Abstract
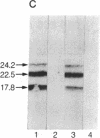
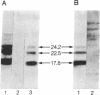
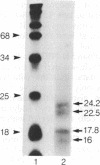
Human basic fibroblast growth factor (bFGF) is an angiogenic polypeptide mitogen present in a wide variety of mesoderm- and neuroectoderm-derived tissues. bFGF cDNA and genomic clones predict a 17.8-kDa (155-amino acid) gene product based on the presence of a single putative translational initiator ATG codon. However, a bFGF protein isolated from human placenta contains two additional amino acids NH2-terminal to the predicted initiator methionine. We report here that the human cell line SK-HEP-1 coexpresses four molecular forms (17.8, 22.5, 23.1, and 24.2 kDa) of bFGF. The 17.8-kDa bFGF protein is translationally initiated at the previously predicted methionine (AUG) codon, whereas the 22.5-, 23.1-, and 24.2-kDa proteins initiate at unusual non-AUG codons. The higher molecular weight forms are colinear NH2-terminal extensions of the 18-kDa bFGF.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Whang J. L., Tumolo A., Mergia A., Friedman J., Gospodarowicz D., Fiddes J. C. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986 Oct;5(10):2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 1988 Jan;7(1):245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Smith A., Bergmann J. E., Rose J. K. Isolation of stable mouse cell lines that express cell surface and secreted forms of the vesicular stomatitis virus glycoprotein. J Cell Biol. 1983 Nov;97(5 Pt 1):1381–1388. doi: 10.1083/jcb.97.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Fibroblast growth factor: structural and biological properties. Int J Rad Appl Instrum B. 1987;14(4):421–434. doi: 10.1016/0883-2897(87)90019-5. [DOI] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lobb R. R., Harper J. W., Fett J. W. Purification of heparin-binding growth factors. Anal Biochem. 1986 Apr;154(1):1–14. doi: 10.1016/0003-2697(86)90487-2. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Florkiewicz R. Z., Rose J. K. A single N-linked oligosaccharide at either of the two normal sites is sufficient for transport of vesicular stomatitis virus G protein to the cell surface. Mol Cell Biol. 1985 Nov;5(11):3074–3083. doi: 10.1128/mcb.5.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergia A., Eddy R., Abraham J. A., Fiddes J. C., Shows T. B. The genes for basic and acidic fibroblast growth factors are on different human chromosomes. Biochem Biophys Res Commun. 1986 Jul 31;138(2):644–651. doi: 10.1016/s0006-291x(86)80545-9. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Joseph-Silverstein J., Manejias R., Rifkin D. B. Mr 25,000 heparin-binding protein from guinea pig brain is a high molecular weight form of basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5778–5782. doi: 10.1073/pnas.84.16.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Rifkin D. B. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S. Translation initiation at an ACG triplet in mammalian cells. J Biol Chem. 1987 Aug 25;262(24):11847–11851. [PubMed] [Google Scholar]
- Rose J. K., Adams G. A., Gallione C. J. The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2050–2054. doi: 10.1073/pnas.81.7.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A., Brewer M. T., Thompson R. C., Moscatelli D., Presta M., Rifkin D. B. A form of human basic fibroblast growth factor with an extended amino terminus. Biochem Biophys Res Commun. 1987 Apr 29;144(2):543–550. doi: 10.1016/s0006-291x(87)80001-3. [DOI] [PubMed] [Google Scholar]
- Squires C. H., Childs J., Eisenberg S. P., Polverini P. J., Sommer A. Production and characterization of human basic fibroblast growth factor from Escherichia coli. J Biol Chem. 1988 Nov 5;263(31):16297–16302. [PubMed] [Google Scholar]