled to come back to the laboratory 5 days later to complete the second session of the study. They were instructed not to smoke any marijuana between the two sessions and were informed that a urine toxicology screen would be performed to confirm their abstinence.
Session 2
When participants arrived at the laboratory for the second session, they were again breathalyzed to ensure that they did not have alcohol in their system. They also provided another urine sample for THC analysis. Participants then completed measures concerning their mood and craving for marijuana. Finally, they were exposed to the cue and again completed the craving and mood measures.
Cue exposure
Based on participants' preference, they were exposed to either a used bong or pipe while sitting alone at a desk. They listened to an audio tape that instructed them to focus their attention on the bong/pipe, smell the bong/pipe, and imagine what it would be like to smoke marijuana out of the bong/pipe. The exposure lasted approximately 2.5 minutes. Participants completed measures of craving and mood before and after exposure to the cue.
Individual difference measures
A demographics questionnaire was used to collect information on age, sex, marital status, socio-economic status, occupation, income, education and race/ethnicity.
A drug use history questionnaire was used to assess the frequency of life-time and recent use for cocaine, amphetamine, opiates, sedatives, hallucinogens and alcohol.
A time-line follow-back (TLFB) interview was used to assess daily substance use for the 30 days prior to the screening session [42]. The TLFB is a calendar-assisted structured interview that provides the subject with temporal cues to increase the accuracy of recall. This interviewer-administered instrument has demonstrated test–retest reliability and validity [43].
The Marijuana Dependence Checklist (MDC) is based on DSM-IV criteria and consists of the following: (i) wanted or tried to cut down on cannabis use but could not; (ii) the same amount of cannabis had less effect or the same effect took more; (iii) spent a great deal of time getting, using or getting over the effects of cannabis; (iv) used cannabis more often or in larger amounts than wanted to; (v) kept from engaging in work, school or recreation; (vi) cannabis-related psychological problems; and (vii) cannabis-related health problems. The sum of the symptoms endorsed is a proxy measure of the extent of marihuana use disorder (abuse and dependence symptoms).
The Alcohol Consumption Questionnaire (ACQ) was used to assess alcohol consumption over the past year (12 months). The ACQ asks participants to indicate the type of alcohol usually consumed, amount of alcohol usually consumed in a ‘typical’ drinking session, largest amount of alcohol ever consumed and how often alcohol is used.
Experimental measures
The Marijuana Withdrawal Checklist (MWC) was used to collect information on withdrawal symptoms during both sessions at baseline. The MWC is a multiple-item scale of marijuana withdrawal [26,44].
The Profile of Mood States (POMS) was used to collect information on mood changes. The POMS is a reliable and valid measure of affect [45,46].
The Craving and Mood Questionnaire (CMQ) consists of five items that were rated on a scale of 0–100 and were combined to form a marijuana craving scale. The five items (modified to measure craving for marijuana) are: ‘I crave marijuana right now’, ‘I have an urge for marijuana’, ‘I have a desire for marijuana right now,’ ‘if it were possible, I would smoke marijuana now’ and ‘all I want right now is marijuana’.
THC metabolite analysis
In order to determine whether individuals were compliant with the marijuana abstinence instructions, urine samples were collected pre- (session 1) and post-5-day abstinence period (session 2). Collected samples were frozen immediately and shipped on ice to the Center for Human Toxicology at the University of Utah Health Sciences Center for quantitative THC metabolite analysis. A gas chromatograph-mass spectrometer using electron impact ionization (GC/MS EI) and selective ion monitoring (SIM) was used to quantitate levels of 11-nor-9-carboxy–Δ9-tetrahydrocannabinol (THC–COOH), the primary metabolite of marijuana. Briefly, a four-point standard curve (0, 7.5, 15 and 30 ng/ml) was generated for THC–COOH in drug-free urine. The curve was run in duplicate, quality control samples in triplicate and samples singly. Prior to extraction, 30 ng of internal standard (d3-?9 THC–COOH) was added to all samples, standards and controls. Quantitation was achieved by calculating the peak-area ratios of derivatized THC–COOH/internal standard. Observing a reduction in THC–COOH urine levels between the pre- and post-abstinence period was used to confirm abstinence from marijuana use during the 5-day abstinence period.
Compliance
To confirm compliance, and exclude those who were non-compliant, with the marijuana abstinence instructions, urine samples collected at baseline and 5 days abstinent were analyzed for THC–COOH metabolite levels. Individuals were excluded from the statistical analyses if their day 5 THC–COOH levels were not 50% lower than their baseline THC–COOH levels. Using the exclusion criteria noted above, 35 of the 140 participants were found to be non-compliant and as such were excluded from statistical analyses, leaving this study with a sample size of 105 individuals. However, we compared these 35 subjects who did not maintain compliance to those who did. χ2 analyses were used to compare the groups on sex and frequency of the CNR1 and FAAH SNPs. There were no differences on these variables (P > 0.05); t-tests were used to compare the groups on marijuana use variables. Although not significant, the non-compliant group was slightly higher than the compliant group in terms of the marijuana dependence score [mean = 5.8, standard deviation (STD) = 2.4 versus mean = 5.2, STD = 2.3] and slightly lower in quantity (mean = 50.5, STD = 36.6; mean = 56.5, STD = 31.1) and frequency of use (mean = 25.7, STD = 5.5; mean = 27.7, STD = 3.9). The difference in frequency use approached significance (P = 0.06). Compliant participants demonstrated a mean decrease of urine THC–COOH by 68% between baseline and day 5 of abstinence (data not shown).
DNA extraction and genetic analysis
To facilitate the third aim, DNA from cheek swabs was collected and extracted following published procedures [22,47,48]. The CNR1 rs2023239 and FAAH rs324420 SNPs were genotyped using 5′-nuclease assays (TaqMan®, Foster City, CA, USA) purchased from ABI in conjunction with the ABI 7500 thermocycler [49]. To ensure accurate genotyping results, accuracy was assessed by re-genotyping 25% of the total sample at random for both SNPs, and was found to be 100% accurate.
Overview
Pre-test baseline differences and frequencies for the CNR1 and FAAH SNPs are presented in Table 1. The frequencies of the SNPs were consistent with previous reports and both SNPs were in Hardy–Weinberg equilibrium. Because, for each SNP, only a few individuals were homozygous for the minor allele, these individuals were combined with the heterozygote group to form one group that was then compared with the group that was homozygous for the common allele. The FAAH and CNR1 SNPs were not in linkage disequilibrium. Analysis of the effects of cannabis withdrawal utilized a 2 (abstinence: pre-abstinence versus post-abstinence) × 2 [genotype: CNR1 T/T versus CNR1 T/C (T/C and C/C) or FAAH C/C versus C/A (C/A and A/A)] mixed analyses of variance where trial was a within-subjects factor and genotype was a between-subjects factor. Analysis of the effects of cue-exposure utilized a 3 (cue: pre-abstinence, pre-cue exposure versus post-abstinence, pre-cue exposure versus post-abstinence, post-cue exposure) × 2 (genotype: CNR1 T/T versus CNR1 T/C or FAAH C/C versus C/A) mixed analysis of variance where cue was a within-subjects factor and genotype was a between-subjects factor. SAS version 9.1 was used to conduct all analyses.
Table 1.
Pre-test differences for cannabinoid receptor 1 (CNR1) rs2023239 single nucleotide polymorphism (SNP)—(T/T versus T/C), and for fatty acid amide hydrolase (FAAH) rs324420 SNP—(C/C versus C/A) participants [means and standard deviations of model constructs for sexually active adolescents (n = 147)].
| Variable† | CNR1 T/T (n = 74) | CNR1 T/C (n = 31) | Test for difference | FAAH C/C (n = 70) | FAAH C/A (n = 35) | Test for difference |
|---|---|---|---|---|---|---|
| Gender (% male) | 75.7% | 74.19% | χ2(1) = 0.026; P = 0.87 | 75.7% | 74.3% | χ2(1) = 0.026; P = 0.87 |
| Race (% Caucasian) | 85.7% | 93.3% | χ2(4) = 5.28; P = 0.26 | 86.8% | 90.6% | χ2(4) = 0.84, P = 0.93 |
| Age | 19.31 (1.53) | 19.58 (1.46) | t (103) = −0.83, ns | 19.3 (1.48) | 19.57 (1.58) | t (103) = −0.87, NS |
| Baseline THC level (ng/ml) | 326.31 (363.46) | 419.6 (402.6) | t (103) = −1.16, ns | 360.26 (378.95) | 341.03 (374.92) | t (103) = 0.25, NS |
| Marijuana dependency checklist (MDC) (possible range of scale: 0–10) | 4.95 (2.37) | 5.94 (2.05) | t (103) = −2.06, P < 0.045 | 5.19 (2.34) | 5.34 (2.30) | t (103) = −0.33, NS |
| Frequency of marijuana use for past month (possible range of scale: 0–30 days) | 27.45 (3.79) | 28.29 (4.18) | t (102) = −1.00, ns | 27.51 (3.9) | 28.09 (3.96) | t (102) = −0.70, NS |
| Frequency of alcohol use for past month (possible range of scale: 0–30 days) | 11.86 (6.48) | 14.61 (7.36) | t (102) = −1.90, P = 0.06 | 13.26 (7.02) | 11.5 (6.37) | t (102) = 1.23, NS |
| Quantity of cigarette use for past month (# cigarettes) | 82.58 (153.45) | 136.05 (219.45) | t (102) = −1.41, P = 0.16 | 94.76 (181.3) | 106.26 (168) | t (102) = −0.31, NS |
| Quantity of marijuana use for past month (# joints) | 51.66 (29.32) | 68.05 (32.7) | t (102) = −2.52, P = 0.01 | 57.42 (30.22) | 54.74 (32.69) | t (102) = 0.41, NS |
| Frequency of marijuana use per day | 3.34 (1.84) | 4.31 (1.71) | t (82) = −2.27, P = 0.026 | 3.65 (1.94) | 3.63 (1.70) | t (82) = 0.07, NS |
| Age of first marijuana use | 14.7 (1.63) | 14.48 (1.46) | t (103) = 0.65, ns | 14.46 (1.52) | 15 (1.65) | t (103) = −1.68, P = 0.10 |
| Age of onset of regular marijuana use | 16.34 (1.63) | 16.5 (1.58) | t (103) = −0.47, ns | 16.23 (1.64) | 16.69 (1.53) | t (103) = −1.36, P = 0.17 |
| Number of years of regular marijuana smoking | 3.21 (1.73) | 3.97 (3.35) | t (103) = −1.52, P = 0.13 | 3.35 (1.74) | 3.6 (3.25) | t (103) = −0.51, NS |
| Attempt to cut down on marijuana smoking (range of scale: 0–1) | 0.649 (0.481) | 0.710 (0.461) | t (103) = −0.60, ns | 0.686 (0.468) | 0.629 (0.490) | t (103) = 0.58, NS |
Standard deviations appear in parentheses below the means of continuous variables. NS: not significant; THC: Δ9-tetrahydrocannabinol.
Participant characteristics
Demographic information and pre-test comparisons are presented in Table 1. The first set of analyses tested for differences among CNR1 (T/T versus T/C) and FAAH (C/C versus C/A) genotype groups on baseline demographics and marijuana use variables that might confound the main analyses. There were no differences for either of these genes on age, race or gender. There were, however, differences on a few marijuana use measures, such that CNR1 T/C individuals scored significantly higher on the MDC and reported significantly higher quantity and frequency of marijuana use per month and day, respectively. These variables were not used as covariates because this study utilized an intermediate phenotype approach (aka, endophenotype), and as such it is expected that the genetic variable(s), the intermediate phenotype(s) and the clinical phenotype(s) are all related. The premise of this approach is that genetic variables contribute to withdrawal and cue-elicited craving, which contributes in turn to frequency of use and dependence. The correlation between MDC and the withdrawal score after abstinence was r(100) = 0.27, P < 0.05, and the correlation between MDC and craving after cue exposure was r(102) = 0.19, P = 0.058.
Results
Withdrawal
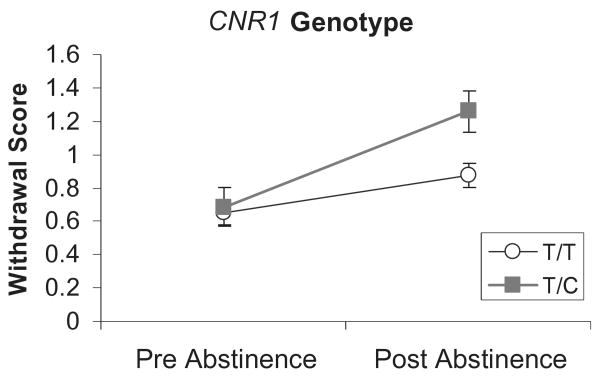
Analyses of the withdrawal scale revealed a significant effect for abstinence, F(1,96) = 35.55, P < 0.0001, such that withdrawal increased significantly after 5 days of abstinence. Analysis of the CNR1 SNP revealed a significant abstinence × genotype interaction, F(1,96) = 6.71, P < 0.012, indicating that T/C individuals demonstrated greater withdrawal after abstinence (see Fig. 1). There was a trend towards a main effect for the CNR1 SNP P < 0.082. Analysis of the FAAH SNP did not reveal any significant effects.
Figure 1.
Withdrawal scores pre- and post-5-day abstinence from marijuana by cannabinoid receptor 1 (CNR1) genotype. Values represent the mean ± 1 standard error of the mean. Individuals with the T/C genotype demonstrated significantly greater post-abstinence withdrawal scores compared to T/T individuals
Effects of withdrawal on mood
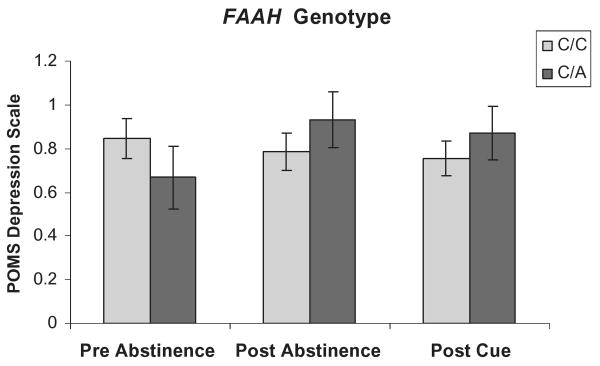
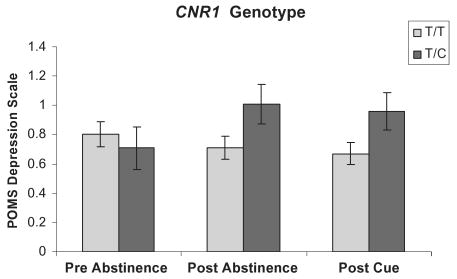
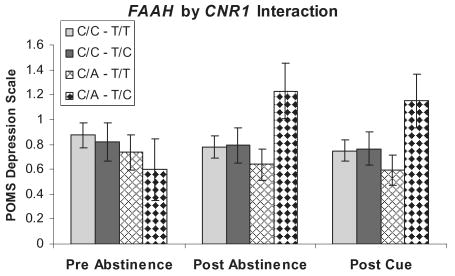
Ratings on the POMS subscale of depression/negative affect were higher after 5 days of abstinence. The FAAH C/A carriers (Fig. 2) trended toward a significant abstinence × genotype interaction, F(2,194) = 3.31, P < 0.056, while CNR1 T/C carriers (Fig. 3) demonstrated a significant abstinence × genotype interaction, F(2,194) = 5.22, P < 0.02. However, exposure to a marijuana cue had no effect. Furthermore, there was a significant additive interaction effect of these two genotypes on negative affect such that individuals that were FAAH C/A carriers and CNR1 T/C carriers reported greater negative affect than any other genotype combination F(2,194) = 3.55, P < 0.047 (Fig. 4).
Figure 2.
Mood effects on the Profile of Mood States depression scale at pre-abstinence, post-abstinence and post-cue exposure from marijuana by fatty acid amide hydrolase (FAAH) genotype. Values represent the mean ± 1 standard error of the mean. Individuals with the FAAH C/A genotype displayed a trend toward greater negative affect at post-abstinence and post-cue exposure from marijuana
Figure 3.
Mood effects on the Profile of Mood States depression scale at pre-abstinence, post-abstinence and post-cue exposure from marijuana by cannabinoid receptor 1 (CNR1) genotype. Values represent the mean ± 1 standard error of the mean. Individuals with the CNR1 T/C genotype demonstrated significantly greater negative affect at post-abstinence and post-cue exposure from marijuana
Figure 4.
Mood effects on the Profile of Mood States depression scale at pre-abstinence, post-abstinence and post-cue exposure from marijuana by fatty acid amide hydrolase–cannabinoid receptor 1 (FAAH–CNR1) genotype. Values represent the mean ± 1 standard error of the mean. Individuals possessing the FAAH–CNR1 genotype C/A–T/C demonstrated a significant gene (gene additive interaction and displayed greater negative affect after marijuana withdrawal at post-abstinence and post-cue exposure from marijuana
Effects of cue exposure
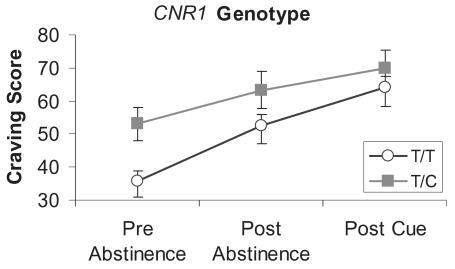
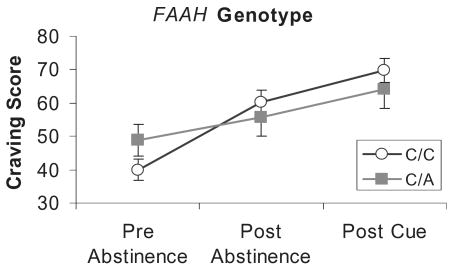
Analyses of the craving scale revealed a significant effect for abstinence, F(2,194) = 30.24, P < 0.0001, indicating that craving increased significantly after abstinence. Analysis of the CNR1 SNP revealed a significant main effect, F(2,194) = 4.3, P < 0.041, but no interaction, indicating that craving was significantly greater at pre-abstinence, post-abstinence and post-cue exposure from marijuana for the CNR1 T/C group (see Fig. 5). Analysis of the FAAH SNP revealed a significant abstinence × genotype interaction, F(2,194) = 3.90, P < 0.04, but no significant main effect for the FAAH SNP. The FAAH C/C group demonstrated a greater increase in craving after abstinence compared to the FAAH C/A group (see Fig. 6). Exposure to the marijuana cues did not impact significantly any of the mood scales.
Figure 5.
Craving scores pre-abstinence, post-abstinence and post-cue exposure from marijuana by cannabinoid receptor 1 (CNR1) genotype. Values represent the mean ± 1 standard error of the mean. Individuals with the T/C genotype demonstrated significantly greater pre-abstinence, post-abstinence and post-cue craving scores compared to T/T individuals
Figure 6.
Craving scores pre-abstinence, post-abstinence and post-cue exposure from marijuana by fatty acid amide hydrolase (FAAH) genotype. Values represent the mean ± 1 standard error of the mean. Individuals with the C/C genotype demonstrated significantly greater increase in withdrawal scores compared to C/A individuals and fatty acid amide hydrolase (FAAH)
Discussion
Consistent with previous findings [44], the present study indicates that abstinence from marijuana use precipitates withdrawal in daily smokers. Abstinence from marijuana use also produces significant increases in withdrawal, craving and negative affect. In addition, the findings from the present study indicate that exposure to marijuana cues (e.g. sight and smell of a used marijuana bong) increase craving above and beyond craving levels due to withdrawal. The results indicate that 5 days of abstinence has a highly significant impact on variables such as withdrawal, negative affect and craving. Given that significant withdrawal, negative affect and craving are often conceptualized as predictors of treatment success, these variables may represent useful targets, both in the context of gene association studies as well as studies that seek to identify medications that may be used to treat cannabis dependence.
With respect to the use of these variables as endophenotypes, the findings also provide some ‘proof of concept’ in the form of significant associations between these variables and putatively functional SNPs that have been associated previously with substance abuse. The findings indicate that a T to C SNP in the CNR1 gene is a significant predictor of withdrawal after abstinence, as well as a predictor of overall levels of craving. A previous study has suggested that individuals with the C substitution may have an alternative CNR1 transcript that is associated with a higher risk for developing substance abuse problems [36]. Consistent with this previous work, the present study indicates that individuals possessing one or more C alleles are more likely to experience greater withdrawal, negative affect and higher levels of craving to smoke more marijuana. In addition, CNR1 C-carriers had 20% higher marijuana dependency checklist scores and used 30% more joints per month as T/T subjects. Furthermore, the present study, consistent with a previous study linking the FAAH SNP with substance abuse [38], observed a significant association between this SNP and craving, such that individuals with the C/C genotype demonstrated significantly greater craving after abstinence than did individuals with at least one copy of the A allele.
These findings may have both etiological and treatment implications. For example, individuals with the CNR1 T/C genotype may be more likely to develop dependence and/or more likely to have trouble establishing abstinence or reducing marijuana use. However, longitudinal studies will be needed to clarify whether this genetic variable actually influences the trajectory of marijuana use/dependence. In addition, treatment studies that incorporate this information are needed to determine whether these (or other) genetic variants may influence treatment outcomes and determine whether alternative treatments may be indicated for these individuals.
Finally, future studies should include diagnostic interviews to document abuse/dependence and also examine comorbid anxiety disorders. Furthermore, future studies are needed to determine whether these SNPs may be associated with differential sensitivity to the acute effects of marijuana. Previous studies have suggested that sensitivity to marijuana plays an important role in the development of marijuana use problems [50,51]. Clearly, the subjective experience of marijuana use in terms of changes in mood, euphoria and reward may play an important role in the trajectory of marijuana use. It stands to reason that SNPs that alter either the regulation of cannabinoid signaling (e.g. the FAAH SNP) or create an alternative form of the CB1 receptor (e.g. the CNR1 SNP) may also alter the subjective effects of marijuana use. Future research needs to examine these genetic variables in the context of marijuana administration in order to determine whether these SNPs alter the acute effects of marijuana use.
In conclusion, the cannabis dependence endophenotypes, craving and withdrawal, are important factors in the etiology and treatment of cannabis dependence and, given growing recognition of the underlying physiological sequalae that coincide with long-term cannabis use, these phenotypes are likely to lend themselves to the identification of underlying genetic factors that have direct implications for treatment approaches.
Acknowledgments
This research was supported by a grant from the National Institute on Alcoholism and Alcohol Abuse (AA015331) (H. H.), (AA012238) (K. H.).
Footnotes
Declarations of interest: None.
References
- 1.Grove WM, Eckert ED, Heston L, Bouchard TJ, Jr, Segal N, Lykken DT. Heritability of substance abuse and antisocial behavior: a study of monozygotic twins reared apart. Biol Psychiatry. 1990;27:1293–304. doi: 10.1016/0006-3223(90)90500-2. [DOI] [PubMed] [Google Scholar]
- 2.Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, et al. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet. 1996;67:473–7. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 3.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–72. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 4.Gynther LM, Carey G, Gottesman II, Vogler GP. A twin study of non-alcohol substance abuse. Psychiatry Res. 1995;56:213–20. doi: 10.1016/0165-1781(94)02609-m. [DOI] [PubMed] [Google Scholar]
- 5.Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, et al. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998;55:982–8. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- 6.Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57:261–9. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–95. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 9.Pickens RW, Johanson CE. Craving: consensus of status and agenda for future research. Drug Alcohol Depend. 1992;30:127–31. doi: 10.1016/0376-8716(92)90017-7. [DOI] [PubMed] [Google Scholar]
- 10.Kozlowski LT, Wilkinson DA. Use and misuse of the concept of craving by alcohol, tobacco, and drug researchers. Br J Addict. 1987;82:31–45. doi: 10.1111/j.1360-0443.1987.tb01430.x. [DOI] [PubMed] [Google Scholar]
- 11.Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York: Guilford Press; 1985. [Google Scholar]
- 12.Anton RF. Alcohol craving—a renaissance. Alcohol Clin Exp Res. 1999;23:1287–8. doi: 10.1111/j.1530-0277.1999.tb04348.x. [DOI] [PubMed] [Google Scholar]
- 13.Sinha R, O'Malley SS. Craving for alcohol: findings from the clinic and the laboratory. Alcohol Alcohol. 1999;34:223–30. doi: 10.1093/alcalc/34.2.223. [DOI] [PubMed] [Google Scholar]
- 14.Verheul R, van den BW, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. 1999;34:197–222. doi: 10.1093/alcalc/34.2.197. [DOI] [PubMed] [Google Scholar]
- 15.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 16.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive–sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 17.Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. J Abnorm Psychol. 1988;97:118–32. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- 18.Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–52. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- 19.Carter BL, Tiffany ST. Cue–reactivity and the future of addiction research. Addiction. 1999;94:349–51. [PubMed] [Google Scholar]
- 20.Tiffany ST, Carter BL, Singleton EG. Challenges in the manipulation, assessment and interpretation of craving relevant variables. Addiction. 2000;95:S177–87. doi: 10.1080/09652140050111753. [DOI] [PubMed] [Google Scholar]
- 21.Hutchison KE, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Exp Clin Psychopharmacol. 1999;7:250–6. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D, Almeida A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology (Berl) 2001;155:27–34. doi: 10.1007/s002130000629. [DOI] [PubMed] [Google Scholar]
- 23.Hutchison KE, LaChance H, Niaura R, Bryan A, Smolen A. The DRD4 VNTR polymorphism influences reactivity to smoking cues. J Abnorm Psychol. 2002;111:134–43. doi: 10.1037//0021-843x.111.1.134. [DOI] [PubMed] [Google Scholar]
- 24.Hutchison KE, Wooden A, Swift RM, Smolen A, McGeary J, Adler L, et al. Olanzapine reduces craving for alcohol: a DRD4 VNTR polymorphism by pharmacotherapy interaction. Neuropsychopharmacology. 2003;28:1882–8. doi: 10.1038/sj.npp.1300264. [DOI] [PubMed] [Google Scholar]
- 25.Glautier S, Drummond DC. Alcohol dependence and cue reactivity. J Stud Alcohol. 1994;55:224–9. doi: 10.15288/jsa.1994.55.224. [DOI] [PubMed] [Google Scholar]
- 26.Singleton EG, Trotman AJ, Zavahir M, Taylor RC, Heishman SJ. Determination of the reliability and validity of the Marijuana Craving Questionnaire using imagery scripts. Exp Clin Psychopharmacol. 2002;10:47–53. doi: 10.1037//1064-1297.10.1.47. [DOI] [PubMed] [Google Scholar]
- 27.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- 28.Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–24. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- 29.Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–22. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- 30.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 31.Kouri EM, Pope HG., Jr Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8:483–92. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- 32.Kouri EM, Pope HG, Jr, Lukas SE. Changes in aggressive behavior during withdrawal from long-term marijuana use. Psychopharmacology (Berl) 1999;143:302–8. doi: 10.1007/s002130050951. [DOI] [PubMed] [Google Scholar]
- 33.Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug Alcohol Depend. 1998;50:27–37. doi: 10.1016/s0376-8716(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 34.Wiesbeck GA, Davids E, Wodarz N, Thome J, Weijers G, Jakob F, et al. Alcohol withdrawal and dopamine receptor sensitivity after prolonged abstinence. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:1171–80. doi: 10.1016/s0278-5846(96)00104-2. [DOI] [PubMed] [Google Scholar]
- 35.Diana M, Melis M, Muntoni AL, Gessa GL. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci USA. 1998;95:10269–73. doi: 10.1073/pnas.95.17.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maldonado R, Rodriguez de FF. Cannabinoid addiction: behavioral models and neural correlates. J Neurosci. 2002;22:3326–31. doi: 10.1523/JNEUROSCI.22-09-03326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang PW, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, et al. Human cannabinoid receptor 1: 5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry. 2004;9:916–31. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- 38.Herman AI, Kranzler HR, Cubells JF, Gelernter J, Covault J. Association study of the CNR1 gene exon 3 alternative promoter region polymorphisms and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141:499–503. doi: 10.1002/ajmg.b.30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci USA. 2002;99:8394–9. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egertova M, Giang DK, Cravatt BF, Elphick MR. A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc Biol Sci. 1998;265:2081–5. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onaivi ES, Leonard CM, Ishiguro H, Zhang PW, Lin Z, Akinshola BE, et al. Endocannabinoids and cannabinoid receptor genetics. Prog Neurobiol. 2002;66:307–44. doi: 10.1016/s0301-0082(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 42.Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–19. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 43.Sobell LC, Sobell MB. Convergent validity: an approach to increasing confidence in treatment outcome conclusions with alcohol and drug abusers. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating Alcohol and Drug Abuse Treatment Effectiveness: Recent Advances. New York: Pergamon; 1980. pp. 177–83. [Google Scholar]
- 44.Sobell MB, Sobell LC, VanderSpek R. Relationships between clinical judgement, self-report and breath analysis measures of intoxication in alcoholics. J Consult Clin Psychol. 1979;47:204–06. doi: 10.1037//0022-006x.47.1.204. [DOI] [PubMed] [Google Scholar]
- 45.Budney AJ, Moore BA. Development and consequences of cannabis dependence. J Clin Pharmacol. 2002;42:S28–33. doi: 10.1002/j.1552-4604.2002.tb06000.x. [DOI] [PubMed] [Google Scholar]
- 46.Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: d-amphetamine. Psychopharmacology (Berl) 1980;71:275–9. doi: 10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- 47.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 48.Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav Genet. 1997;27:251–7. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- 49.Walker AH, Najarian D, White DL, Jaffe JF, Kanetsky PA, Rebbeck TR. Collection of genomic DNA by buccal swabs for polymerase chain reaction-based biomarker assays. Environ Health Perspect. 1999;107:517–20. doi: 10.1289/ehp.99107517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–9. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 51.Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug Alcohol Depend. 2000;59:S41–60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]