INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a neuropsychiatric condition that affects both children and adults. In a representative sample of children 8 to 15 years of age in the U.S., approximately 9% met the criteria for ADHD, as specified in Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).1 In cross-national surveys, the prevalence of ADHD among children in other countries ranged from 5.8% in Canada to 11.2% in India.2
In children with ADHD, symptoms may be manifested as significant social, emotional, and academic problems, including low self-esteem, poor peer relationships, delinquency, and substance abuse. The persistency of ADHD from childhood into adulthood is often underappreciated. Increasingly, the literature clearly demonstrates that ADHD symptoms, particularly those of inattention and many associated impairments, persist into adulthood in a high proportion of cases.3–5 Approximately 65% of people with childhood ADHD continue to exhibit symptoms as adults.6
Results of the National Comorbidity Survey Replication (NCSR), the first definitive epidemiologic study of ADHD, estimated the prevalence of ADHD to be 4.4% among adults in the U.S., but only 10.9% of adults with ADHD had received treatment for it in the previous 12 months.7 In the World Health Organization World Mental Health Survey Initiative, which included respondents 18 to 44 years of age in 10 countries, estimates of ADHD prevalence averaged 3.4%, ranging from 1.2% in Spain to 7.3% in France.8
The validity of ADHD as a disorder in adults has been supported by evidence from studies of clinical correlates, family history, treatment response, and laboratory measures.9 Heritability plays a role, with 76% of ADHD cases having a genetic component.10 Neuroanatomic studies in children with ADHD have shown delayed maturation in the prefrontal cortex, an area known to be involved with executive function and working memory.11 Furthermore, structural imaging studies in children and adults with ADHD have identified smaller volumes in the frontal cortex, cerebellum, and subcortical structures.12
The effects of ADHD on deportment and school performance in children are well established. However, an analysis of the impact of ADHD on workplace productivity and costs to employers has focused on the need to increase the diagnosis and treatment of ADHD in adults.13 The study, conducted by Kessler et al. at a large manufacturing firm and published in 2009, found that ADHD was associated with a 4% to 5% reduction in on-the-job productivity, the equivalent of losing 10 to 12 work days in a 250-day work year. The cost to employers for lost work performance was estimated to be $4,336 per employee with ADHD, which translates to $8,241 per 100 workers, assuming a conservative 1.9% prevalence of adult ADHD.13
These findings suggest that treatment of this disabling condition in adults may have a substantial economic benefit to employers as well as to the patients themselves. Furthermore, an analysis of pharmacy and medical claims data obtained from a large managed care plan revealed that annual total costs for adults with ADHD were $3,020 per person, slightly more than the total annual cost for adults with seasonal allergies and less than those incurred by adults with depression or diabetes.14
HISTORY OF THERAPY
General Background on Stimulants
Stimulants have been used to treat symptoms of ADHD for more than 50 years, and in 2006, a treatment algorithm developed by the Texas Consensus Conference Panel on Pharmacotherapy of Childhood ADHD confirmed the use of these agents as a first-line treatment.15,16 The use of stimulant medications in the treatment of ADHD, however, is not without controversy.
In 1999, a four-group, parallel-design study—the Multimodal Treatment Study of Children with ADHD (MTA)—was conducted.17 The children (N = 579) were randomly assigned to receive 14 months of medication management, behavioral treatment (including parent, school, and child components), combined treatment, or the standard care by community providers. All four groups showed marked reductions in symptoms over the course of the study; however, medication management alone and with behavioral therapy were clinically and statistically superior to behavioral treatment or community care alone in reducing ADHD symptoms.17
In a follow-up study by Molina et al.,18 which used mixed-effects regression models with planned contrasts at six and eight years, the original study authors17 concluded that the type or intensity of 14 months of treatment for ADHD in childhood (at age 7 to 9.9 years) did not predict functioning six to eight years later. The results of the long-term data require critical evaluation, given that there was ultimately a mix of treatments over time, making it difficult to interpret the outcome from any single treatment.
In addition to concerns about the efficacy of stimulant treatment for ADHD, there are several safety concerns as well. In May 2006, based on recommendations from the FDA’s Drug Safety and Risk Management Advisory Committee and the Pediatric Advisory Committee, the FDA recommended that manufacturers of ADHD medications revise their product labeling to reflect concerns about adverse cardiovascular and psychiatric events such as sudden death in patients with underlying serious heart problems or defects; aggressive behaviors; visual, tactile, or auditory hallucinations; psychosis; and mania.19,20
The use of stimulants for children with ADHD has also been associated with suppression of growth. In a 36-month follow-up of the MTA study population,17 children newly treated with these agents showed growth suppression of approximately 2 cm compared with unmedicated children.21 Growth suppression occurred during the first 24 months and then stabilized without rebounding.21 A planned 12-year follow-up of this study population may elucidate the long-term growth suppression, if any, experienced by children receiving stimulant treatment for ADHD.
Although the mechanism of action of drugs indicated for the treatment of ADHD is unknown, the therapeutic efficacy of ADHD medications is likely to be dependent on their relative ability to elevate synaptic catecholamine concentrations.22 Both methylphenidate and amphetamine, the two types of stimulants commonly used to treat ADHD, are thought to enhance the efflux and function of both noradrenaline and dopamine in the central nervous system (CNS).22 In addition, the increased dopamine efflux produced by stimulant medications is not limited to cortical regions. Both types of stimulants also have a rapid onset of action with no ceiling on drug effect.22 As molecular entities, methylphenidate and amphetamine are considered equally effective; however, some patients respond better to one drug than to others.23,24 Thus, identifying treatment that is effective for and tolerable to individual patients often requires the use of trial drugs and drug substitution.15
The development of different long-acting stimulant formulations has improved convenience and has extended the effects through once-daily dosing. In addition tothe methylphenidate transdermal patch, which is indicated for ADHD in children, four long-acting, mechanically formulated oral stimulants and one long-acting, chemically formulated prodrug are indicated for the treatment of ADHD in children and adults:
dexmethylphenidate HCl extended-release (ER) capsules (Focalin XR, Novartis): beaded technology, immediate-release (IR), and enteric-coated25
methylphenidate HCl ER tablets (Concerta, McNeil Pediatrics): osmotic release26
methylphenidate HCl ER capsules (Metadate CD, UCB; and Ritalin LA, Novartis): beaded technology, IR, and ER27,28
mixed salts of a single-entity amphetamine product, ER (MAS-XR; Adderall XR, Shire U.S. Inc.): beaded technology, IR, and enteric-coated29
lisdexamfetamine dimesylate (LDX; Vyvanse capsules, Shire U.S. Inc.): prodrug, in vivo conversion of LDX to d-amphetamine30
LDX as a Prodrug
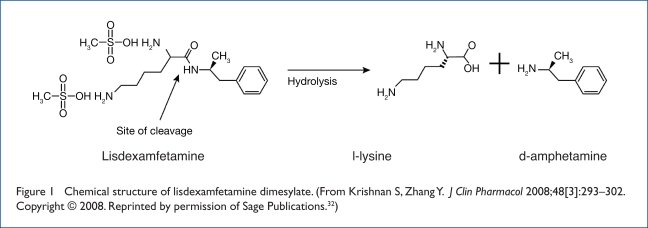
As the first chemically formulated prodrug stimulant,30 LDX represents a new class of long-acting agents for the treatment of ADHD. The concept of prodrugs was proposed by Albert in 1958,31 who described the alteration of the physiochemical properties of drugs to make them pharmacologically inactive until metabolized in the body to an active drug element. Unlike other formulations that rely on mechanical release of active drug that may be affected by gastrointestinal (GI) factors such as transit time and pH, LDX as a prodrug has a biological mechanism of drug delivery that uses enzymatic hydrolysis to convert the therapeutically inactive molecule to the active drug, d-amphetamine (Figure 1).32 The naturally occurring amino acid l-lysine is a by-product of the hydrolysis.
Figure 1.
Chemical structure of lisdexamfetamine dimesylate. (From Krishnan S, Zhang Y. J Clin Pharmacol 2008;48[3]:293–302. Copyright © 2008. Reprinted by permission of Sage Publications.32)
Indicated for the treatment of ADHD in patients 6 to 12 years of age since 2007, LDX received FDA approval for the treatment of ADHD in adults in April 2008.30 In addition to long-term efficacy throughout the day in both children and adults with once-daily dosing, the advantages afforded by this biological mechanism of drug delivery include low rates of inter-patient and intrapatient pharmacokinetic variability, reduced risk of pH-mediated food or drug interactions, an unlikely impact on the availability of active drug with changes in GI transit time, and possibly a lower potential for abuse or diversion.
Pharmacology and Mechanism of Action
The therapeutically active metabolite of LDX is d-amphetamine. Although the precise therapeutic mechanism by which d-amphetamine relieves the symptoms of ADHD is not known, inhibition of dopamine and norepinephrine reuptake and release of these monoamines into extraneuronal space are thought to be involved.30
Pharmacokinetics and Pharmacodynamics
LDX is rapidly absorbed in the intestine. Conversion of LDX to the active metabolite d-amphetamine occurs primarily in the blood through enzymatic cleavage after active absorption of LDX from the GI lumen. The time to maximum concentration (Tmax) of the prodrug molecule (LDX) was one hour.30 In a study of 18 pediatric patients 6 to 12 years of age, the Tmax of d-amphetamine was 3.5 hours following a single oral dose of LDX 30, 50, or 70 mg.30
The bioavailability of d-amphetamine after oral administration of LDX was evaluated in an open-label, randomized, single-dose, three-treatment, three-period, crossover study in 18 healthy volunteers 18 to 55 years of age.32 After administration of a single LDX dose of 70 mg under three dose conditions (fasting and with capsule only; fasting and with solution containing capsule contents; and intact capsule after a high-fat meal), systemic exposure of d-amphetamine was bioequivalent, as measured by drug plasma concentration-time plots and maximum drug concentration. The 70-mg dose is expected to be therapeutically equivalent to the amphetamine base content of 30-mg MAS-XR (20.8 mg vs. 18.8 mg, respectively), which is known to be therapeutically active in children with ADHD.32
In a randomized, multicenter, double-blind, three-treatment, three-period crossover study, Biederman et al. determined the interpatient pharmacokinetic variability of d-amphetamine after giving LDX and MAS-XR to children 6 to 12 years of age with a primary diagnosis of ADHD.33,34 LDX demonstrated low interpatient variability of pharmacokinetic measures, indicating consistent delivery of d-amphetamine. The Tmax for d-amphetamine following an LDX 70-mg dose was 5.06 hours (range, 4.5–6 hours), with a coefficient of variation of 15.33%.
In the MAS-XR 30-mg group, the Tmax for d-amphetamine was 6.6 hours (range, 3–12 hours), with a coefficient of variation of 52.77%. Coefficients of variation for peak plasma concentration (Cmax) were 20.34% with LDX and 43.96% with MAS-XR, suggesting that systemic exposure to d-amphetamine was more predictable after oral administration of 70 mg of LDX than 30 mg of MAS-XR.33,34 Low interpatient and intrapatient variability has been shown in adults as well.35
The dosage-formulation effect was studied in a three-treatment, three-period, single-dose, open-label, crossover pharmacokinetic study in 18 adults (mean age, 31.6 years).36 Shojaei et al. administered a single oral dose of LDX 70 mg to each subject as an intact capsule, a solution, or an intact capsule after a high-fat meal. Systemic exposure of d-amphetamine, in the area-under- the curve (AUC) concentration and Cmax, was bio-equivalent when LDX was administered in a solution or as an intact capsule with or without food. These findings suggest that the dosage formulation does not affect the pharmacokinetic profile of d-amphetamine following the oral administration of LDX. After a high-fat meal, however, Tmax was prolonged by approximately one hour.32
In an in vitro study, the pH solubility profile of LDX was determined in buffered aqueous solutions using an assay specific for LDX. The environmental pH did not affect the solubility profile of LDX within the biological pH range (pH, 1–8), suggesting that gastric pH variation does not affect the absorption of LDX.36
Haffey et al. compared the pharmacokinetics of LDX and MAS-XR, alone or with omeprazole (Prilosec, AstraZeneca),37 a proton pump inhibitor that has been shown to decrease basal gastric acid output by as much as 94%.38 In 24 adults 18 to 45 years of age, total exposure was unaffected by omeprazole for both LDX and MAS-XR. However, for MAS-XR, which uses a pH-sensitive beaded technology, the median Tmax was decreased by 2.25 hours when MAS-XR and omeprazole were coadministered (Tmax = 2.75 hours), compared with MAS-XR administered alone (Tmax = 5 hours). No median Tmax difference was found with coadministration of LDX and omeprazole. This suggests that there is no drug interaction with medications that lower GI pH with the prodrug LDX.37
Nonclinical in vivo and in vitro studies designed to investigate the absorption39 and hydrolysis39,40 of LDX using rodent and human tissues suggest that absorption of LDX occurs primarily in the small intestine and that conversion of LDX into active d-amphetamine occurs primarily in the blood. In the rodent, intact LDX was readily absorbed through duodenal, jejunal, and ileal intestinal segments and underwent pre-systemic enzymatic conversion to active d-amphetamine.39 In the presence of rat and human whole blood, LDX was converted to amphetamine. However, conversion did not occur in plasma or human white blood cells or platelets.39,40 Studies of in vitro enzymatic conversion by human blood cell fractions demonstrated that LDX was converted into active d-amphetamine by red blood cells.39,40
CLINICAL TRIALS
The approval of LDX for children with ADHD was based on results from three controlled clinical trials and one open-label trial.33,41–43 The indication for adult ADHD was supported by data from a large, double-blind, placebo-controlled trial by Adler et al. (see page 283).44
For the studies,33,41–43 children were required to satisfy DSM-IV Text Revision (DSM-IV-TR) criteria for a diagnosis of the combined or predominantly hyperactive–impulsive subtype of ADHD, and adults had to meet six of the nine DSM-IV-TR subtype criteria. General primary inclusion criteria included a history of treatment with a stable regimen of stimulant medication and the ability to function at an age-appropriate intellectual level. Exclusion criteria were as follows:
the presence of comorbid illness that could interfere with participation in or completion of the study or that could affect the efficacy or tolerability of the study drugs
a documented allergy or intolerance to any study drug
concomitant medications with CNS effects
a history of drug abuse
a current comorbid psychiatric disorder (e.g., psychosis or bipolar disorder) that would contraindicate treatment with the study drug or that would confound efficacy or safety assessments
a history of seizures within the previous two years
tic disorders
cardiac disorders (not specified except for a prolonged QTc interval, cardiac structural abnormality, or a condition that might afffect cardiac performance)
significant laboratory abnormalities (not specified except hyperthyroidism)
significant deviation from normal weight
contraceptive restrictions such as the double-barrier method, intrauterine devices, or pharmacologically effective hormaonal contraceptives
Pediatric Studies
Biederman et al.33
The efficacy of LDX and MAS-XR was examined in a simulated classroom setting. In a randomized, double-blind, placebo-controlled and active-controlled crossover study, 52 children with ADHD 6 to 12 years of age satisfied DSM-IV-TR criteria for a diagnosis of the combined or predominantly hyperactive–impulsive subtype of ADHD. Each child received LDX, MAS-XR, and placebo for one week. The study authors measured responses to treatment using three standard efficacy scales during observations made over a period of approximately 12 hours:
the validated Swanson, Kotkin, Agler, M-Flynn, and Pelham Deportment (SKAMP-D) rating scale, which uses an independent observer to measure classroom symptoms of ADHD45
the Permanent Product Measure of Performance (PERMP), a validated tool consisting of 400 age-appropriate math questions administered in a 10-minute time period46
the validated Clinical Global Impressions (CGI) scale for symptom severity and improvement47
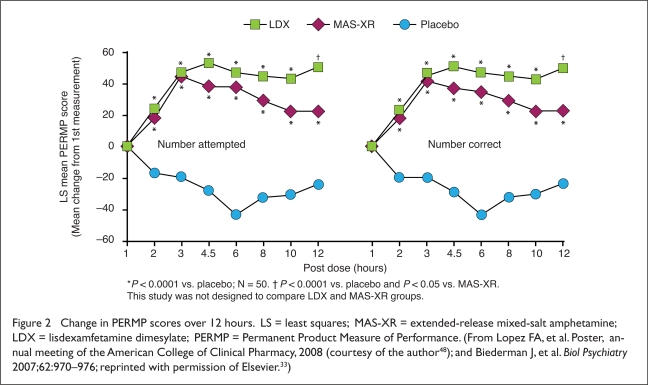
LDX 30, 50, or 70 mg and MAS-XR 10, 20, or 30 mg significantly improved measures of efficacy on all three scales compared with placebo.33 Least-squares mean scores on the PERMP-Attempted (PERMP-A) were 133.3 for LDX, 133.6 for MAS-XR, and 88.2 for placebo (P < 0.0001 for both active treatments vs. placebo) (Figure 2).33,48 Similarly, least-squares mean scores on the PERMP-Correct (PERMP-C) were 129.6 for LDX, 129.4 for MAS-XR, and 84.1 for placebo (P < 0.0001 for both active treatments vs. placebo).
Figure 2.
Change in PERMP scores over 12 hours. LS = least squares; MAS-XR = extended-release mixed-salt amphetamine; LDX = lisdexamfetamine dimesylate; PERMP = Permanent Product Measure of Performance. (From Lopez FA, et al. Poster, annual meeting of the American College of Clinical Pharmacy, 2008 (courtesy of the author48); and Biederman J, et al. Biol Psychiatry 2007;62:970–976; reprinted with permission of Elsevier.33)
No meaningful abnormalities were observed in diastolic blood pressure (BP), pulse, heart rate, QRS interval, or corrected QT (QTc) interval.33 Adverse events (AEs) occurring in 2% of patients or more during the double-blind treatment period were insomnia, decreased appetite, and anorexia with LDX, and decreased appetite, abdominal and upper abdominal pain, vomiting, and insomnia with MAS-XR.33 Of the 52 children enrolled, 50 completed the study. Two patients discontinued the study during the first double-blind treatment week while receiving placebo. One subject discontinued therapy because of gastroenteritis, and one participant was lost to follow-up.
A post hoc analysis of PERMP-A and PERMP-C ratings revealed that the duration, as measured by the change in score at each hour from first measurement at one hour post dose, favored both active treatments at all time points starting two hours post dose (P < 0.0001 for both active treatments vs. placebo) (see Figure 2).33,48
An additional post hoc analysis of CGI scores was conducted using McNemar’s test to assess differences in the proportion of children receiving LDX or MAS-XR whose scores were rated as “very much improved” on the CGI–Improvement (CGI–I) scale.47 LDX therapy resulted in a significantly higher proportion of children whose scores were rated as “very much improved” compared with MAS-XR (P < 0.05).33,49 Numerically, 32% of LDX patients, 16% of MAS-XR patients, and 2% of placebo controls were rated as “very much improved” on the CGI–I scale. 33
Biederman et al.41
The efficacy of LDX was evaluated in a large, multicenter, randomized, double-blind, forced-dose titration, parallel-group study in 290 children 6 to 12 years of age with ADHD.41 Oral doses of 30, 50, or 70 mg/day of LDX were administered with forced-dose titration or placebo once daily in the morning for four weeks. The primary efficacy measure was the score on the ADHD Rating Scale IV (ADHD–RS-IV),50 a validated instrument51 based on investigator-conducted parent interviews. Significantly greater improvements from baseline ADHD–RS-IV scores were noted with each of the three LDX doses measured throughout the day compared with placebo (P < 0.001 for all comparisons). LDX resulted in significant improvement in both the inattention and the hyperactivity subscales of the ADHD–RS.
At the end of the study, the effect sizes of treatment with LDX, based on the ADHD–RS-IV, were 1.21, 1.34, and 1.60 for LDX 30, 50, and 70 mg, respectively. Moreover, significant improvements from baseline were observed on the validated Conners’ Parent Rating Scale (CPRS–R)52 throughout the day and up to 6 p.m.41 Compared with placebo, CGI scores also improved significantly from baseline with all LDX doses at the treatment endpoint.47
More than 95% of all AEs were rated as mild to moderate in intensity. Most of these events began in the first week of treatment, and the onset of new events abated over the four-week study period. The most common AEs, occurring in more than 5% of patients with active treatment, were decreased appetite and weight, insomnia, upper abdominal pain, headache, irritability, vomiting, and nausea.41 No significant changes in mean electrocardiographic parameters, including corrected QT intervals, laboratory values, and systolic and diastolic BP, were observed with LDX. A significant increase in heart rate was observed in the LDX groups, compared with the placebo group; the highest placebo-adjusted increase was four to five beats per minute (bpm), observed in the LDX 70-mg group at the study’s endpoint. No meaningful differences in heart rate were found at each of the four treatment weeks.
Twenty-one treated patients discontinued the study because of AEs, including six (8%) in the LDX 30-mg group, four (5%) in the LDX 50-mg group, 10 (14%) in the LDX 70-mg group, and one (1%) in the placebo group.41
Faraone53,54
To further assess the large effect sizes observed with LDX treatment in the Biederman study,41 Faraone et al. recalculated the effect sizes and used them in meta-analyses.53,54 The investigators calculated effect sizes, expressed as standard mean differences, by taking the mean drug effect minus the mean placebo effect and dividing the result by the pooled standard deviation of the groups. In a meta-analysis of stimulant therapy for ADHD, LDX was found to have an effect size larger than that previously reported for other long-acting stimulants, including MAS-XR.54
The analysis examined two potential artifacts that might have inflated the LDX effect size:
precision of measurement, as indexed by standard deviation (SD) of endpoint scores
the baseline effect size when compared with the endpoint placebo effect
It was determined that the large LDX effect size could not be attributed to unusually high precision of measurement or to an unusually low placebo effect size, suggesting that the relatively large effect size of LDX was a result of drug efficacy itself.54 The effect size of LDX was 1.39 (95% confidence interval [CI], 1.03–1.76) for 30 mg/day, 1.42 (95% CI, 1.05–1.79) for 50 mg/day, and 1.73 (95% CI, 1.35–2.11) for 70 mg/day. Among stimulants using the same outcome measure, this was the largest effect size, although the 95% CI overlapped with those of other studies.
Wigal et al.42
LDX efficacy was also documented in a third study of 129 children 6 to 12 years of age in a simulated classroom setting.42 In a randomized, double-blind, placebo-controlled crossover study by Wigal et al., changes from the baseline SKAMP-D, SKAMP-Attention (SKAMP-A),45 and PERMP46 scores up to 13 hours post dose were significantly greater for children receiving LDX than placebo (P < 0.005). LDX showed efficacy at each post-dose time point (1.5–13.0 hours), as measured by SKAMP-D, SKAMP-A, and total scores, and from 2.5 to 13 hours for the SKAMP quality of work subscale (all P values were less than 0.005). Results on the PERMP scales were consistent with these observations.
AEs were consistent with those of other pediatric studies with LDX.41 Treatment-emergent AEs occurring in 10% of subjects or more during the dose-optimization period were lability of affect, decreased appetite, headache, insomnia, irritability, and upper abdominal pain. During the double-blind crossover period, there were no new treatment-emergent AEs. No serious AEs or deaths were reported. All treatment-related AEs leading to discontinuation occurred before the double-blind crossover period; those occurring in more than one subject and leading to discontinuation were abdominal pain in two patients, nausea in two, vomiting in two, fatigue in two, irritability in two, anorexia in two, psychomotor hyperactivity in two, and insomnia or sleep disorder in four.
Suicidal ideation, related to four days of exposure to LDX and assessed as mild in severity, was reported during dose optimization in an 11-year-old boy with no other clinical conditions at baseline. There were no dose-related changes in vital signs during the dose-optimization period; however, vital signs increased slightly from baseline for both patients treated with LDX and placebo controls during the crossover period. Maximum mean (SD) increases from baseline in BP were 4.2 (9.2) mm Hg for systolic BP and 4.7 (8.5) mm Hg for diastolic BP, both in the 70-mg LDX group at eight hours after the dose was given. The maximum mean (SD) increase in pulse rate was 9.9 (9.8) bpm in the 70-mg LDX group at 12.5 hours post dose compared with 6.6 (12.9) bpm for the placebo group and 6.6 (13.6) bpm for all active doses of LDX combined at the same time point. Consistent with other clinical studies of LDX, data for the ECG interval exhibited no clinically meaningful trends.42
Findling et al.43
The long-term tolerability and efficacy of LDX were confirmed in an open-label, 12-month, single-arm study of 272 children 6 to 12 years of age with a DSM-IV diagnosis of ADHD. The initial dose of LDX was 30 mg/day. The children continued with this dose, or the dose was increased to 50 or 70 mg/day over a four-week period as needed. ADHD–RS total scores improved significantly (above 60%, P < 0.0001) compared with baseline over the 12-month treatment period.50 The improvement over baseline ADHD–RS total score was significant at the first on-therapy visit at week 1 and was maintained throughout the 12-month treatment period. AEs were rated generally mild to moderate in intensity and were consistent with those observed in short-term studies.
Treatment-emergent AEs occurring in 5% or more of subjects, most often within the first four weeks of therapy, included decreased appetite, headache, weight loss, insomnia, upper abdominal pain, upper respiratory tract infection, irritability, nasopharyngitis, vomiting, cough, and influenza. Insomnia and vomiting occurred more often in those receiving higher doses of LDX. Five serious AEs were reported in four patients, but none were considered related to study medication. No deaths were reported. Overall, 25 of 272 patients (9%) discontinued the study drug because of AEs; aggression, irritability, and decreased appetite were the most commonly reported reasons.
At the end of the study, the mean increase from baseline in height was 1.5 inches (P < 0.05) and 0.6 pounds in weight (P was not significant). As with other stimulants, slowing of growth was observed when these changes were normalized to account for expected growth. As was the case in the short-term pediatric studies, treatment with LDX produced small but clinically nonsignificant changes in cardiovascular parameters similar to those seen in other studies with stimulants.
For vital signs measured at the study’s endpoint, mean (SD) increases from baseline in BP were 0.7 (10) mm Hg for systolic BP and 0.6 (8.3) mm Hg for diastolic BP. The mean (SD) increase in pulse rate was 1.4 (13.7) bpm.43 No clinically meaningful changes in ECG measures were observed, and there were no apparent trends in vital sign outliers.
Regular monitoring of vital signs during treatment with LDX and other ADHD medications is recommended because of possible outlying measures. No clinically significant changes in laboratory values or in physical findings were observed.43
Wigal et al.42
The impressions of parents and guardians were assessed in a laboratory school study of LDX in children 6 to 12 years of age. The study was designed to evaluate the efficacy and safety of LDX 30, 50, or 70 mg/day. Parents completed the Medication Satisfaction Questionnaire, a non-validated scale. They were asked to rate their level of satisfaction with LDX and to compare LDX with their child’s previous ADHD drug treatment.
Parents of participants in the intent-to-treat (ITT) population (n = 113) reported being “very satisfied” (76%) or “moderately satisfied” (23%) with LDX.
Studies in Adults
Adler et al.44
The efficacy and safety of LDX in adults were evaluated in a randomized, double-blind, placebo-controlled, parallel-group, four-week study with forced-dose escalation in 420 adults 18 to 55 years of age.44 Patients received LDX 30, 50, or 70 mg/day or placebo for four weeks. At the end of the study, changes in ADHD–RS50 scores were significantly greater for each LDX dose than for placebo (P < 0.001 vs. placebo). Relative to placebo, significantly more subjects in each LDX group had a reduction of 30% or more in ADHD–RS total scores beginning at week 1 and at each week thereafter (P < 0.01 for all comparisons).
At the end of the study, the effect sizes of treatment with LDX, based on the ADHD–RS raw mean change scores, were 0.73 with LDX 30 mg/day, 0.89 with LDX 50 mg/day, and 0.99 with LDX 70 mg/day. Safety and tolerability profiles of LDX were similar in adults and children.
Among adults in the LDX groups, AEs with an incidence of greater than 5% and twice that of placebo included decreased appetite, anorexia, dry mouth, insomnia, nausea, diarrhea, jittery feelings, and anxiety. Most AEs occurred during the first week of treatment, when all patients randomly assigned to active treatment were receiving LDX 30 mg.
Rates of therapy discontinuation attributable to AEs among adults were low (6% with LDX and 2% with placebo). Treatment-related AEs occurring alone or in combination in more than one subject and leading to discontinuation were insomnia in eight patients, tachycardia in three, irritability in two, headache in two, increased BP in four, anxiety in two, and dyspnea in three. No effects on QTc Fridericia (QTcF) measurements and no clinically meaningful trends for systolic BP or diastolic BP were observed with LDX.44,55
Least-squares mean (95% CI) changes in systolic BP were −0.5 (−2.6, 1.5) mm Hg with placebo; 0.8 (−0.7, 2.3) mm Hg with LDX 30 mg/day; 0.3 (−1.2, 1.8) mm Hg with LDX 50 mg/day; and1.3 (−0.2, 2.7) mm Hg with LDX 70 mg/day.
Least-squares mean changes (95% CI) in diastolic BP were 1.1 (−0.5, 2.7) mm Hg with placebo; 0.8 (−0.4, 2.0) mm Hg with LDX 30 mg/day; 1.1 (−0.1, 2.3) mm Hg with LDX 50 mg/day; and 1.6 (0.4, 2.7) mm Hg with LDX 70 mg/day.
Using the Pittsburgh Sleep Quality Index (PSQI), Adler et al. observed no statistically significant changes in sleep quality, as measured by PSQI total score, in any of the treatment groups.44,56 The PSQI total score included component scores for subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and dysfunction in the daytime.
Brams57
Brams also assessed LDX in adults with ADHD in a simulated workplace environment.57 This randomized, double-blind, placebo-controlled, crossover study evaluated the efficacy and safety of LDX 30, 50, and 70 mg compared with placebo in 142 adults with ADHD. LDX demonstrated significant improvement when compared with placebo in average total PERMP scores (312.9 and 289.5, respectively; P < 0.0001). At each post-dose assessment from two to 14 hours, the LDX group also had significantly better mean PERMP total scores than the placebo group (P < 0.01 for all). Decreased appetite, dry mouth, headache, insomnia, upper respiratory tract infection, irritability, nausea, anxiety, and jittery feelings were the most frequently reported AEs in more than 5% of patients during the dose-optimization phase of the study.
Weisler et al.58
The long-term tolerability and efficacy of LDX were documented in a 12-month, open-label, single-arm extension of the four-week study reported by Adler et al.44 Weisler et al. enrolled 349 adults with ADHD, 18 to 55 years of age, who had completed the short-term study. LDX doses were optimized for all subjects over four weeks and were continued for 11 months with dose adjustments allowed. Most AEs were rated as mild or moderate in severity and occurred early in the course of treatment. The most common treatment-emergent AEs, experienced by more than 10% of subjects, were upper respiratory tract infection, insomnia, headache, dry mouth, decreased appetite, and irritability.
LDX was also associated with statistically significant improvements in sleep quality, as assessed by global PSQI scores (P < 0.0001). A PSQI score above 5 is generally considered definitive to distinguish poor sleepers from good sleepers.59
At the study’s endpoint, small but statistically significant increases (mean [SD] change from baseline) in pulse rate (3.2 [11.6] bpm), systolic BP (3.1 [10.7] mm Hg), and diastolic BP (1.3 [7.6] mm Hg), were noted; however, these changes were consistent with the known effects of stimulants, as stated in the labeling for these medications regarding serious cardiovascular events.25–30
One patient discontinued the study with an elevated glucose level, which was considered to be unrelated to the study drug. For the ITT population, significant improvements in ADHD–RS50 total scores from baseline were observed at all visits. At the endpoint, the mean (SD) change in total score was −24.8 (11.7; P < 0.0001) and 84.1% of the ITT population was considered to have improved CGI–I47 measures from baseline scores.58
LABELING FOR ABUSE POTENTIAL
All stimulants indicated for the treatment of ADHD are controlled substances. However, abuse liability varies with the delivery system used in these formulations.60 Although the rate of onset is an important consideration for the impact of the formulation on the potential for abuse, as often shown in such studies, the greatest abuse-related effects occurred relatively close to the time point associated with Cmax and Tmax.60 Conversion of the LDX prodrug to its active component requires enzymatic hydrolysis that results in a slow rise in serum d-amphetamine level, which provides a possibly reduced potential for diversion or abuse.61 LDX includes data on abuse liability in the product label; however, similar to other stimulants approved in the U.S. to treat ADHD, LDX is classified as a Schedule CII compound.
Jasinski and Krishnan61,62
Several attributes of LDX have been evaluated to further characterize its abuse liability. In a randomized, double-blind, three-way crossover study,61 Jasinski and Krishnan administered intravenous (IV) 50-mg doses of LDX and 20-mg doses of d-amphetamine (with an equivalent amphetamine base content) to adults with a history of stimulant abuse who did not have ADHD. IV LDX 50 mg did not have significantly different abuse-related “drug-liking” (or “drug-likable”) effects compared with placebo. In contrast, approximately equivalent doses of IV IR d-amphetamine 20 mg did have significantly more drug-liking effects than placebo.
The pharmacokinetic profile of d-amphetamine following IV LDX 50 mg also demonstrated a delayed Tmax (2.5 hours) in contrast to IV IR d-amphetamine 20 mg (0.8 hours). Relative to an equivalent dose of IR d-amphetamine, the Cmax of
d-amphetamine was lower for LDX (105 and 38.9 ng/mL, respectively).61 As expected, IV IR d-amphetamine 20 mg demonstrated a rapid rise in serum level by Tmax and elevation by Cmax in contrast to dose-equivalent IV LDX 50 mg, whose Tmax was slower by three-fold and whose Cmax was 63% lower.
In another study of adult abusers of stimulants by the same authors, LDX 50 and 100 mg, taken orally, had reduced abuse-related drug-liking effects compared with IR d-amphetamine (40 mg, an amphetamine-based dose equivalent to LDX 100 mg).62 However, a higher dose of LDX (150 mg, equivalent to d-amphetamine 60 mg) resulted in a significant difference in abuse-related drug-liking scores compared with placebo.
Ermer et al.63
Ermer et al. examined the pharmacokinetic properties of LDX administered to adults intranasally or orally. The plasma concentration, compared with time curves, for d-amphetamine were similar when LDX was given by either route. Thus, the rate of elevation in the d-amphetamine serum level was identical regardless of the route of administration. This seems counterintuitive, because a more rapid rise in serum level with an intranasal administration would be expected. It might be that the active therapeutic agent, d-amphetamine, is made available to the system only after LDX conversion in the body. The exposure to d-amphetamine by the two routes was bio-equivalent.
ADHERENCE TO THERAPY
Non-adherence to prescribed treatment is common with most chronic medical conditions.64 In adults with ADHD, who tend to be forgetful, non-adherence can be particularly problematic. Moreover, patients may require more than a single dose of medication if symptoms are not controlled throughout the day.
One approach to measuring adherence to a treatment regimen is to calculate the daily average consumption (DACON) value of the index medication, the need for augmentation with a second medication, and persistence with prescription refills. The DACON value is the ratio of the average quantity supplied versus quantity consumed during the same period or, more simply put, the number of doses taken per day.
Christensen et al. conducted a retrospective study of medical and pharmacy claims data from January 2007 to April 2008 to compare DACON, persistence with therapy, and the use of selected long-acting ADHD medications in adults and to examine the relationship between these variables and ADHD-related pharmacy costs.65 Selected agents included LDX, MAS-XR, methylphenidate ER, dexmethylphenidate ER, and atomoxetine (Strattera, Lilly).
Adults receiving LDX as their initial medication for ADHD had the lowest DACON scores (1.06) and were more likely to have a score of less than 1. They also had the longest persistence with their index medication (116.5 days) compared with adults initiating treatment with other long-acting ADHD medications (74.9–115.4 days).65
Although LDX was associated with higher pharmacy costs (median price, $543; P < 0.0001 vs. other drugs), patients receiving LDX had the highest number of prescription fills, the longest persistence, and low per-prescription costs compared with patients who used other study drugs.65
DISCUSSION
Benefits of LDX
As the first chemically formulated prodrug stimulant, LDX has unique attributes over existing treatments for ADHD. Low interpatient variability for key pharmacokinetic parameters suggests consistent systemic exposure for most patients; this might be a result of the absence of a mechanized delivery system.
Unlike other stimulants that use a pH-dependent mechanical formulation (beads) to deliver active medication, LDX absorption and conversion are not affected by variations in gastric pH; therefore, LDX is not prone to pH-mediated food or drug interactions. This factor is particularly relevant for adults, many of whom take prescribed or over-the-counter drugs that alter gastric pH. Changes in GI transit time are also unlikely to affect the bioavailability of the active drug d-amphetamine.
LDX capsules may be taken whole, or the capsule may be opened and the entire contents dissolved in a glass of water,30 providing the only liquid long-acting stimulant preparation. This route of administration may be particularly helpful in children who may have difficulty swallowing capsules or tablets and who need a long duration of action from the medication.
Stimulant medications such as LDX that have barriers to the extraction of the active drug may be a useful addition to a physician’s armamentarium of therapeutic choices. Abuse-liability studies of LDX found a lower drug-liking effect than an equivalent oral dose of immediate-release (IR) d-amphetamine.61–63 Intravenously, LDX showed a delayed Tmax and comparable abuse-related drug-liking scores, when compared with placebo, offering the advantage of a prolonged effect with reduced abuse-related drug liking within the recommended dosage range. Doses that are greater than twice the recommended maximum can result in higher drug-likability scores. Furthermore, intranasal administration resulted in a pharmacokinetic profile of d-amphetamine exposure similar to that of oral administration.
The drug’s long duration of efficacy was established in three controlled trials in children and one controlled trial in adults. In two pediatric studies, efficacy was demonstrated with LDX up to 6 p.m. on parent rating scales.41 Efficacy was consistently maintained from the first post-dose time point (1.5 hours) up to and including the last time point assessed (13 hours) on the primary and most secondary efficacy measures.42 In a work-place-like setting, the duration of effect in adults was observed for 14 hours after the dose was received.57 Efficacy was achieved with a safety and tolerability profile consistent with that of long-term stimulant use.
The large effect size of LDX is believed to be a result of the drug’s efficacy itself. Although drug efficacy might not be directly related to pharmacokinetic characteristics, these large effects on symptoms may be related to more predictable bioavailability of the active moiety, d-amphetamine, when hydrolyzed from the inactive prodrug, LDX.
Adverse Events and Precautions
In clinical trials, the most common AEs associated with LDX in children 6 to 12 years of age, at an incidence of 5% or more, were decreased appetite, dizziness, dry mouth, irritability, insomnia, upper abdominal pain, nausea, vomiting, and decreased weight. The most common AEs associated with LDX in adults were upper abdominal pain, diarrhea, nausea, fatigue, jittery feelings, irritability, anorexia, decreased appetite, headaches, anxiety, and insomnia.
Consistent with other CNS stimulant medications, LDX labeling carries a precaution regarding serious cardiovascular events, including sudden death, increases in BP, psychiatric AEs such as treatment-emergent psychotic or manic symptoms in patients with no prior history, or exacerbation of symptoms in patients with pre-existing psychosis, seizures, visual disturbances, exacerbation of tics, and long-term growth suppression. Psychotic AEs have been identified as adverse reactions during post-approval use of LDX and other psychostimulants, and these events were rarely reported at an incidence of less than 1% in the clinical trials.
CONCLUSION
ADHD is a common neuropsychiatric disorder that often persists from childhood into adulthood, causing costly reductions in workplace productivity. Although stimulants remain the first-line treatment for ADHD,15 some patients respond better to one drug than to others.23,24
As a therapeutic option for patients with ADHD, LDX has relevance for health care professionals and P&T committees. Compared with other long-acting drugs indicated for ADHD, LDX is associated with lower daily average consumption, less need for augmentation, and greater persistence with therapy.65 Its lower potential for abuse, when compared with that of short-acting ADHD agents,61–63 also helps to address a serious public health concern.
Available as a first-line therapy for ADHD in adults and children 6 to 12 years of age, LDX has less interpatient and intra-patient variability in pharmacokinetic characteristics of d-amphetamine derived from the inactive prodrug. It is efficacious at doses of 30 to 70 mg daily in both children and adults. Its effects last for 13 hours in children and 14 hours in adults, and its safety and tolerability profile is similar to that of other stimulant medications.
Acknowledgments
Editorial assistance was provided by Ann Sherwood, Robert Gregory, William Perlman, and Rosa Real, all from Excerpta Medica in Bridgewater, New Jersey.
Footnotes
Disclosure. Dr. Goodman has received grant and research support from Forest Labs, Shire, McNeil, and Cephalon. He has received honoraria from GlaxoSmithKline, Forest Labs, Eli Lilly, Shire, McNeil, Wyeth, and Novartis. He is on the speakers’ bureau for Glaxo-SmithKline, Forest Labs, Eli Lilly, Shire, McNeil, Wyeth, and Novartis. Dr. Goodman is also a consultant for GlaxoSmithKline, Forest Labs, Eli Lilly, Shire, McNeil, and Novartis. Preparation of this manuscript was supported by Shire Development, Inc., in Wayne, Pennsylvania.
REFERENCES
- 1.Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of U.S. children. Arch Pediatr Adolesc Med. 2007;161:857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- 2.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: Is it an American condition? World Psychiatry. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss M, Hechtman LT, Weiss G. ADHD in Adulthood: A Guide to Current Theory, Diagnosis, and Treatment. Baltimore: Johns Hopkins University Press; 1999. [Google Scholar]
- 4.Kessler RC, Adler LA, Barkley R, et al. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: Results from the National Comorbidity Survey Replication. Biol Psychiatry. 2005;57:1442–1451. doi: 10.1016/j.biopsych.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkley RA, Murphy KR, Fischer M. ADHD in Adults: What the Science Says. New York: Guilford Press; 2008. [Google Scholar]
- 6.Dulcan M, Work Group on Quality Issues Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(Suppl 10):85S–121S. doi: 10.1097/00004583-199710001-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayyad J, de Graaf R, Kessler R, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402–409. doi: 10.1192/bjp.bp.106.034389. [DOI] [PubMed] [Google Scholar]
- 9.Faraone SV, Biederman J, Spencer T, et al. Attention-deficit/hyper-activity disorder in adults: An overview. Biol Psychiatry. 2000;48:9–20. doi: 10.1016/s0006-3223(00)00889-1. [DOI] [PubMed] [Google Scholar]
- 10.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyper-activity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faraone SV. Etiology and pathophysiology of adult attention-deficit/hyperactivity disorder. Prim Psychiatry. 2004;11:28–40. [Google Scholar]
- 13.Kessler RC, Lane M, Stang PE, Van Brunt DL. The prevalence and workplace costs of adult attention deficit hyperactivity disorder in a large manufacturing firm. Psychol Med. 2009;39:137–147. doi: 10.1017/S0033291708003309. [DOI] [PubMed] [Google Scholar]
- 14.Hinnenthal JA, Perwien AR, Sterling KL. A comparison of service use and costs among adults with ADHD and adults with other chronic diseases. Psychiatr Serv. 2005;56:1593–1599. doi: 10.1176/appi.ps.56.12.1593. [DOI] [PubMed] [Google Scholar]
- 15.Pliszka SR, Crismon ML, Hughes CW, et al. Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention-Deficit/Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: Revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:642–657. doi: 10.1097/01.chi.0000215326.51175.eb. [DOI] [PubMed] [Google Scholar]
- 16.Pliszka S, AACAP (American Academy of Child and Adolescent Psychiatry) Work Group on Quality Issues Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- 17.The MTA Cooperative Group A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 18.Molina BS, Hinshaw SP, Swanson JM, et al. the MTA Cooperative Group MTA at 8 years: Prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA Minutes of the Drug Safety and Risk Management Advisory Committee, February92006. Available at: www.fda.gov/ohrms/dockets/ac/06/minutes/2006-4202M1_final-Minutes.pdf Accessed September 28, 2009.
- 20.FDA Minutes of the Pediatric Advisory Committee, March222006. Available at: www.fda.gov/ohrms/dockets/ac/06/minutes/2006-4210m_Minutes%20PAC%20March%2022%202006.pdf Accessed September 28, 2009.
- 21.Swanson JM, Elliott GR, Greenhill LL, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46:1015–1027. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- 22.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: Insights on efficacy and safety. Neuropharmacology. 2009;57(7–8):608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA Study: Relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry. 1996;34:1304–1313. doi: 10.1097/00004583-199610000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Arnold LE, Christopher J, Huestis R, Smeltzer DJ. Methylphenidate vs. dextroamphetamine vs. caffeine in minimal brain dysfunction: Controlled comparison by placebo washout design with Bayes’ analysis. Arch Gen Psychiatry. 1978;35:463–473. doi: 10.1001/archpsyc.1978.01770280073008. [DOI] [PubMed] [Google Scholar]
- 25.Focalin XR, package insert. East Hanover, N.J.: Novartis; Mar, 2010. [Google Scholar]
- 26.Concerta, package insert. Titusville, N.J.: McNeil Pediatrics; Nov, 2009. [Google Scholar]
- 27.Metadate CD, package insert. Smyrna, Ga: UCB, Inc.; Jun, 2009. [Google Scholar]
- 28.Ritalin LA, package insert. East Hanover, N.J.: Novartis; Apr, 2009. [Google Scholar]
- 29.Adderall XR, package insert. Wayne, Pa: Shire US Inc.; Mar, 2010. [Google Scholar]
- 30.Vyvanse, package insert. Wayne, Pa: Shire US Inc.; Dec, 2009. [Google Scholar]
- 31.Albert A. Chemical aspects of selective toxicity. Nature. 1958;182:421–423. doi: 10.1038/182421a0. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan S, Zhang Y. Relative bioavailability of lisdexamfetamine 70-mg capsules in fasted and fed healthy adult volunteers and in solution: A single-dose, crossover pharmacokinetic study. J Clin Pharmacol. 2008;48(3):293–302. doi: 10.1177/0091270007310381. [DOI] [PubMed] [Google Scholar]
- 33.Biederman J, Boellner SW, Childress A, et al. Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: A double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry. 2007;62:970–976. doi: 10.1016/j.biopsych.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Ermer JC, Shojaei AH, Biederman J, Krishnan S. Improved inter-patient pharmacokinetic variability of lisdexamfetamine dimesylate compared with mixed amphetamine salts extended release in children aged 6 to 12 years with attention-deficit/hyperactivity disorder. Poster presented at the American Psychiatric Association annual meeting; May 19–24, 2007; San Diego. [Google Scholar]
- 35.Ermer J, Homolka R, Martin P, et al. Lisdexamfetamine dimesylate: Linear dose proportionality, low inter- and intrasubject variability, and safety in an open-label single-dose pharmacokinetic study in healthy adult volunteers. J Clin Pharmacol; Poster presented at the American College of Clinical Pharmacy annual meeting; October 19–22, 2008; Louisville, Ky. Feb, 2010. (electronic version). [DOI] [PubMed] [Google Scholar]
- 36.Shojaei A, Ermer JC, Krishnan S. Lisdexamfetamine dimesylate as a treatment for ADHD: Dosage formulation and pH effects. Poster presented at the American Psychiatric Association annual meeting; May 19–24, 2007; San Diego. [Google Scholar]
- 37.Haffey M, Buckwalter M, Zhang P, et al. Effects of omeprazole on the pharmacokinetic profiles of lisdexamfetamine dimesylate and extended-release mixed amphetamine salts in adults. Postgrad Med. 2009;121:11–19. doi: 10.3810/pgm.2009.09.2048. [DOI] [PubMed] [Google Scholar]
- 38.Prilosec, package insert. Wilmington, DE: AstraZeneca; Mar, 2010. [Google Scholar]
- 39.Pennick M. Absorption of lisdexamfetamine dimesylate and hydrolysis to form the active moiety, d-amphetamine. Poster presented at the annual meeting of the New Clinical Drug Evaluation Unit; June 29–July 2, 2009; Hollywood, Fla. (in press). [Google Scholar]
- 40.Pennick M.Hydrolytic conversion of lisdexamfetamine dimesylate to the active moiety, d-amphetamine Poster presented at the Annual Scientific Convention and Meeting of the Society of Biological PsychiatryMay 14–16, 2009Vancouver; Absorption of the prodrug lisdexamfetamine dimesylate and its subsequent enzymatic conversion to the active moiety d-amphetamine. Neuropsychiatric Dis Treat (in press). [Google Scholar]
- 41.Biederman J, Krishnan S, Zhang Y, et al. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: A phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther. 2007;29:450–463. doi: 10.1016/s0149-2918(07)80083-x. [DOI] [PubMed] [Google Scholar]
- 42.Wigal SB, Kollins SH, Childress AC, et al. 311 Study Group A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Mental Health. 2009;3:17–30. doi: 10.1186/1753-2000-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Findling RL, Childress AC, Krishnan S, McGough JJ. Long-term effectiveness and safety of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. CNS Spectrums. 2008;13:614–620. doi: 10.1017/s1092852900016898. [DOI] [PubMed] [Google Scholar]
- 44.Adler LA, Goodman DW, Kollins SH, et al. 303 Study Group Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69:1364–1373. doi: 10.4088/jcp.v69n0903. [DOI] [PubMed] [Google Scholar]
- 45.Wigal SB, Gupta S, Guinta D, Swanson JM. Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacol Bull. 1998;34:47–53. [PubMed] [Google Scholar]
- 46.Swanson J, Wigal S, Greenhill L, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacol Bull. 1998;34:55–60. [PubMed] [Google Scholar]
- 47.Guy W.ECDEU Assessment Manual for Psychopharmacology Revised Rockville, Md: National Institute of Mental Health, U.S. Department of Health, Education, and Welfare, Pub. No. ADM 76-338. 1976218–222. [Google Scholar]
- 48.Lopez FA, Childress AC, Curtiss S. Improvement in attention-deficit/hyperactivity disorder symptoms in children with lisdexamfetamine dimesylate versus extended-release mixed amphetamine salts and placebo in an analog classroom. Poster presented at the annual meeting of the American College of Clinical Pharmacy; October 19–22, 2008; Louisville, Ky. [Google Scholar]
- 49.Scheckner B, Schreckengost J, Favit A. Physician perception of clinical improvement with lisdexamfetamine dimesylate in children aged 6 to 12 years with attention-deficit/hyperactivity disorder. Poster presented at the annual meeting of the American Psychiatric Association; May 3–8, 2008; Washington, DC. [Google Scholar]
- 50.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale–IV: Checklists, Norms, and Clinical Interpretations. New York: Guilford Press; 1998. [Google Scholar]
- 51.Faries DE, Yalcin I, Harder D, Heiligenstein JH. Validation of the ADHD Rating Scale as a clinician-administered and scored instrument. J Atten Disord. 2001;5:107–115. [Google Scholar]
- 52.Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS–R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 53.Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8:4. [PMC free article] [PubMed] [Google Scholar]
- 54.Faraone SV, Schreckengost J. Lisdexamfetamine dimesylate effect size in children with attention-deficit/hyperactivity disorder. Poster presented at the American Academy of Child and Adolescent Psychiatry annual meeting; October 23–28, 2007; Boston. [Google Scholar]
- 55.Adler L, Weisler R, Goodman D, et al. Short-term effects of lisdexamfetamine dimesylate on cardiovascular parameters in a 4-week clinical trial in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2009;70(12):1652–1661. doi: 10.4088/JCP.09m05335pur. [DOI] [PubMed] [Google Scholar]
- 56.Adler LA, Goodman D, Weisler R, et al. Effect of lisdexamfetamine dimesylate on sleep in adults with attention-deficit/hyperactivity disorder. Behav Brain Func. 2009;5:34. doi: 10.1186/1744-9081-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brams M. Efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder in the adult workplace environment. Presented at the annual meeting of the New Clinical Drug Evaluation Unit; June 29–July 2, 2009; Holly-wood, Fla. [Google Scholar]
- 58.Weisler R, Young J, Mattingly G, et al. Long-term safety and efficacy of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. Poster 206, presented at the U.S. Psychiatric and Mental Health Congress; October 30–November 2, 2008; San Diego. [Google Scholar]
- 59.Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 60.Mansbach RS, Moore RA., Jr Formulation considerations for the development of medications with abuse potential. Drug Alcohol Depend. 2006;83S:S15–S22. doi: 10.1016/j.drugalcdep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Jasinski DR, Krishnan S. Human pharmacology of intravenous lisdexamfetamine dimesylate: Abuse liability in adult stimulant abusers. J Psychopharmacol. 2009;23:410–418. doi: 10.1177/0269881108093841. [DOI] [PubMed] [Google Scholar]
- 62.Jasinski D, Krishnan S. Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse. J Psychopharmacol. 2009;23:419–427. doi: 10.1177/0269881109103113. [DOI] [PubMed] [Google Scholar]
- 63.Ermer J, Dennis K, Haffey M, et al. Pharmacokinetics of intranasal versus oral administration of lisdexamfetamine dimesylate in healthy adults. Poster presented at the American Psychiatric Association annual meeting; May 16–21, 2009; San Francisco. [Google Scholar]
- 64.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437–443. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christensen LL, Sasané R, Hodgkins P, Harley CR. Treatment burden and costs of lisdexamfetamine dimesylate (LDX) users compared with users of other long-acting treatments in patients with attention-deficit hyperactivity disorder. Poster presented at the 14th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR); May 16–20, 2009; Orlando. [Google Scholar]