Abstract
The expression of carbonic anhydrase IX (CAIX), a marker for hypoxic tumors, is correlated with poor prognosis in breast cancer patients. We show herein that the MDA-MB-231 cells, a “triple-negative,” basal B line, express exclusively CAIX, while a luminal cell line (T47D) expresses carbonic anhydrase XII (CAXII). CAIX expression in the basal B cells is both density-and hypoxia-dependent and is correlated with carbonic anhydrase activity. Evidence is provided that CAIX contributes to extracellular acidification through studies on pH, lactic acid production, and CAIX inhibition. Together, these studies suggest that CAIX expression and activity is associated with metabolic dysfunction in MDA-MB-231 cells.
Keywords: Carbonic anhydrase IX, Breast cancer, Metabolic phenotype
INTRODUCTION
Neoplastic cells undergo a metabolic transition which allows them to survive and grow in the hostile environment created by the decreased blood flow associated with the disordered vascularization of tumors (1, 2). The most striking feature of tumor cells is the production of excessive amounts of lactic acid, due to the increased conversion of glucose to lactic acid via glycolysis. This occurs even in the presence of oxygen (termed “aerobic glycolysis”) as discovered by Otto Warburg over 70 years ago [see (3)]. In part, lactic acid production is related to the overexpression of GLUT1 in human cancer (4–6), in addition to the increased expression of key glycolytic enzymes (6, 7). While it was originally thought that the intracellular pH of tumor cells would be reduced relative to normal cells, as is well known now, it is the pH of the interstitial fluid that drops. This is a result of the expression of several proteins whose function is to export protons from the cytosol to the extracellular space. These include the lactate transporter (7), vacuolar ATPase (8, 9), and Na+/H+ exchanger (10). This combination of events leading to tumor acidification is defined as the glycolytic (metabolic) phenotype of cancer cells. This is of great interest to cancer biologists because cancer cells are resistant to the toxic effects of acidification (11). Further, acidification of the microenvironment of tumors alters the efficacy of chemotherapy and radiation therapy (2) and is associated with tumor progression and increased invasion (11).
Recent studies have shown that tumors generated from glycolysis-deficient cells still create acidic microenvironments (12). This suggests that lactic acid is not the only cause of acidification in vivo. In that regard, metabolic profiling of glycolysis-impaired cells compared to parental cells revealed that CO2 may be a significant source of acidity in tumors (13). CO2 is an acidic oxide that reacts with water to form carbonic acid. In vivo, this reaction is catalyzed by members of the carbonic anhydrase (CA) family of enzymes that mediate the reversible hydration of CO2 to bicarbonate:
Of the 15 isoforms of CA, CAIX appears to be the best candidate for playing a role in regulating the acidity of the tumor environment. CAIX was originally identified in HeLa cells (14), although its normal tissue distribution is limited primarily to stomach and intestinal epithelial cells (15, 16). Further, it is induced by hypoxia (17, 18) and considered a marker for hypoxic tumors (19). CAIX is a membrane protein whose catalytic domain faces the interstitial space. Its specific activity is similar to that of CAII (20), a cytosolic form of this enzyme whose activity and structure have been well studied. Interestingly, ectopic expression of CAIX, alone, enhances cellular proliferation (14), while blocking its action with derivatives of the classical sulfonamide inhibitors (21) or by RNAi technology (22) reduces proliferation.
The number of available breast cancer cell lines is relatively small, and only a few have been extensively studied. Yet it has been suggested that these lines are likely to reflect the features of cancer cells in vivo (23). Based on this, we chose two widely studied lines to investigate the hypothesis that CAIX expression in breast cancer cells contributes to metabolic dysfunction. The T47D line is derived from a ductal carcinoma and is estrogen receptor positive (24). Analysis of transcriptional activity reveals that these cells align with luminal markers (25). When injected into nude mice, these cells form a solid tumor but do not readily metastasize (23). The MDA-MB-231 line is derived from an adenocarcinoma and expresses the receptor for EGF (24). These cells align with a group defined as basal B which represent the “triple-negative” tumors (estrogen and progesterone receptor negative and HER2 negative) (25). When injected into nude mice, these cells form tumors and aggressively metastasize (23). We have also used the MCF10A line which is derived from fibrotic tissue and is frequently used as a control for cancer cell lines. These cells are estrogen receptor negative, EGF receptor negative, and HER2 negative but E-cadheren positive. These cells do not form tumors in vivo. We will show that these cells are good metabolic controls but interestingly express CAIX.
The goal of the following studies was to determine if CAIX expression and activity contribute to the cells ability to acidify their environment. We discovered that, among the cell lines tested, the membrane-associated CA family members are differentially expressed. However, high expression of CAIX in MDA-MB-231 cells, along with that of cytoplasmic CAII, shows strong correlation with the ability to acidify the medium. This predicts that the most aggressive breast cancer cells will express CAIX, which is consistent with recent studies demonstrating increased mortality of breast cancer patients with tumors that express CAIX (26). These observations warrant further investigation in a wider sampling of cell lines.
EXPERIMENTAL PROCEDURES
Cell culture
The MDA-MB-231 (MDA) cell line was provided by Dr. Kevin Brown (University of Florida) and was plated at a density of 1,000 cells/cm2 DMEM (Gibco, 12100-061) containing 10% fetal bovine serum (FBS; Valley Biomedical, #BS3033). This is equivalent to plating 80,000 cells per 10-cm plate or 10,000 cells/mL. The T47D line was provided by Dr. Keith Robertson (University of Florida) and was plated at a density of 2,000 cells/cm2 McCoy’s medium (Gibco, #16600) containing 10% FBS and 0.2 units/mL bovine insulin (Elanco, #4020). The MCF 10A (MCF) line was purchased from ATCC and was plated at a density of 2,000 cells/cm2 Mammary epithelial basal medium (Cambrex Bioscience, #CC3151) supplemented with 0.1 µg/mL cholera toxin (Calbiochem, #227035). It should be pointed out that ∼40% of the cells fail to adhere during the first 24 hr, regardless of cell line. Thus, it is important to evaluate cell number at the time the experiment is performed for equivalency across cell types. MDA-MB-231 cells were provided fresh medium every 2 days. T47D and MCF10A cells were allowed to grow for 3 days after plating before providing fresh medium and then every 2 days thereafter. For each cell type, normoxic cells were compared to those treated with desferroxamine mesylate (DFO; an iron chelator which mimics hypoxia) or exposed to hypoxic conditions. Normoxic and DFO-treated cells were incubated in a humidified atmosphere at 37°C in 5% CO2. Hypoxic conditions (1% O2, 5% CO2, and balance N2) were maintained in humidified modulator incubator chambers (MIC-101) purchased from Billups-Rothenberg, Inc. Medium pH was determined using a handheld pH meter (IQ Instruments, model IQ120). Lactate and glucose concentration in the medium was determined using a VITROS DT II Bioanalyzer (Millipore Instruments).
Cell number determination
For determining growth curves, cells were released from plates with cell dissociation buffer (Gibco, #13151-014). An aliquot of cells was suspended in isotonic diluent (Fisher, #H60620). Cell number was determined using a Coulter counter (Beckman Coulter Corp., Model ZM).
Reverse transcriptase–polymerase chain reaction (RT-PCR) assay
Cells were washed with phosphate buffered saline (PBS) at room temperature, and total RNA was isolated using a kit purchased from Qiagen (RNeasy Mini Kit, #74104). Ratios at 260/280 nm were used to evaluate quality and estimate concentration of RNA. For amplification reactions, components for both cDNA synthesis and PCR were combined in a single reaction (SuperScript One-Step RT-PCR with Platinum Taq, #10928-042) along with an aliquot of RNA (0.1–0.2 µg) and gene-specific primers. RNA isolated from human tissue was purchased from Clontech and used to verify if the PCR reactions were successful. This also revealed that the product size from these control PCR reactions was equivalent to the predicted size for each CA family member. Primers were designed to span intron–exon boundaries to assure that they would not amplify genomic DNA. Forward (F) and reverse (R) primers, designed using Primer3, are listed in Table 1.
Table 1.
Primers for RT-PCR
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | PCR Product Size (bp) |
|---|---|---|---|
| CA2 | CAATGGTCATGCTTTCAAC | AGCACAATCCAGGTCACACA | 450 |
| CA4 | CACTGGTGCTACGAGGTTCA | CAGTCCTCCTCCAGAAATGC | 252 |
| CA9 | GTCTCGCTTGGAAGAAATCG | AGAGGGTGTGGAGCTGCTTA | 200 |
| CA12 | AGTACAAAGGCCAGGAAGCA | CTTGCACTTGGGAGAAGGAG | 300 |
| CA14 | TGGAGACTGCAGAGGGAGAT | GCCCTCATACGTCCAGTGTT | 359 |
| GAPDH | GTCAGTGGTGGACCTGACCT | AGGGGTCTACATGGCAACTG | 420 |
Lysate preparation
After treatment as indicated in figure legends, cells were placed on ice, washed with cold PBS, and lysed in lysis buffer [1% Triton X-100, 50-mM Tris-HCl (pH 7.5), 150-mM NaCl, 1-mM EDTA, 1-mM EGTA, 0.5-mM sodium orthovanadate, 25-mM NaF]. Protease inhibitor (Roche Diagnostics, #11836170001) was added to the solution according to the manufacturer’s instructions. Cell lysates were clarified by centrifugation at 16,300 × g for 15 min at 4°C. Protein concentration in the clarified lysates was determined using the bicinchoninic acid (BCA) assay kit from Pierce (#23235).
Membrane isolation
Cells were washed three times with 5-mL Krebs–Ringer phosphate (KRP) buffer (pH 7.4) at 37°C and incubated for 10 min. Cells were then homogenized in a Tris-based buffer (TESp) containing 20 mM Tris–HCl (pH7.4), 255-mM sucrose, 1-mM EDTA, and protease inhibitor (Roche Complete Mini EDTA-free, #11836 170 001). A total membrane fraction was collected at 212,000 × g. This pellet was washed once and recollected by centrifugation. The final pellet was resuspended in a small volume of TESp. Protein concentration was determined using a modification of the Lowry assay (27). Subcellular fractions were collected as previously published (28). Briefly, cells (from plates that were 5–10 cm) were washed in KRP and then scraped into TESp buffer at 18°C. Cells were homogenized at the same temperature using a steel block/ball bearing system as designed by Balch and Rothman (29). The homogenate was subjected to a combination of differential and sucrose gradient centrifugation as described by Weber et al. (30), which provided a plasma membrane fraction (PM), a high-density membrane fraction (ER and Golgi), and a low-density fraction (endosomal). Final pellets were resusupended in TESp and protein measured using the modified Lowry assay.
Carbonic anhydrase (CA) activity assay
An Extrell EXM-200 mass spectrophotometer was used to measure the rate of exchange of 18O from CO2 and bicarbonate to water at chemical equilibrium as previously described (31). This exchange is catalyzed by, and is a measure of, CA activity.
Glucose transport activity assay
Uptake of [3H]-deoxyglucose to measure glucose transport rates has been previously described (32). Briefly, cells adherent to 35-mm plates were washed in KRP at 37°C and then incubated with 40-µM cytochalasin B or dimethyl sulfoxide (DMSO) for 10 min. This was followed by a 10-min incubation with 0.2-mM [3H]-deoxyglucose. Cells were finally washed with ice-cold PBS. After drying, the cells were lysed in 0.1% sodium dodecyl sulfate (SDS), and aliquots were counted by scintillation spectrometry.
SDS polyaerylamide gel electrophoresis (SDS-PAGE) and western blot analysis
These procedures have been described in previous publications (32). Briefly, aliquots of concentrated cytosolic fractions and/or membrane fractions were loaded onto 10% polyacry-lamide SDS gels under reducing conditions, and proteins were separated by electrophoresis. Proteins were transferred to nitrocellulose and detected by enhanced chemiluminescence (Amersham, #RPN2109). Two antibodies were used to identify CAIX. For extracts containing the cytosolic fraction (lysates), we used the mouse monoclonal antibody (M75) which recognizes the proteoglycan domain of CAIX. This antibody was developed by Pastorek et al. (14). The clone for this antibody was provided to one of the authors (E.O.) who expressed and purified the antibody. For membrane fractions, we used a polyclonal antibody against a C-terminal peptide (Novus Biologicals). The specific antibody is identified in the figure legends. The CAXII antibody was generated in goats against a recombinant protein containing amino acids 25–291 (R&D Systems). The CAII antibody is a polyclonal made against the entire protein (Novus Biologicals). The GLUT1 antibody is a rabbit polyclonal that was generated in our lab and previously characterized (32). Na+/K+ATPase α antibody, a marker for plasma membranes, is a rabbit polyclonal antibody against an internal sequence (Santa Cruz). The antibody to GRP78, a protein resident to ER, is a rabbit polyclonal, which was generated in our lab and previously characterized (33). Images were captured using Adobe Photoshop (5.0 LE) and densitometric analysis was performed using Un-Scant-It 6.1 (Silk Scientific Corp.). Statistical differences between groups were calculated using one-way ANOVA (Sigma STAT 3.5) unless otherwise noted. For means in the graphs without a common letter, p < .05.
RESULTS
Growth of cultured human breast cancer cells
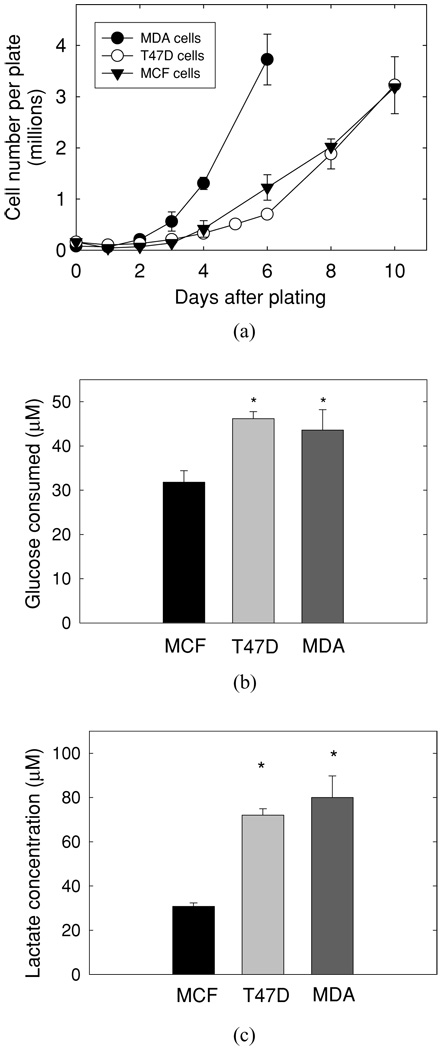
To compare the expression of proteins potentially involved in the development of the glycolytic phenotype, it is important to conduct experiments in which cells are at similar densities. Thus, we first assessed the growth rate of the T47D, MDA-MB-231, and MCF10A cell lines to guide the timing of our experiments [Figure 1(a)]. As might be expected, the MDA-MB-231 cells grew more rapidly than either the T47D or the MCF10A cells despite their lower plating density. These growth curves allowed us to select specific days after plating to evaluate metabolic features. For example, we found that the density of MDA-MB-231 cells at 2 days post plating was equivalent to the density of MCF10A and T47D cells at 3 days post plating. In each case, this represented about 50% confluence. In the experiments that follow, cells that are called subconfluent were analyzed on these days. Likewise, the MDA-MB-231 cells at day 4 post plating were equivalent to the density of MCF10A and T47D cells at day 7 post plating. This stage of growth represented confluent, but not overgrown, cells.
Figure 1.
Characterization of human breast cancer lines. (a) MDA-MB-231 (MDA), T47D, and MCF10A (MCF) cells were plated and grown as described in Material and Methods. At specific times after plating, cell number was determined using a Coulter Counter ZM. Each set of data represent at least three independent experiments. The data are reported as the mean ± SD. MDA = MDA-MB-231 cells; T47D = T47D cells; MCF = MCF10A cells, (b) Confluent cells [MCF cells (day 7), T47D cells (day 7), and MDA cells (day 4)] were washed and given fresh medium containing 15-mM glucose. After 4 hr, medium was collected for determining glucose concentration. Data represent the mean ± SD of a single experiment in which n = 6, p < .001 versus MCF cell line, (c) Medium from confluent MCF, T47D, and MDA cells, treated as in (b), was collected and assayed for lactate concentration. Data represent the mean ± SD of a single experiment in which n = 6, p < .003 versus MCF cells. *n = 6.
At confluence, the MDA-MB-231 and T47D cells consumed significantly more glucose and secreted significantly more lactate than did the MCF10A cells [Figure 1(b) and (c)]. In the MCF10A cells, 50% of the glucose consumed was used to make lactate. It is not atypical of cultured cells to shift glucose metabolism toward lactate production. The T47D and MDA-MB-231 cells shifted this flux even further, as 80% and 94% of the glucose consumed, respectively, was used to make lactate.
Density- and hypoxia-dependent expression of mRNA of different isoforms of CA
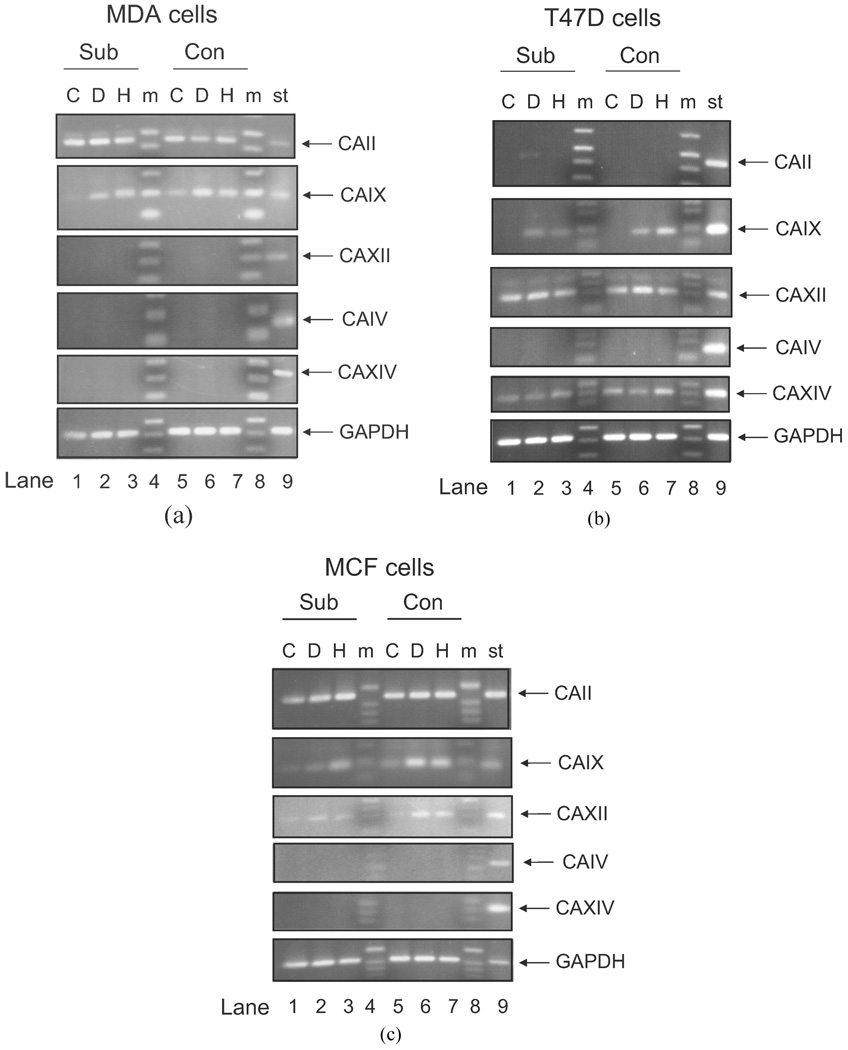
While CAIX is the most likely membrane-associated CA family member to contribute to the regulation of extracellular pH (34), the expression of other isoforms could confound interpretation of activity measurements. For that reason, we examined the expression of all four of the membrane-associated CA family members (CAIV, CAIX, CAXII, and CAXIV mRNA), in each cell line, using semiquantitative RT-PCR. These data are shown in Figure 2 along with CAII, the ubiquitous cytosolic form of this family. Surprisingly, CAIX mRNA was observed in all three cell lines and showed sensitivity to hypoxia. CAXII mRNA was not observed in MDA-MB-231 cells but was expressed in the T47D and MCF10A lines. In the T47D cell line, there was no increase in CAXII mRNA in response to hypoxia, but its expression was induced in the MCF10A line. CAIV mRNA was not detected in any of the cell lines. CAXIV mRNA was only expressed in the T47D cells and was not sensitive to DFO or hypoxia. The message for CAII was detected in both the MDA-MB-231 and MCF10A cells but not T47D cells. CAII expression was not sensitive to DFO or hypoxia.
Figure 2.
Comparison of CA mRNA expression in human breast cancer lines. RNA was isolated from subconfluent and confluent MDA-MB-231, T47D, and MCF10A cells exposed or not to DFO (100 µM) or to hypoxic conditions (1% oxygen) for 16 hr. RT-PCR was performed as described. C = control; D = DFO; H = hypoxia; m = markers; St = RT-PCR of RNA isolated from select human tissue. The standard RNA for CAIV came from heart; the standard for CAIX came from stomach; the standard for CAXII came from kidney; the standard for CAXIV, CAII, and GAPDH came from liver. GAPDH is used as a control for the RT-PCR reaction. These data represent at least two independent sets of cells.
Density- and hypoxia-dependent expression of CA protein in breast cancer cells
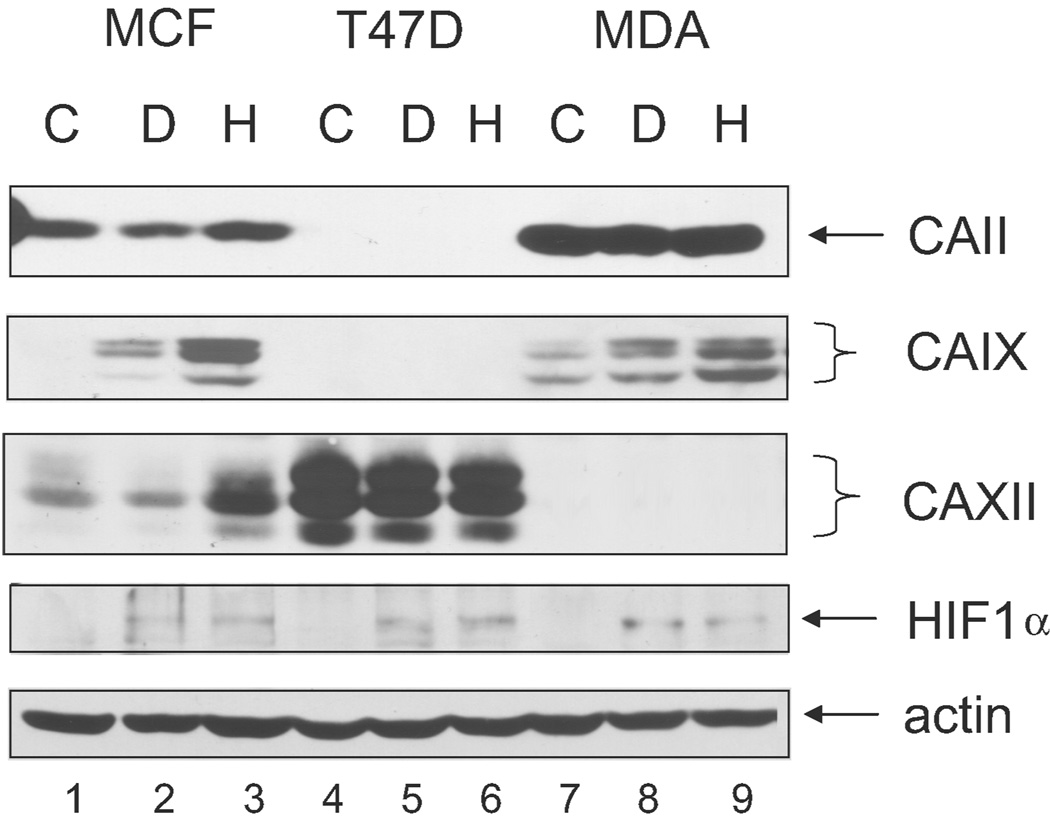
Ultimately, it is protein expression that will lead to a physiological change, so we next measured CA expression by western blot analysis. In confluent cells, CAIX protein was only detected in the MDA-MB-231 cells (Figure 3). As observed by others, CAIX migrated as a doublet of 54 kDa and 58 kDa. Each of these bands appeared to be glycosylated based on its sensitivity to N-glycosidase F (data not shown). Both MCF10A and MDA-MB-231 cells showed enhanced CAIX expression in response to DFO and hypoxia. Over three experiments, the fold increase over the control in the MDA-MB-231 cells was 1.9 in the presence of DFO and 2.4 after hypoxia exposure. Quantification in the MCF10A cells was not possible because the control lane was blank. T47D cells expressed no CAIX protein either constitutively or in response to DFO or hypoxia. Yet these cells, as did the MDA-MB-231 and MCF10A cells, showed an increase in the expression of the transcription factor, HIF1α. CAXII protein was strongly expressed in the T47D cells and observed as three bands. Each of these bands was also sensitive to N-glycosidase F treatment (data not shown). Again, expression of CAXII in T47D cells was not sensitive to DFO or hypoxia. MCF10A cells expressed less CAXII, and its expression appeared elevated in response to hypoxia but not DFO. CAXII was not detected in the MDA-MB231 cells. CAII was strongly expressed in the MDA-MB-231 but detectable in MCF10A cells, although in neither cell line was its expression altered by DFO or hypoxia. We did not further explore the expression of CAIV protein, for which no message was detected in any of the cells, or CAXIV protein, for which the message was only observed in the T47D cells.
Figure 3.
Expression of CA protein in human breast cancer lines. Confluent cells were exposed to DFO or hypoxia as in Figure 2 and then lysed. Equal protein (100 µg) was analyzed by western blot analysis using the M75 monoclonal antibody. Identical results were obtained in independent duplicate experiments. MCF = MCF10A cells; T47D = T47 D cells; MDA = MDA-MB-231 cells; C = control; D = DFO; H = hypoxia.
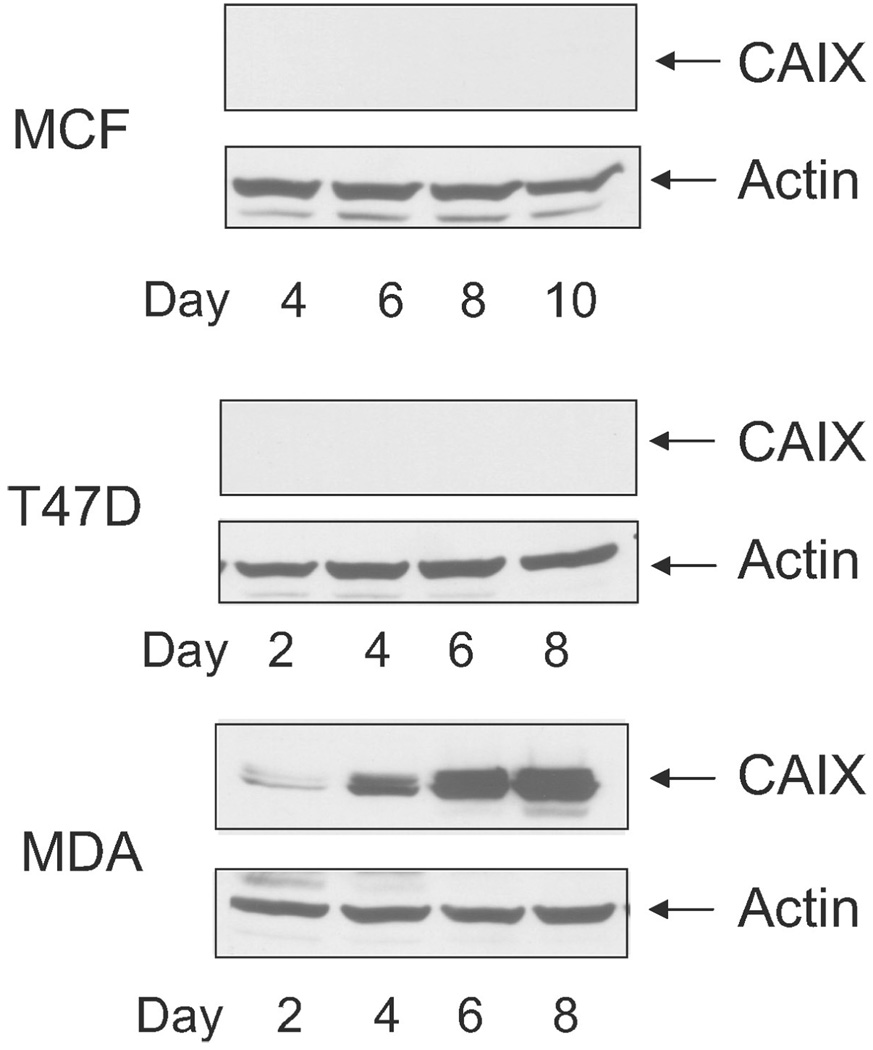
We also examined density-dependent expression of CAIX in cell lysates (Figure 4). MCF10A cells expressed no CAIX in subconfluent cells, and the expression was not induced by increasing cell density [Figure 4(a)]. T47D cells showed no CAIX expression [Figure 4(b)]. In MDA-MB-231 cells, we observed little expression in subconfluent cells but a substantial increase in response to density [Figure 4(c)]. Thus, only the MDA-MB-231 cells showed both density- and oxygen-dependent regulation of CAIX at the protein level.
Figure 4.
Density-dependent expression of CAIX in breast cancer cells. Cells were collected at specific times after plating. Cells were lysed, and equal protein was analyzed by western blot analysis using the M75 monoclonal antibody. Identical results were obtained in independent duplicate experiments. MCF = MCF10A cells; T47D = T47 D cells; MDA = MDA-MB-231 cells.
Localization of CAIX and CAII in MDA-MB-231 cells
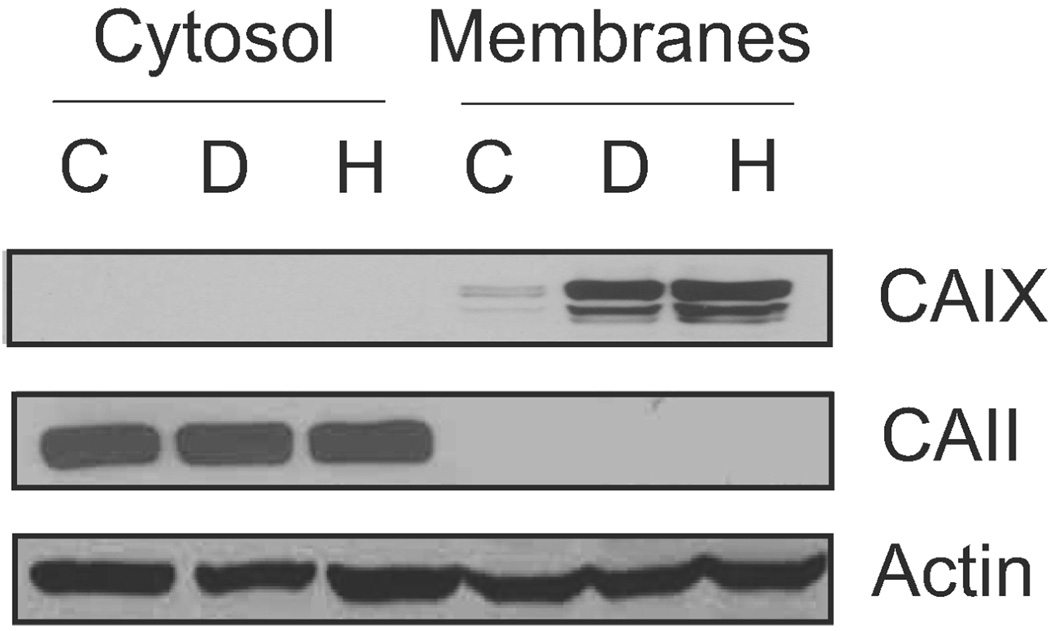
Based on the RT-PCR and protein data, we conclude that the MDA-MB-231 cells express only one of the membrane-associated CA family members. This gave us the opportunity to explore its specific regulation and activity. Confluent MDA-MB-231 cells were separated into total membrane and cytosolic fractions [Figure 5(a)]. CAIX was only detected in the membrane fraction, and its expression increased significantly in response to DFO or hypoxia. CAII, as expected, was localized to the cytoplasmic compartment and was not sensitive to DFO or hypoxia. While there are data that show that CAII interacts with anion transporters in the plasma membrane (35), we observed little CAII association with the total membrane fraction.
Figure 5.
Expression and localization of CAIX. Confluent MDA cells were collected and separated into total membrane and cytoplasmic fractions after exposure or not to DFO or hypoxia. Equal protein was analyzed by western blot analysis for CAIX (using the M75 monoclonal antibody), CAII, and actin expression. C = control; D = DFO; H = hypoxia. These data represent two independent experiments.
Subcellular localization and regulation of CAIX
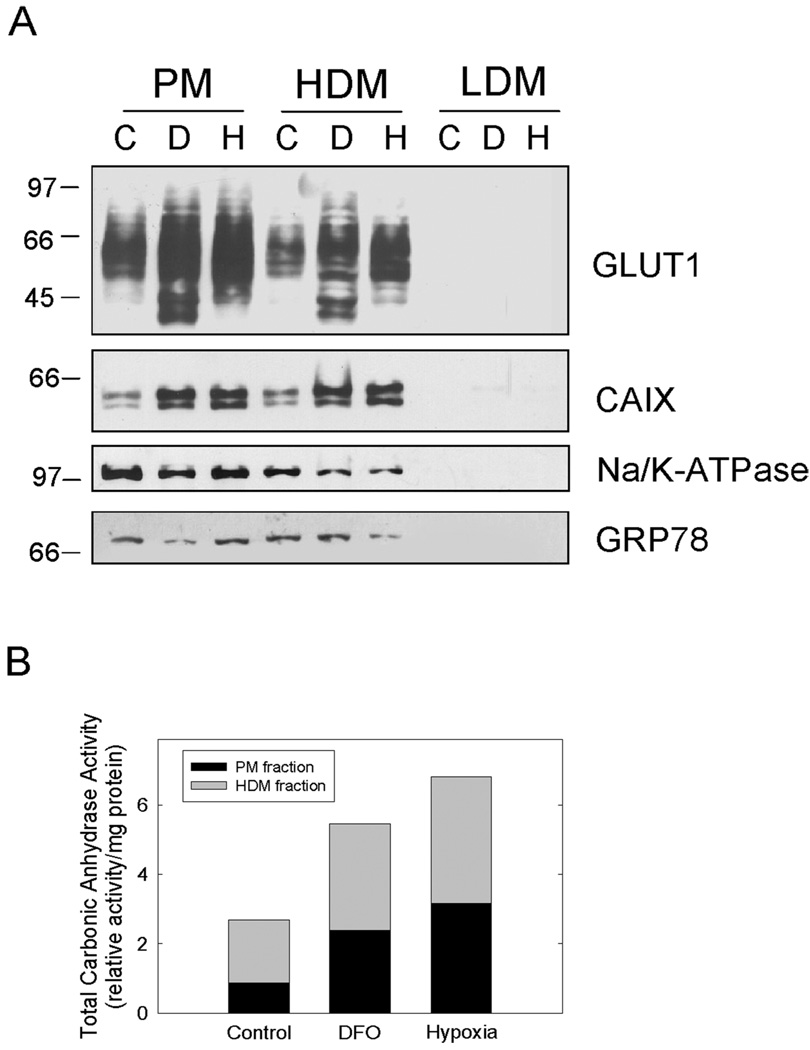
To determine the activity of CAIX, we first isolated subcellular membrane fractions from confluent MDA-MB-231 cells [Figure 6(a)]. We observed CAIX in two of the three membrane fractions that were collected. The marker proteins (Na+/K+ATPase and GRP78) indicated that there was substantial cross contamination between the plasma membrane and high-density (ER/Golgi) fractions. While the purity of the fractions was not particularly impressive, both pools showed enhanced expression of CAIX in response to DFO or hypoxia. We also examined the expression of the GLUT1 glucose transporter. It is well known that the expression of GLUT1 is sensitive to hypoxia, which was confirmed here. In two experiments, the increase in GLUT1 expression in response to DFO ranged from a 2.6- to 5.9-fold increase over controls, while hypoxia increased expression by 2.1- to 5.1-fold. Membranes collected at high centrifugal force, representing an endosomal low-density fraction (LDM), showed no CAIX or GLUT1 expression. Using the membrane fractions that did contain CAIX, we measured CA activity using 18O isotopic exchange between CO2 and water by mass spectrometry. Figure 6(b) shows that CA activity was increased by both DFO and hypoxia (2.0-fold and 2.5-fold, respectively) over control membranes.
Figure 6.
Subcellular localization and activity of CAIX. (a) Confluent MDA-MB-231 cells were collected after treatment with either DFO or hypoxia. Subcellular fractions were collected as described in the methods. Equal protein was separated by SDS-PAGE, transferred to nitrocellulose, and probed for GLUT1, CAIX, Na/K-ATPase, and GRP78. In these experiments, the CAIX antibody used in western blotting was purchased from Novus Biologicals. These data represent two independent experiments. PM = plasma membranes; HDM = high density membranes; LDM = low density membranes. (b) Plasma membrane (PM) and high-density membrane (HDM) fractions isolated as in (a) were assayed for CA activity using 18O exchange. The data are reported as the relative total activity under each condition. The black bars represent PM, and the gray bars represent HDM.
Effect of hypoxia on metabolic indices
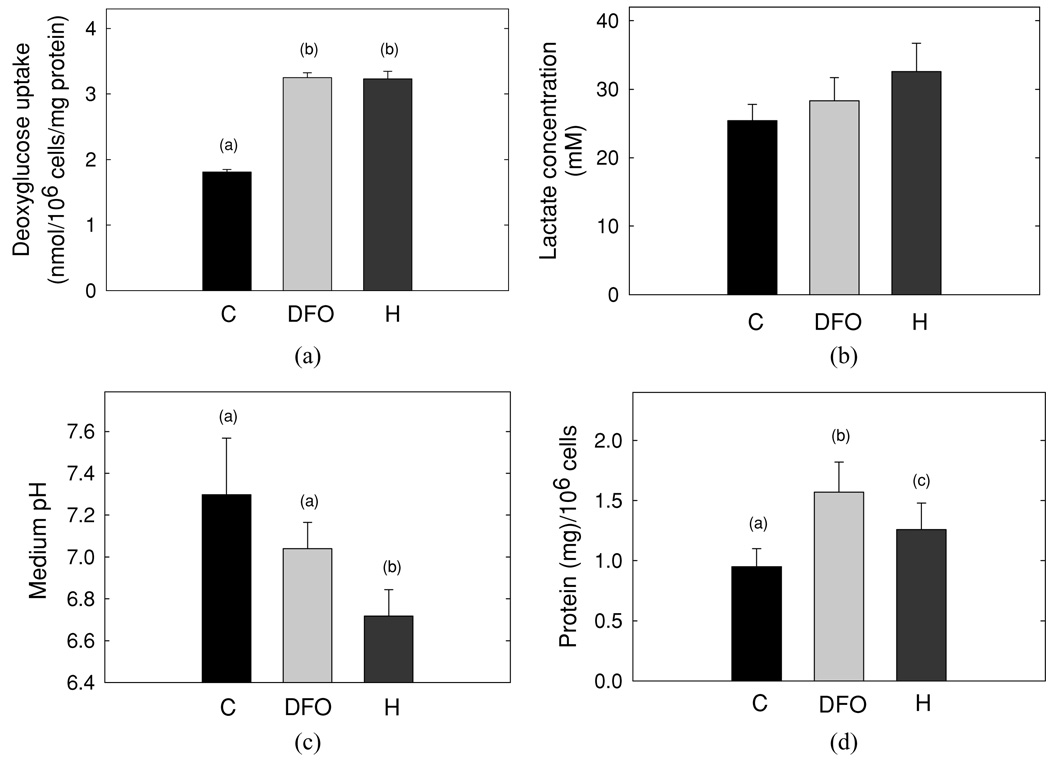
To examine the effect of DFO and hypoxia on breast cancer cell metabolism, we first looked at glucose transport activity [Figure 7(a)]. Our results show that the rate of glucose uptake was a direct reflection of GLUT1 expression. Thus, transport activity increased in cells exposed to either DFO or hypoxic conditions. With increased GLUT1 in response to DFO or hypoxia, the concentration of lactic acid increased in the medium [Figure 7(a)], although these changes did not achieve statistical significance. Hypoxia significantly decreased extracellular pH [Figure 7(c)], which could not be accounted for by lactic acid alone.
Figure 7.
Effect of hypoxia on metabolic activity in MDA cells. (a) MDA-MB-231 cells, at day 3 post plating, were exposed to DFO or hypoxia, washed, and assayed for glucose transport activity. Each data point in a given experiment was performed in duplicate. The duplicates were then averaged and compiled with the means of replicate experiments (n = 3). The data are reported as the mean ± SD. (b) Lactate concentration was determined in medium that was collected from MDA cells treated as in (a). Data are reported as the mean ± SD from three independent experiments. (c) The levels of pH measured in medium that was collected from MDA cells treated as in (a) are shown. Data are reported as the mean ± SD from four independent experiments, (d) Protein content and cell number were determined in MDA cells treated as in (a). Data are reported as the mean protein concentration per 106 cells ± SD from four independent experiments.
Effect of CA inhibition
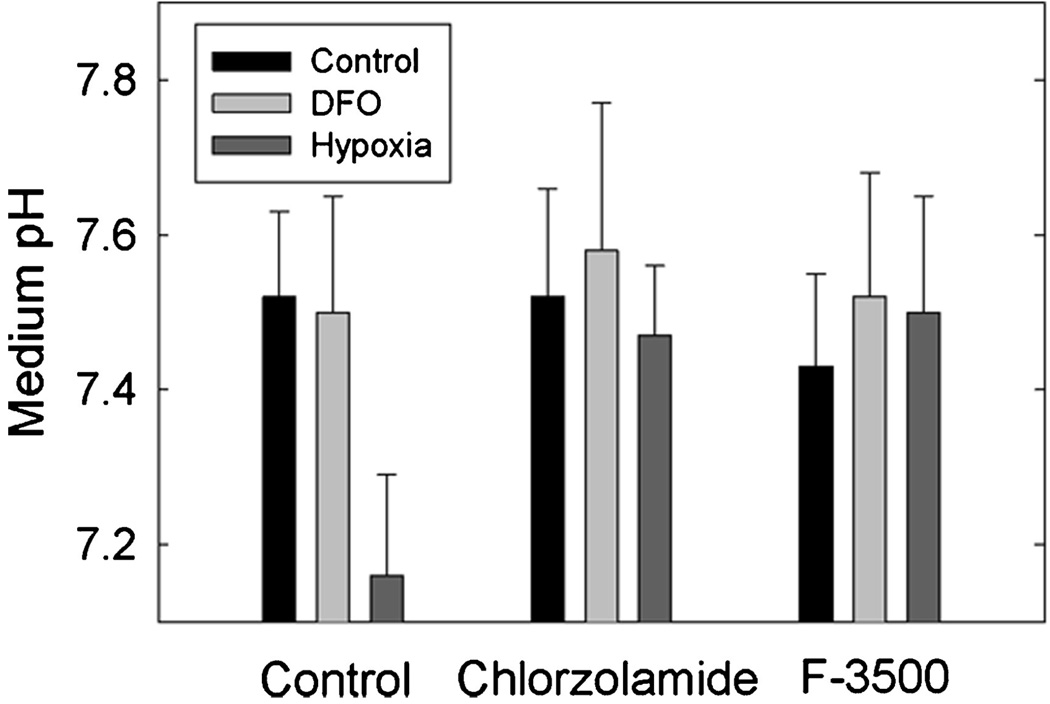
Finally, to test the specific role of CAIX on changes in extracellular pH, we utilized both a general CA inhibitor (chlorozolamide) and a membrane impermeant inhibitor (F-3500), originally designed by Conroy, Wynns, and Maren (36). The membrane impermeant inhibitor is essentially specific for CAIX, as this is the only cell surface CA expressed in the MDA-MB-231 cells. In three independent experiments, both inhibitors blocked the decrease in pH in response to hypoxia, as shown in Figure 8(a). The differences between the absence and presence of inhibitors in hypoxic cells fell just short of statistical significance, although the trend in each of the three experiments was identical. Thus, these data suggest that CAIX activity contributes to the extracellular acidification of MDA-MB-231 cells.
Figure 8.
Effect of CA Inhibitors on medium pH. Cells were treated 2 days post plating with DFO, or exposed to 1% oxygen, for 16 hr in the absence or presence of 100-µM chlorozolamide or F3500. The pH of the medium was tested at the end of the incubation, which will be noted is higher, overall, than that in Figure 7 because of the lower density of the cells. Data are reported as the mean ± SD, n = 3.
DISCUSSION
Oxygen levels decrease across a tumor as distance from blood vessels increases (37). The hypoxic microenvironment of tumors is associated with poor response to radiation and chemotherapy, genetic instability, resistance to apoptosis, and increased risk of invasion and metastasis (38–41). Hypoxia-inducible CAIX expression has been studied in a number of tumor types, and its expression in many is correlated with poor clinical outcomes. In a recent retrospective study in breast cancer tissue, it was shown that CAIX protein expression, detected by immunohistochemistry, was associated with poor survival (42). Poor survival is generally related to metastatic disease.
Few studies (if any) have actually examined cellular CAIX activity directly and instead use CA inhibitors to measure its effect, indirectly, on medium pH (43, 44) or invasion (45). RNAi technology against CAIX has also been employed, successfully showing that partial CAIX knockdown affects cell growth (22). Our data are important in that we show for the first time that the MDA-MB-231 cells express only one of the membrane-associated CA family members, CAIX, allowing us to demonstrate, directly, that CAIX activity increased in response to hypoxia and DFO. This is also the first study that directly measures the activity of endogenous CAIX in cancer cells. The change in activity is directly correlated to the increase in protein expression but could be inhibited by an impermeant CA inhibitor which is essentially specific for CAIX. This indicates that not only is CAIX expressed, it is active and the only membrane CA in the MDA-MB-231 cells that can participate in extracellular acidification.
Gillies and Gatenby (46) have argued that intermittent hypoxia leads to upregulation of glycolysis in early in situ cancers and that this feature is further selected for because it provides some advantage to cancer progression. This glycolytic phenotype is more complex than just upregulation of the enzymes that control glycolytic rate. Rather it is a series of events (often heterogenous in nature) that lead to permanent changes in protein expression that drives both glycolysis and the upregulation of proton exporters. Enhanced glycolytic activity results in intracellular acidification while upregulation of the proton export machinery contributes to extracellular acidification avoiding intracellular proton toxicity. It is not really understood why cancer cells are able to adjust their sensitivity to low pH while normal cells surrounding cancer cells die off (46), but it clearly contributes to their metastatic potential (11, 47). Both GLUT1 and CAIX have the potential to regulate pH. Increased GLUT1 expression leads to increased glucose metabolism leading to an increase in lactic acid production. In our hands, the MDA-MB-231 cells had the highest growth rate and the highest rate of lactic acid production among the cells that were tested. In terms of glycolysis, 94% of the glucose consumed was converted to lactic acid. By comparison, the MCF10A cells converted only about 50% of the glucose to lactic acid. Thus, not only was glucose uptake increased in MDA-MB-231 cells relative to MCF10A cells but there also was a shift in metabolic flux which typifies cancer cells. Metabolic activity in T47D cells was intermediate between these cell types. Hypoxia further enhanced glucose uptake and anaerobic glycolysis in MDA-MB-231 cells, but the intrinsic metabolic behavior of these cells was preestablished. Interestingly, the drop in medium pH in response to hypoxia could not be accounted for by lactic acid production alone. This lends credence to the possibility that the induction of CAIX contributes independently to acidification. The ability of an impermeant CA inhibitor confirms this contribution. While the MCF10A cells did show inducible expression of CAIX, they clearly did not exhibit the same intrinsic metabolic phenotype as the MDA-MB-231 cells. Thus despite their common basal B lineage, MDA-MB-231 cells can be distinguished metabolically from MCF10A cells in culture. This may be a contributing factor in their in vivo behavior.
It was also surprising that the MCF10A cells showed hypoxia-dependent expression of both CAIX mRNA and CAIX protein, as these cells are neither tumorigenic nor metastatic. The study by Neve and colleagues (25) perhaps provides some clues for this observation, as the MCF10A cell line, an immortal but nontransformed cell line, shares transcriptional characteristics that define basal progenitor cells suggesting that these cells may represent a multipotent lineage. Thus, the gene transcripts that define the basal B group are found in both MDA-MB-231 and MCF10A cell types despite their very different in vivo behaviors. The Neve study did not reveal that CAIX fell out as a marker for the basal B cells which is not surprising, as CAIX expression in subconfluent cells, used for the analysis, is absent.
Another surprise was the high level of CAII mRNA expression in the MDA-MB-231 cells, which was verified at the protein level and which we are the first to show. It has been proposed that CAII associates with the Cl−/bicarbonate exchanger (AE) in the plasma membrane (48) and that together with CAIV forms a “metabolon” to accelerate the rate of AE-mediated bicarbonate transport (49). A similar metabolon has been proposed for CAIX (50). It is envisioned that CAII detoxifies protons generated from metabolic acids by combining them with bicarbonate to form H2O and CO2. The CO2 diffuses out of the cell in which CAIX mediates its reaction with extracellular water to reform bicarbonate and proton. The proton contributes to extracellular acidification, and the bicarbonate is brought back into the cell through the AE to repeat the cycle. Obviously, this model requires co-expression of CAIX and CAII. This condition is satisfied in the MDA-MB-231 but not in the T47D cells. The MDA-MB-231 cells show strong acidification of the medium in response to hypoxia at either subconfluent or confluent states, while the T47D cells do not acidify medium at subconfluence and only weakly at confluence in response to hypoxia (data not shown).
The lack of constitutive or induced CAIX protein expression in T47D cells is consistent with the less aggressive phenotype of these cells in vivo. However, there is an anomaly between mRNA and protein expression in the T47D cells. The RT-PCR data reveal that CAIX mRNA is transcribed in response to DFO and hypoxia, and yet no protein was translated. In a genomic study across several cell lines, Ivanov et al. (51) showed that T47D cells are negative for CAIX but positive for CAXII. Their result is consistent with our control data for this cell line. They also showed that lobular (ductal) carcinomas (from which T47D cells derive) exhibit focal staining for CAXII but no staining for CAIX. It might be imagined that at least some regions of the tumor would be hypoxic, from which we infer that hypoxia does not regulate CAIX expression, in vivo, in this type of tumor. Only a few investigators have compared CAIX expression at both the mRNA and protein levels within the same study. Several of those showed similar expression of CAIX mRNA and protein in response to hypoxia (18, 52, 53). However, Stockwin et al. (9) showed a 36-fold induction of CAIX mRNA in hypoxia-adapted malignant melanoma but only a 1.8-fold change in CAIX protein. On a more global level, it is also important to point out that mRNA expression does not necessarily lead to protein synthesis, as was elegantly shown by the genomic/proteomic analysis in yeast conducted by Leroy Hood’s laboratory (54). In their experiments, the correlation (r) between transcription and translation was only 0.61. Only 30 of 289 proteins, identified in a screening in which nutrient availability was perturbed, showed similar induction at both gene and protein levels. Activation of 15 “metabolic” genes led to no change in protein expression. These authors interpreted this to mean that these genes, and probably many others, underwent posttranscriptional regulation. Of course, the T47D cells could have a nucleotide insertion or deletion which would allow amplification of mRNA using RT-PCR but prevent translation of that message. Certainly it is not due to lack of HIF1α, as this transcription factor was stabilized (upregulated) in response to hypoxia in all three cell lines that we examined.
While T47D cells do not express CAIX, they strongly express CAXII. CAXII expression, but not that of CAIX, exhibits a positive correlation with estrogen receptor expression (55) which is a marker for luminal breast cancer cells. Patients with luminal tumors have a more positive prognosis than those with either HER2 or basal phenotypes. Thus CAXII activity may regulate the tumor microenvironment in a unique manner. While these cells do not express CAII, there are other soluble CAs that could participate in a CAXII-specific metabolon which may contribute to a less aggressive behavior and a more positive outcome. Variation in the expression of CAIX and CAXII across a wide range of cancer tissues (56) support the existence of tissue-specific metabolons which may ultimately regulate tumor progression.
In summary, we show for the first time that the MDA-MB-231 cells, which represent the triple-negative breast cancer phenotype, show inducible expression of CAIX, while the less aggressive luminal line, T47D cells, expresses primarily CAXII. CAIX activity is induced by hypoxia in MDA-MB-231 cells, and its inhibition blocks the drop in extracellular pH. We also show for the first time that MDA-MB-231 cells express high levels of CAII which may contribute to the flux of protons and bicarbonate across the plasma membrane in concert with CAIX, as has been hypothesized. While this suggests that CAIX and CAII may play key roles in acidification, a wider sampling of basal B cells will determine if this role is more generally applicable. If so, then CAIX may offer a new target for therapeutic intervention because of its limited tissue distribution and exofacial orientation.
ACKNOWLEDGEMENTS
This work was supported by a grant received from the Thomas H. Maren Foundation (SCF) and National Institutes of Health grants RO1 DK045035 (SCF) and GM25154 (DNS). The authors thank Dr. Patricia Moussatche for her critique of the manuscript and gratefully acknowledge the excellent technical assistance of Ms. Xiao Wei Gu and Mr. Emilio Madrigal. In addition, the authors would like to acknowledge the assistance of three talented undergraduate students: Jordan Teper, Ryan O’Donnelly, and Patricia Gomez.
Footnotes
DECLARATION OF INTEREST
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- 1.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem. Sci. 1999;24:68–69. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 2.Cairns R, Papandreou I, Denko N. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Res. 2006;4:61–70. doi: 10.1158/1541-7786.MCR-06-0002. [DOI] [PubMed] [Google Scholar]
- 3.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 4.Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995;15:2895–2898. [PubMed] [Google Scholar]
- 5.Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Wide expression of the human erythrocyte glucose transporter GLUT1 in human cancers. Cancer Res. 1996;56:1164–1167. [PubMed] [Google Scholar]
- 6.Brown R, Wahl RL. Overexpression of Glut-1 glucose transporter in human breast cancer: an immunohistolochemical study. Cancer. 1993;72:2979–2985. doi: 10.1002/1097-0142(19931115)72:10<2979::aid-cncr2820721020>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways in cancer cells and stromal cells in colorectal cancer: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:623–627. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 8.Sennoune SR, Bakunts K, Martinez GM, Chua-Tuan JL, Kebir Y, Attaya MN, Martinez-Zaguilan R. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol. 2004;286:C1443–C1452. doi: 10.1152/ajpcell.00407.2003. [DOI] [PubMed] [Google Scholar]
- 9.Stockwin LH, Blonder J, Bumke MA, Lucas DA, Chan KC, Conrads TP, Issaq HJ, Veenstra TD, Newton DL, Rybak SM. Proteomic analysis of plasma membrane from hypoxia-adapted malignant melanoma. J Proteome Res. 2006;5:2996–3007. doi: 10.1021/pr0601739. [DOI] [PubMed] [Google Scholar]
- 10.McLean LA, Roscoe J, Jorgensen NK, Gorin FA, Cala PM. Malignant gliomas display altered pH regulation by NHE1 compared to nontransformed astrocytes. Am J Physiol. 2000;278:C676–C688. doi: 10.1152/ajpcell.2000.278.4.C676. [DOI] [PubMed] [Google Scholar]
- 11.Stubbs M, McSheehy PMJ, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6:15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 12.Yamagata M, Hasuda K, Stamato T, Tannock IF. The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br J Cancer. 1998;77:1726–1731. doi: 10.1038/bjc.1998.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res. 2002;8:1284–1291. [PubMed] [Google Scholar]
- 14.Pastorek J, Pastoreková S, Callebaut I, Mornon JP, Zelník V, Opavsky R, Zat’ovicová M, Liao S, Portetelle D, Stanbridge EJ, Závada J, Burny A, Kettmann R. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix–loop–helix DNA binding segment. Oncogene. 1994;9:2877–2888. [PubMed] [Google Scholar]
- 15.Pastorekova S, Parkkila S, Parkkila A, Opavsky R, Zelnik V, Saarnio J, Pastorek J. Carbonic anhydrase IX, MN/CAIX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology. 1997;112:398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 16.Saarnio J, Parkkila S, Parkkila A-K, Waheed A, Casey MC, Zhou XY, Pastoreková S, Pastorek J, Karttunen T, Haukipuro K, Kairaluoma MI, Sly WS. Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in epithelial cells with the highest proliferative capacity. J Histochem Cytochem. 1998;46:497–504. doi: 10.1177/002215549804600409. [DOI] [PubMed] [Google Scholar]
- 17.Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wykoff CC, Pastorek J, Ratcliffe PJ, Stratford IJ, West CML. Carbonic anhydrase (CAIX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–6399. [PubMed] [Google Scholar]
- 18.Wykoff CC, Beasley NJP, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. Hypoxia-inducible expression of tumor-associated carbonic anhydrase. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 19.Potter C, Harris AL. Hypoxia inducible carbonic anhydrase IX, marker of tumor hypoxia, survival pathway and therapy target. Cell Cycle. 2004;3:164–167. [PubMed] [Google Scholar]
- 20.Wingo T, Tu C, Laipis PJ, Silverman DN. The catalytic properties of human carbonic anhydrase IX. Biochem Biophys Res Comm. 2001;288:666–669. doi: 10.1006/bbrc.2001.5824. [DOI] [PubMed] [Google Scholar]
- 21.Supuran CT, Briganti F, Tilli S, Chegwidden WR, Scozzafava A. Carbonic anhydrase inhibitors: sulfonamides as antitumor agents? Bioorg Med Chem. 2001;9:703–714. doi: 10.1016/s0968-0896(00)00288-1. [DOI] [PubMed] [Google Scholar]
- 22.Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. 2004;64:6160–6165. doi: 10.1158/0008-5472.CAN-03-2224. [DOI] [PubMed] [Google Scholar]
- 23.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 24.Engel LW, Young NA. Human breast carcinoma cells in continuous culture: a review. Cancer Res. 1978;38:4327–4339. [PubMed] [Google Scholar]
- 25.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe J, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo W, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS, Billingham L, James ND, Spooner D, Poole CJ, Rea DW, Palmer DH. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer. 2007;96:104–109. doi: 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markwell MAK, Haas SM, Lieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 28.Fisher MD, Frost SC. Translocation of GLUT1 does not account for elevated glucose transport in glucose-deprived 3T3-L1 adipocytes. J Biol Chem. 1996;271:11806–11809. doi: 10.1074/jbc.271.20.11806. [DOI] [PubMed] [Google Scholar]
- 29.Balch WE, Rothman JE. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch Biochem Biophys. 1985;240:413–425. doi: 10.1016/0003-9861(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 30.Weber TM, Joost HG, Simpson IA, Cushman SW. In: Methods for assessment of glucose transport activity and the number of glucose transporters in isolated rat adipose cells and membrane fractions, in Receptor Biochemistry and Methodology (The Insulin Receptor) Kahn RC, Harrison LC, editors. New York: Alan R. Liss; 1988. pp. 171–187. [Google Scholar]
- 31.Wingo T, Tu C, Laipis PJ, Silverman DN. The catalytic properties of human carbonic anhydrase IX. Biochem Biophys Res Comm. 2001;288:666–669. doi: 10.1006/bbrc.2001.5824. [DOI] [PubMed] [Google Scholar]
- 32.Kitzman HH, Jr, McMahon RJ, Williams MG, Frost SC. Effect of glucose deprivation on GLUT1 expression in 3T3-L1 adipocytes. J Biol Chem. 1993;268:1320–1325. [PubMed] [Google Scholar]
- 33.Kitzman HH, Jr, McMahon RJ, Aslanian AM, Fadia PM, Frost SC. Differential regulation of GRP78 and GLUT1 expression in 3T3-L1 adipocytes. Mol Cell Biochem. 1996;162:51–58. doi: 10.1007/BF00250995. [DOI] [PubMed] [Google Scholar]
- 34.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26:299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- 35.Vince JW, Reithmeier RAF. Carbonic anhydrase II binds to the carboxyl-terminus of human band 3, the erythrocyte exchanger. J Biol Chem. 1998;273:28430–28437. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 36.Conroy CW, Wynns GC, Maren TH. Synthesis and properties of two new membrane-impermeant high-molecular-weight carbonic anhydrase inhibitors. Bioorg Chem. 1996;24:262–272. [Google Scholar]
- 37.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 38.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumour oxygenation predicts for likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 39.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminshed apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 40.Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and radiation response in human tumors. Semin Radiat Oncol. 1996;6:3–9. doi: 10.1053/SRAO0060003. [DOI] [PubMed] [Google Scholar]
- 41.Kim CY, Tsai MH, Osmanian C, Graeber TG, Lee JE, Giffard RG, DiPaolo JA, Peehl DM, Giaccia AJ. Selection of human cervical epithelial cells that possess reduced apoptotic potenial to low-oxygen conditions. Cancer Res. 1997;57:4200–4204. [PubMed] [Google Scholar]
- 42.Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS, Billingham L, James ND, Spooner D, Poole CJ, Rea DW, Palmer DH. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer. 2007;96:104–109. doi: 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cecchi A, Hulikova A, Pastorek J, Pastorekova S, Scozzafava A, Winum J-Y, Montero J-L, Supuran CT. Carbonic anhydrase inhibitors: design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J Med Chem. 2005;48:4841. doi: 10.1021/jm0501073. [DOI] [PubMed] [Google Scholar]
- 44.Svastova E, Hulíková A, Rafajová M, Zat’ovicová M, Gibadulinová A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, Pastoreková S. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577:439–445. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 45.Parkkila S, Rajaniemi H, Parkkila A, Kivela J, Waheed A, Pastorekova S, Pastorek J, Sly WS. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Nat Acad Sci USA. 2000;97:2220–2224. doi: 10.1073/pnas.040554897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 47.Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisplinary study. Cancer Res. 2006;66:5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 48.Sterling D, Brown NJD, Supuran CT, Casey JR. The functional and physical relationship between the DRA bicarbonate transporter and carbonic anhydrase II. Am J Physiol. 2002;283:C1522–C1529. doi: 10.1152/ajpcell.00115.2002. [DOI] [PubMed] [Google Scholar]
- 49.Sterling D, Alvarez BV, Casey JR. The extracellular component of a transport metabolon: extracellular loop 4 of the human AE1 exchanger binds carbonic anhydrase IV. J Biol Chem. 2002;277:25239–25246. doi: 10.1074/jbc.M202562200. [DOI] [PubMed] [Google Scholar]
- 50.Potter C, Harris AL. Hypoxia inducible carbonic anhydrase IX, marker of tumor hypoxia, survival pathway and therapy target. Cell Cycle. 2004;3:164–167. [PubMed] [Google Scholar]
- 51.Ivanov S, Liao S-Y, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rafajová M, Zatovicova M, Kettmann R, Pastorek J, Pastorekova S. Induction by hypoxia combined with low glucose or low bicarbonate and high posttranslational stability upon reoxygenation contribute to carbonic anhydrase IX expression in cancer cells. Int J Oncol. 2004;24:995–1004. [PubMed] [Google Scholar]
- 53.Kaluzova M, Kaluz S, Lerman MI, Stanbridge EJ. DNA damage is a prerequisite for p53-mediated proteasomal degradation of HIF-1α in hypoxic cells and downregulation of the hypoxia marker carbonic anhydrase IX. Mol Cell Biol. 2004;24:5757–5766. doi: 10.1128/MCB.24.13.5757-5766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ideker T, Thorsson V, Ranish JA, Christmas R, Buhler J, Eng JK, Bumgarner R, Goodlett DR, Aebersold R, Hood L. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science. 2001;292:929–934. doi: 10.1126/science.292.5518.929. [DOI] [PubMed] [Google Scholar]
- 55.Barnett DH, Sheng S, Charn TH, Waheed A, Sly WS, Lin C-Y, Liu ET, Katzenellenbogen BS. Estrogen receptor regulation of carboinc anhydrase XII through a distal enhancer in breast cancer. Cancer Res. 2008;68:3505–3515. doi: 10.1158/0008-5472.CAN-07-6151. [DOI] [PubMed] [Google Scholar]
- 56.Ivanov S, Liao S-Y, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]