Abstract
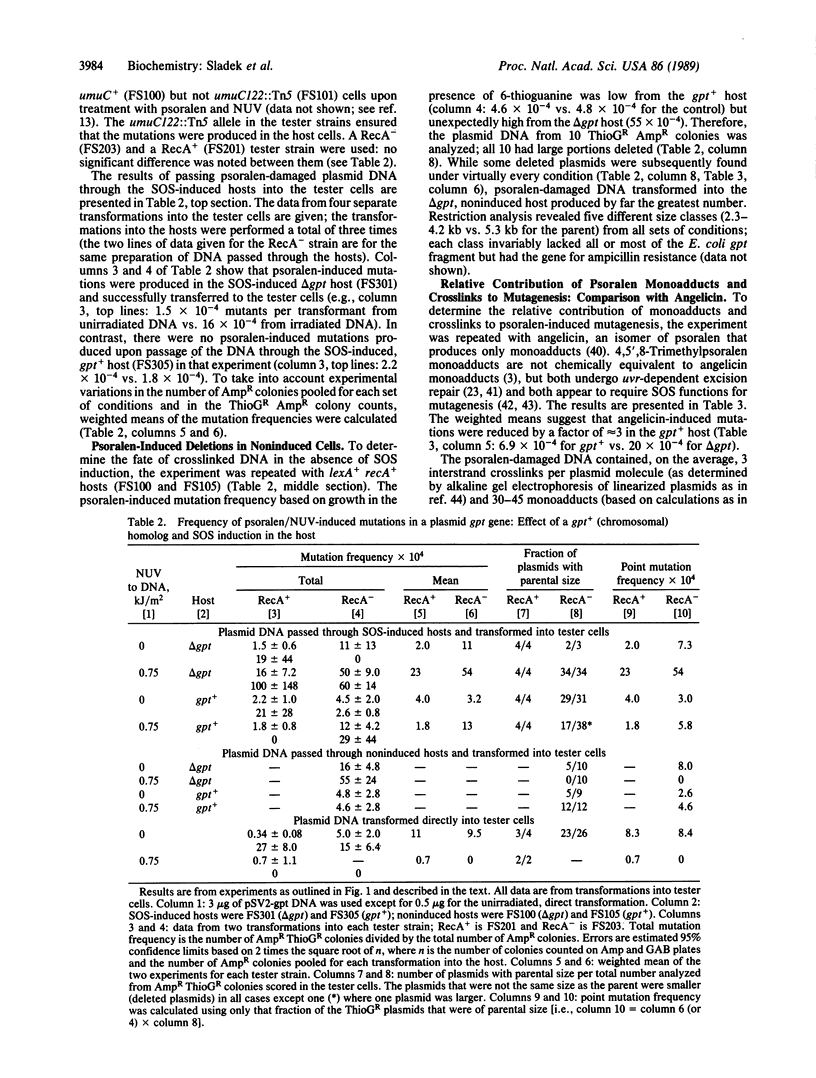
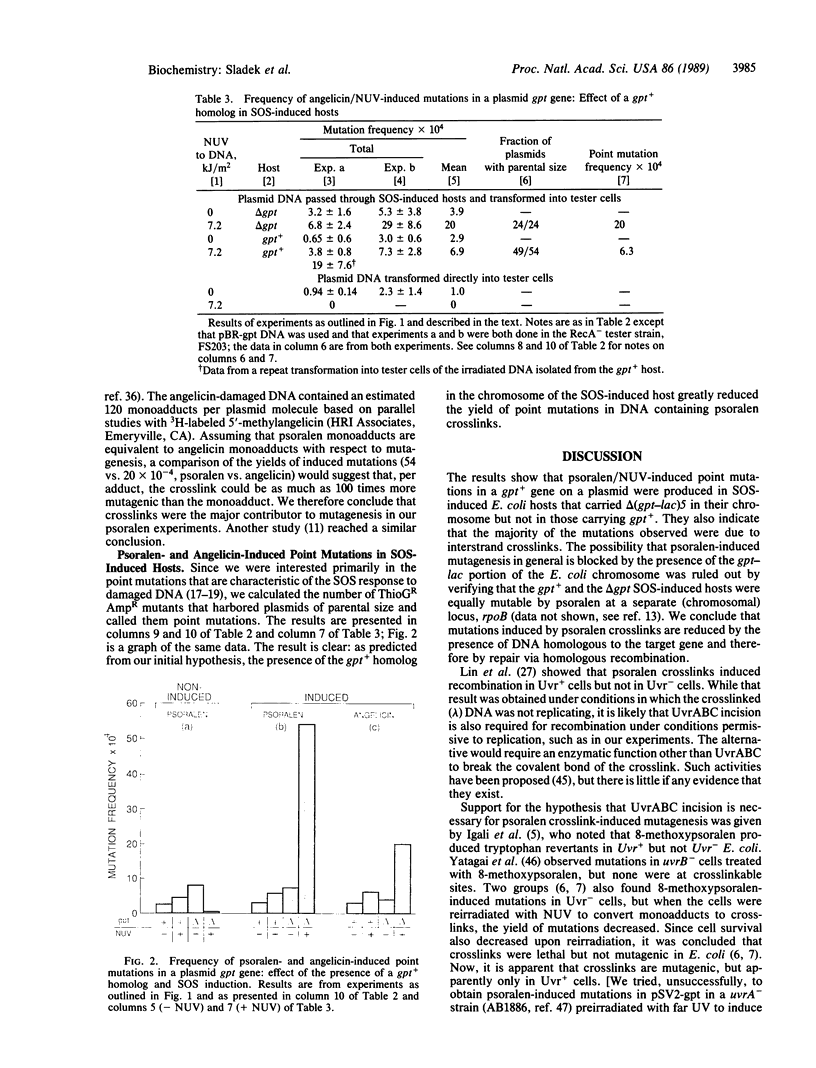
4,5',8-Trimethylpsoralen (psoralen) plus near UV light produces interstrand crosslinks and monoadducts in DNA, both of which are mutagenic. In Escherichia coli, crosslinks are incised by UvrABC excinuclease, an event that can lead to homologous recombination and repair. To determine whether UvrABC incision of crosslinks is a step in the path to mutagenesis as well as repair, the effect of DNA homologous to a target gene on a plasmid was determined. pSV2-gpt DNA was treated with psoralen and transformed into a pair of hosts: one was gpt+, the other was delta (gpt-lac)5. The DNA was extracted and transformed into a tester strain [delta (gpt-lac)5] in which Gpt- mutations in the plasmid were scored. The results show that psoralen-induced mutations were reduced to background levels by the presence of the gpt+ homolog in the host chromosome. delta gpt hosts that were constitutively induced for the SOS response yielded point mutations, whereas noninduced hosts yielded almost exclusively large deletions. Since crosslinks were estimated to be responsible for most of the mutations observed, we conclude that the premutagenic lesion of psoralen crosslinks is recombinagenic and therefore very likely to be the product of UvrABC incision.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwood-Smith M. J., Grant E. Conversion of psoralen DNA monoadducts in E. coli to interstrand DNA cross links by near UV light (320-360 nm): inability of angelicin to form cross links, in vivo. Experientia. 1977 Mar 15;33(3):384–386. doi: 10.1007/BF02002841. [DOI] [PubMed] [Google Scholar]
- Ashwood-Smith M. J., Poulton G. A., Barker M., Mildenberger M. 5-Methoxypsoralen, an ingredient in several suntan preparations, has lethal, mutagenic and clastogenic properties. Nature. 1980 Jun 5;285(5764):407–409. doi: 10.1038/285407a0. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P., Knowles A. Mutation induction and killing of Escherichia coli by DNA adducts and crosslinks: a photobiological study with 8-methoxypsoralen. Chem Biol Interact. 1979 Oct;27(2-3):221–233. doi: 10.1016/0009-2797(79)90127-3. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., von Wright A. Influence of mutations at the rep gene on survival of Escherichia coli following ultraviolet light irradiation or 8-methoxypsoralen photosensitization: evidence for a recA+ rep+-dependent pathway for repair of DNA crosslinks. Mutat Res. 1981 Jul;82(2):229–238. doi: 10.1016/0027-5107(81)90152-4. [DOI] [PubMed] [Google Scholar]
- Cassuto E., Gross N., Bardwell E., Howard-Flanders P. Genetic effects of photoadducts and photocross-links in the DNA of phage lambda exposed to 360 nm light and tri-methylpsoralen or khellin. Biochim Biophys Acta. 1977 Apr 19;475(4):589–600. doi: 10.1016/0005-2787(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Alkaline gel electrophoresis of deoxyribonucleic acid photoreacted with trimethylpsoralen: rapid and sensitive detection of interstrand cross-links. Biochemistry. 1981 Mar 17;20(6):1431–1437. doi: 10.1021/bi00509a005. [DOI] [PubMed] [Google Scholar]
- Cheng S., Van Houten B., Gamper H. B., Sancar A., Hearst J. E. Use of psoralen-modified oligonucleotides to trap three-stranded RecA-DNA complexes and repair of these cross-linked complexes by ABC excinuclease. J Biol Chem. 1988 Oct 15;263(29):15110–15117. [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W., McGuire J. Properties of r mutants of bacteriophage T4 photodynamically induced in the presence of thiopyronin and psoralen. J Virol. 1967 Apr;1(2):260–267. doi: 10.1128/jvi.1.2.260-267.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E., Cairns J. Random components in mutagenesis. Nature. 1982 Sep 23;299(5881):365–367. doi: 10.1038/299365a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K. Persistence of pyrimidine dimers during post-replication repair in ultraviolet light-irradiated Escherichia coli K12. J Mol Biol. 1974 Jul 25;87(1):103–119. doi: 10.1016/0022-2836(74)90563-4. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Boyer H. Susceptibility of the phiX-like phages G4 and G14 to R-EcoRi endonuclease. Virology. 1974 Nov;62(1):270–275. doi: 10.1016/0042-6822(74)90321-3. [DOI] [PubMed] [Google Scholar]
- Grossweiner L. I., Smith K. C. Sensitivity of DNA repair-deficient strains of Escherichia coli K-12 to various furocoumarins and near-ultraviolet radiation. Photochem Photobiol. 1981 Mar;33(3):317–323. doi: 10.1111/j.1751-1097.1981.tb05424.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L., Stedeford J. B. Some properties of excision-defective recombination-deficient mutants of Escherichia coli K-12. J Bacteriol. 1969 Mar;97(3):1134–1141. doi: 10.1128/jb.97.3.1134-1141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igali S., Bridges B. A., Ashwood-Smith M. J., Scott B. R. Mutagenesis in Escherichia coli. IV. Photosensitization to near ultraviolet light by 8-methoxypsoralen. Mutat Res. 1970 Jan;9(1):21–30. doi: 10.1016/0027-5107(70)90067-9. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran P., Ashman C. R., Roberts S., Langenberg J. Nucleotide sequence and analysis of deletion mutants of the Escherichia coli gpt gene in plasmid pSV2 gpt. Gene. 1984 Nov;31(1-3):309–313. doi: 10.1016/0378-1119(84)90228-2. [DOI] [PubMed] [Google Scholar]
- Lin P. F., Bardwell E., Howard-Flanders P. Initiation of genetic exchanges in lambda phage--prophage crosses. Proc Natl Acad Sci U S A. 1977 Jan;74(1):291–295. doi: 10.1073/pnas.74.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H. Carcinogens induce targeted mutations in Escherichia coli. Cell. 1982 Nov;31(1):5–7. doi: 10.1016/0092-8674(82)90398-1. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Low K. B. Specificity of mutagenesis resulting from the induction of the SOS system in the absence of mutagenic treatment. Cell. 1984 Jun;37(2):675–682. doi: 10.1016/0092-8674(84)90400-8. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Mutational specificity in bacteria. Annu Rev Genet. 1983;17:215–238. doi: 10.1146/annurev.ge.17.120183.001243. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Eisenstadt E. Suppressible base substitution mutations induced by angelicin (isopsoralen) in the Escherichia coli lacI gene: implications for the mechanism of SOS mutagenesis. J Bacteriol. 1987 Jun;169(6):2724–2729. doi: 10.1128/jb.169.6.2724-2729.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Nüesch J., Schümperli D. Structural and functional organization of the gpt gene region of Escherichia coli. Gene. 1984 Dec;32(1-2):243–249. doi: 10.1016/0378-1119(84)90052-0. [DOI] [PubMed] [Google Scholar]
- Paramio J. M., Bauluz C., de Vidania R. Lethal and mutagenic effects of 8-methoxypsoralen-induced lesions on plasmid DNA. Mutat Res. 1987 Jan;176(1):21–28. doi: 10.1016/0027-5107(87)90248-x. [DOI] [PubMed] [Google Scholar]
- Peterson K. R., Ossanna N., Thliveris A. T., Ennis D. G., Mount D. W. Derepression of specific genes promotes DNA repair and mutagenesis in Escherichia coli. J Bacteriol. 1988 Jan;170(1):1–4. doi: 10.1128/jb.170.1.1-4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Decuyper-Debergh D., Gamper H. Mutagenesis of the lac promoter region in M13 mp10 phage DNA by 4'-hydroxymethyl-4,5',8-trimethylpsoralen. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7355–7359. doi: 10.1073/pnas.82.21.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Saffran W. A., Cantor C. R. Mutagenic SOS repair of site-specific psoralen damage in plasmid pBR322. J Mol Biol. 1984 Sep 25;178(3):595–609. doi: 10.1016/0022-2836(84)90240-7. [DOI] [PubMed] [Google Scholar]
- Saffran W. A., Cantor C. R. The complete pattern of mutagenesis arising from the repair of site-specific psoralen crosslinks: analysis by oligonucleotide hybridization. Nucleic Acids Res. 1984 Dec 21;12(24):9237–9248. doi: 10.1093/nar/12.24.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Franklin K. A., Sancar G., Tang M. S. Repair of psoralen and acetylaminofluorene DNA adducts by ABC excinuclease. J Mol Biol. 1985 Aug 20;184(4):725–734. doi: 10.1016/0022-2836(85)90316-x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Scott B. R., Pathak M. A., Mohn G. R. Molecular and genetic basis of furocoumarin reactions. Mutat Res. 1976;39(1):29–74. doi: 10.1016/0165-1110(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G. Misrepair of overlapping daughter strand gaps as a possible mechanism for UV induced mutagenesis in UVR strains of Escherichia coli: a general model for induced mutagenesis by misrepair (SOS repair) of closely spaced DNA lesions. Mutat Res. 1976 Dec;41(2-3):185–200. doi: 10.1016/0027-5107(76)90091-9. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Strand cleavage at psoralen adducts and pyrimidine dimers in DNA caused by interaction between semi-purified uvr+ gene products from Escherichia coli. Mutat Res. 1981 Jun;82(1):11–22. doi: 10.1016/0027-5107(81)90133-0. [DOI] [PubMed] [Google Scholar]
- Seki T., Nozu K., Kondo S. Differential causes of mutation and killing in Escherichia coli after psoralen plus light treatment: monoadducts and cross-links. Photochem Photobiol. 1978 Jan;27(1):19–24. doi: 10.1111/j.1751-1097.1978.tb07559.x. [DOI] [PubMed] [Google Scholar]
- Sladek F. M., Munn M. M., Rupp W. D., Howard-Flanders P. In vitro repair of psoralen-DNA cross-links by RecA, UvrABC, and the 5'-exonuclease of DNA polymerase I. J Biol Chem. 1989 Apr 25;264(12):6755–6765. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatagai F., Horsfall M. J., Glickman B. W. Defect in excision repair alters the mutational specificity of PUVA treatment in the lacI gene of Escherichia coli. J Mol Biol. 1987 Apr 20;194(4):601–607. doi: 10.1016/0022-2836(87)90237-3. [DOI] [PubMed] [Google Scholar]
- Zhen W. P., Jeppesen C., Nielsen P. E. Repair in Escherichia coli of a psoralen-DNA interstrand crosslink site specifically introduced into T410A411 of the plasmid pUC 19. Photochem Photobiol. 1986 Jul;44(1):47–51. doi: 10.1111/j.1751-1097.1986.tb03562.x. [DOI] [PubMed] [Google Scholar]




