Abstract
Age-associated mitochondrial dysfunction is a major source of reactive oxygen species (ROS) and oxidative modification to proteins. Mitochondrial electron transport chain (ETC) complexes I and III are the sites of ROS production and we hypothesize that proteins of the ETC complexes are primary targets of ROS-mediated modification which impairs their structure and function. The pectoralis, primarily an aerobic red muscle, and quadriceps, primarily an anaerobic white muscle, have different rates of respiration and oxygen-carrying capacity, and hence, different rates of ROS production. This raises the question of whether these muscles exhibit different levels of oxidative protein modification. Our studies reveal that the pectoralis shows a dramatic age-related decline in almost all complex activities that correlates with increased oxidative modification. Similar complex proteins were modified in the quadriceps, at a significantly lower level with less change in enzyme and ETC coupling function. We postulate that mitochondrial ROS causes damage to specific ETC subunits which increases with age and leads to further mitochondrial dysfunction. We conclude that physiological characteristics of the pectoralis vs quadriceps may play a role in age-associated rate of mitochondrial dysfunction and in the decline in tissue function.
Keywords: Oxidative stress, Skeletal muscle, Pectoralis, Quadriceps, Mitochondrial dysfunction, Aging, Carbonylation, 4-Hydroxynonenal, Tyrosine nitration
Introduction
Sarcopenia, or muscle weakness, is a major physiological sign of aging in humans [1-4]. The mitochondrial theory of aging proposes that increasing oxidative stress resulting from progressive mitochondrial dysfunction is a mechanism of mammalian aging and may be an important physiological characteristic of muscle aging [5,6]. It is well established that although ROS are generated by multiple compartments, e.g., the plasma membrane NADPH oxidases [7], peroxisomal lipid metabolism, and cytosolic enzymes such as cyclogenases, the majority (~90%) of ROS is generated by mitochondrial dysfunction [8]. The generation of mitochondrial ROS, in vivo, is linked to the processes of oxidative phosphorylation carried out by the mitochondrial electron transport chain complexes. Thus, leakage of electrons, i.e., mitochondrial ROS, is produced in vivo, from ETC complexes during normal respiration, particularly from Complex I (CI) and Complex III (CIII) [9-11]. Increased ROS production and oxidative stress during the aging process are, therefore, attributed to mitochondrial dysfunction, and the age-associated decline in tissue function is proposed to be a consequence of dysfunctional mitochondria [12-14].
Mitochondrially generated ROS are difficult to measure directly both in vitro and in vivo. Although the production of ROS in CI and CIII has been estimated by electron paramagnetic resonance [15-17], the short half-life of these radicals makes their accurate measurement difficult. Since the products of ROS reaction with proteins are stable and easily measured we have proposed that the amount of modified protein may be indicative of the level of oxidative modification in tissues [13]. Furthermore, since oxidatively modified proteins have been shown to impair the functions of these proteins [18-21] we have initiated these studies to characterize the role of these modifications on the development of mitochondrial dysfunction in aged pectoralis (aerobic) and quadriceps (anaerobic) skeletal muscle.
The covalent oxidative protein modifications caused by reactions with free radicals are more stable and easily detectable, and thus have been used as molecular markers of oxidative stress [17,22-24]. The relative abundance of modified proteins has been used in our laboratory to indicate the level of oxidatively modified proteins that accumulate in aged tissues [22-24]. Protein modifications caused by ROS include the formation of lipid peroxidation adducts (4-hydroxynonenal or HNE and malondialdehyde or MDA) on lysine, histidine, and cysteine, nitration of tyrosine and cysteine, and carbonylation of lysine, arginine, proline, and threonine [18,19,25,26]. Oxidatively modified proteins have been detected by immunoblotting using antibodies specific for these modifications and subsequently identified by mass spectrometry [17,22-24]. In addition, such oxidative modifications to proteins can result in reduction of function associated with aging and age-associated diseases [18,19,21-27]. These oxidative modifications are, therefore, molecular markers that provide insight into the cumulative effects of oxidative stress on the molecular mechanisms of aging and development of age-associated diseases.
In this study we analyzed mitochondria isolated from young, middle-aged, and old mouse pectoralis and quadriceps skeletalmuscles because of their aerobic vs anaerobic physiological characteristics, to determine whether aging affects the activities of their ETC complexes CI–CV. We chose, specifically, the pectoralis which is an adductor, internal rotator, and flexor of the shoulder; its fibers, which are slow twitch (type I), consist of high myoglobin levels which improves the delivery of oxygen, and high mitochondrial content, are primarily red muscle and highly aerobic. Secondly, we chose the quadriceps which consists of fast-twitch type I fibers whose physiological characteristics include fewer mitochondria and less myoglobin. The large store of glycogen and high levels of glycolytic enzymes enable these fibers to respire anaerobically. We tested, therefore, whether specific ETC CI–CV proteins of the highly aerobic pectoralis and primarily anaerobic quadriceps muscles are differentially susceptible to oxidative modification due to their location within the mitochondria, whether the levels of these modifications increase, and whether their functions are altered with aging. To achieve this we identified the oxidatively modified subunits of ETC CI–CV and correlated the levels of protein modification with changes in enzyme activities. Blue-native polyacrylamide gel electrophoresis (BN-PAGE) was used to resolve intact ETC complexes [28] followed by second-dimension denaturing SDS-PAGE to resolve individual complex subunits. Protein abundance of each complex was measured using antibodies to specific complex subunits to quantify age-related changes [22,23]. Using immunobloting with antibodies recognizing specific types of oxidative damage, we detected proteins that were carbonylated, and modified by HNE, MDA, and tyrosine nitration. Proteins shown to be differentially modified were identified by MALDI-TOF-TOF mass spectrometry. These studies identify whether there is a differential susceptibility of highly aerobic skeletal muscle ETC complexes compared to the anaerobic skeletal muscle. Finally, we determined whether there is a direct correlation between increased protein modification on enzyme function associated with the ETC complexes that might suggest a progressive increase in endogenous oxidative stress during aging. Our study systematically identifies the effects of oxidative protein modification on ETC activity, on possible specificity of targeted oxidative modification and whether these modifications play a role in causing symptoms of the aging, of the pectoralis and quadriceps.
Materials and methods
Animals
Young (3–5 months), middle–aged (12–14 months), and old (20–22 months) male C57BL/6 mice, purchased from the National Institute on Aging (Bethesda, MD), were maintained in a pathogen-free facility at 27°C, 45–55% humidity, on a 14/10 light/dark cycle and fed ad libitum on an NIH low-fat diet (Purina) 4% fat by weight.
Mitochondrial isolation
Mice were sacrificed by cervical dislocation and their skeletal muscles were harvested immediately, rinsed in ice-cold PBS, and prepared for subcellular fractionations. Mitochondria were prepared from the pooled muscles of 9 young, 10 middle-aged, and 8 old C57BL/6 male mice. Mitochondrial isolation was carried out at 4°C as described with minor modifications [22,24,29].
Enzyme activities
Enzyme activities were measured at room temperature using a Beckman Coulter DU 530 spectrophotometer (Beckman Coulter, CA). Citrate synthase activity was measured as described [22,30]. Rotenone-sensitive Complex I (CI) activity, malonate-sensitive Complex II (CII) activity, antimycin A-sensitive Complex III (CIII) activity, KCN-sensitive Complex IV (CIV) activity, and oligomycin-sensitive Complex V (CV) activities were assayed as described [22,27,31]. CI–III and CII–III coupled assays were performed as described [25,32-34].
Coenzyme Q levels
Total mitochondrial coenzyme Q was quantified using an HPLC method described previously [22,33,34].
Polyacrylamide gel electrophoresis
BN-PAGE and SDS-PAGE were carried out by established methods [26] with minor modifications as described previously [17,22,28].
Immunobloting
MALDI-TOF-TOF: Sample preparation
Individual ROS-modified protein bands were excised from second-dimension SDS-PAGE run simultaneously with the gels that were immunoblotted and analyzed by the Proteomics Core Facility at UTMB. Gel samples were cut into ~1-mm-size pieces and placed into separate 0.5-ml polypropylene tubes. Ammonium bicarbonate buffer (50mM; 100μl) was added to each tube and the samples were then incubated at 37°C for 30min. After incubation, the buffer was removed, 100μl of water was added to each tube, and the samples were incubated again at 37°C for 30min. After incubation, the water was removed and 100μl of acetonitrile was added to each tube to dehydrate the gel pieces. The samples were vortexed, and after 5min the acetonitrile was removed. Acetonitrile (100μl) was again added to each of the sample tubes and vortexed, and acetonitrile removed after 5min. The samples were then placed in a speedvac for 45min to remove excess solvent.
Lyophilized trypsin (20μg; Promega Corp.) was added to 2ml of 25mM ammonium bicarbonate, pH 8.0. The trypsin solution was then vortexed and added to each sample tube in an amount (~10μl) to just cover the dried gel. The samples were then incubated at 37°C for 6h.
After digestion, 1μl of the sample solution was spotted directly onto a MALDI target plate and allowed to dry. Alpha-cyano-4-hydroxycinnamic acid (1μl; Aldrich Chemical Co.) matrix solution (50/50 acetonitrile/water at 5mg/ml) was then applied on the sample spot and allowed to dry. The dried MALDI spot was blown with compressed air (Decon Laboratories, Inc.) before inserting into the mass spectrometer.
Mass spectrometry
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI TOF-MS) was used for protein identification. Datawere acquired with an Applied Biosystems 4800 MALDI TOF/TOF Proteomics analyzer. Applied Biosystems software package included 4000 Series Explorer (v. 3.6 RC1) with Oracle Database Schema Version (v. 3.19.0), Data Version (3.80.0) to acquire both MS and MS/MS spectral data. The instrument was operated in positive-ion reflectron mode, mass range was 850–3000Da, and the focus mass was set at 1700Da. For MS data, 1000–2000 laser shots were acquired and averaged from each sample spot. Automatic external calibration was performed using a peptide mixture with reference masses 904.468, 1296.685, 1570.677, and 2465.199.
Following MALDI MS analysis, MALDI MS/MS was performed on several (5–10) abundant ions fromeach sample spot. A1-kVpositive-ion MS/MS method was used to acquire data under postsource decay (PSD) conditions. The instrument precursor selectionwindowwas +/– 3Da. For MS/MS data, 2000 laser shots were acquired and averaged from each sample spot. Automatic external calibration was performed using reference fragment masses 175.120, 480.257, 684.347, 1056.475, and 1441.635 (from precursor mass 1570.700).
Applied Biosystems GPS Explorer (v. 3.6) software was used in conjunction with MASCOT to search the respective protein database using both MS and MS/MS spectral data for protein identification. Protein match probabilities were determined using expectation values and/or MASCOT protein scores. MS peak filtering included the following parameters: mass range 800 to 4000Da, minimum S/N filter = 10, mass exclusion list tolerance = 0.5Da, and mass exclusion list (for some trypsin and keratin-containing compounds) included masses 842.51, 870.45, 1045.56, 1179.60, 1277.71, 1475.79, and 2211.1. For MS/MS peak filtering, the minimum S/N filter = 10.
For protein identification, the Mus musculus taxonomy was searched in either the NCBI and/or SwissProtein database. Other parameters included the following: selecting the enzyme as trypsin; maximum missed cleavages = 1; fixed modifications included carbamidomethyl (C) for 2-D gel analyses only; variable modifications included oxidation (M); precursor tolerance was set at 0.2Da; MS/MS fragment tolerance was set at 0.3Da; mass = monoisotopic; and peptide charges were only considered as +1. The significance of a protein match, based on both the peptide mass fingerprint (PMF) in the first MS and the MS/MS data from several precursor ions, is based on expectation values; each protein match is accompanied by an expectation value. The expectation value is the number of matches with equal or better scores that are expected to occur by chance alone. The default significance threshold is P < 0.05, so an expectation value of 0.05 is considered to be on this threshold. We used a more stringent threshold of 10-3 for protein identification; the lower the expectation value, the more significant the score.
Results
Inhibitor-sensitive enzyme activities from pectoralis and quadriceps
To evaluate the effects of aging on mitochondrial ETC complexes derived from the physiologically unique pectoralis (red-aerobic) and quadriceps (white-anaerobic) we compared the enzyme activities of all five complexes as well as the coupled activities of CI–III and CII–III for all three ages (Figs. 1 and 2).
Fig. 1.
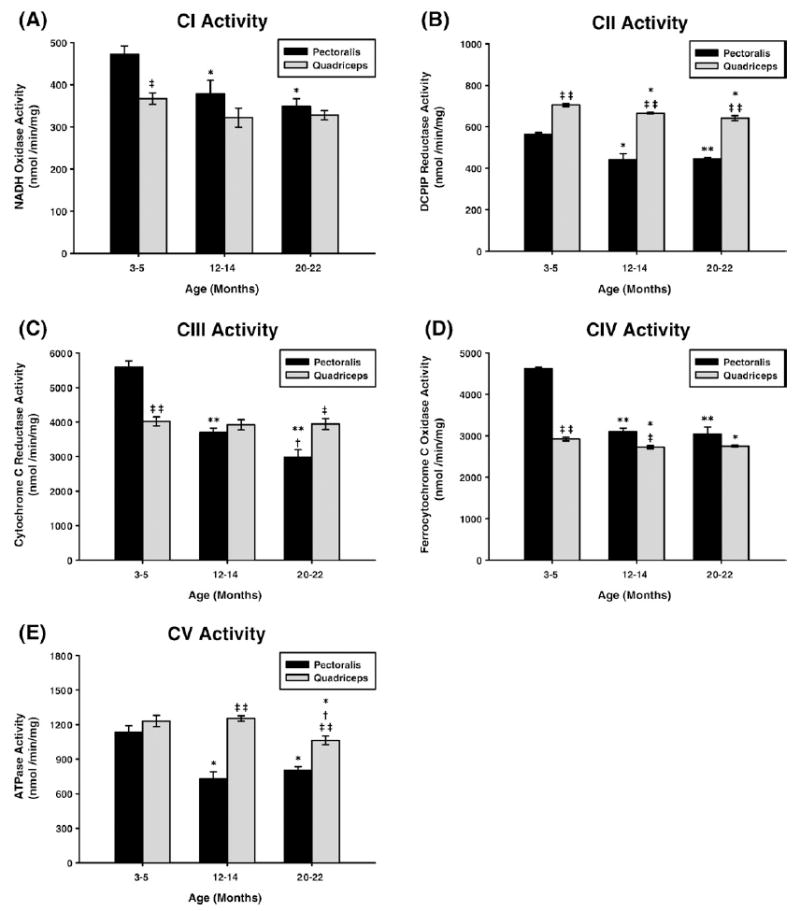
Measurement of ETC complex activities from young (3–5 months), middle-aged (12–14 months), and old (20–22 months) mouse skeletal muscle mitochondria. Individual complex enzyme activities were measured spectrophotometrically as described under Materials and methods. All activity results are averages of 4 assays from the pooled sample±SE for each age group. Citrate synthase assay results were used to normalize mitochondrial proteins. Activities for young (3–5 months), middle-aged (12–14 months), and old (20–22 months) pectoralis and quadriceps ETC CI–CV are plotted as follows: (A) CI activity with aging. Coefficients of variance for pectoralis and quadriceps were 4.2 and 3.8% (young), 6.8 and 6.2% (middle age), and 3.8 and 2.9% (old), respectively. (B) CII activity with aging. Coefficients of variance for pectoralis and quadriceps were 1.3 and 1.1% (young), 5.3 and 0.6% (middle age), and 1 and 2.9% (old), respectively. (C) CIII activity with aging. Coefficients of variance for pectoralis and quadriceps were 3.2 and 3.1% (young), 2.2 and 3.7% (middle age), and 4 and 3.9% (old), respectively. (D) CIV activity with aging. Coefficients of variance for pectoralis and quadriceps were 0.8 and 1.5% (young), 1.9 and 1.2% (middle age), and 3.6 and 0.8% (old), respectively. (E) CV activity with aging. Coefficients of variance for pectoralis and quadriceps were 5.2 and 4% (young), 5.5 and 1.8% (middle age), and 2.9 and 3.1% (old), respectively. * P<0.05 compared to young, ** P<0.001 compared to young, † P<0.05 compared to middle aged, ††P<0.001 compared to middle aged, ‡ P<0.05 compared to pectoralis, and ‡‡ P<0.001 compared to pectoralis.
Fig. 2.
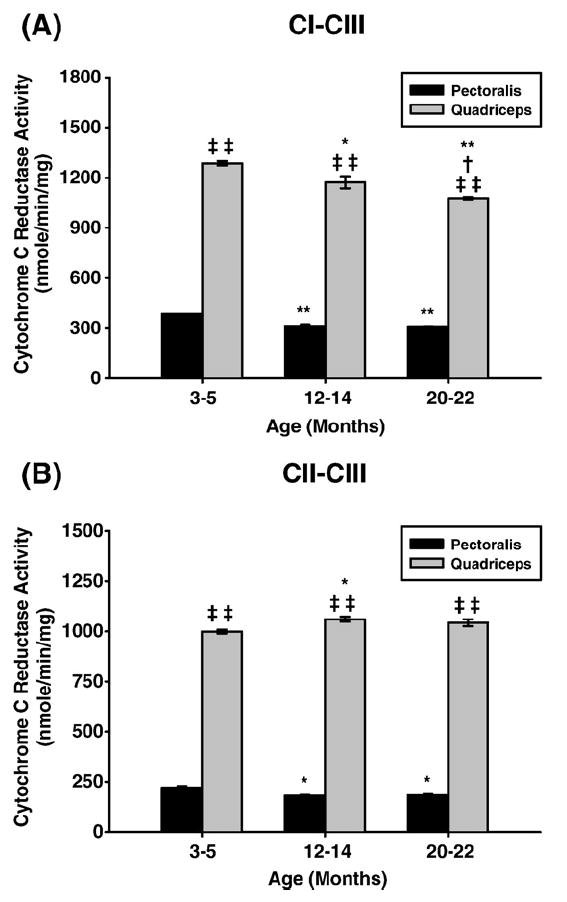
Measurement of coupled mitochondrial ETC complex activities from young (3–5 months), middle aged (12–14 months), and old (20–22 months) mouse skeletal muscle mitochondria. CI–III and CII–III coupled enzyme activities were measured spectrophotometrically as described under Materials and methods. All activity results are averages of 4 assays from the pooled sample±SE for each age group. Citrate synthase assay results were used to normalize mitochondrial proteins. Activities for young (3–5 months), middle-aged (12–14 months), and old (20–22 months) pectoralis and quadriceps ETC CI–III and CII–III are plotted as follows: (A) CI–CIII coupled activity with aging. Coefficients of variance for pectoralis and quadriceps were 0.5 and 1% (young), 2.7 and 2.8% (middle age), and 1 and 0.5% (old), respectively. (B) CII–CIII coupled activity with aging. Coefficients of variance for pectoralis and quadriceps were 4.7 and 0.9% (young), 1.9 and 1.3% (middle age), and 2.8 and 1.7% (old), respectively. *P<0.05 compared to young, **P<0.001 compared to young, †P<0.05 compared to middle aged, ‡P<0.05 compared to pectoralis, and ‡‡P<0.001 compared to pectoralis.
Pectoralis enzyme activities
The data, in all cases, indicate that the enzyme activity in pectoralis decreased from young to middle age and in some cases (CI, CIII) further decline was noted from middle-age to aged mice. Rotenone-sensitive CI activity decreased by 20% from young to middle age and only slightly decreased from middle aged to old (Fig. 1A). Malonate-sensitive CII activity also declined by ~22% in middle age but no further decline was noted in old age (Fig. 1B). Antimycin A-sensitive CIII activity showed a continuous decline, i.e., ~34% decline in middle age and ~47% decline by old age (Fig. 1C). KCN-sensitive CIV activity decreased by ~34% by middle age and no further decline was noted in old animals (Fig. 1D). Oligomycin-sensitive CV activity again showed a pattern similar to CII and CIV, i.e., ~35% decrease in middle age and no further decline in old age (Fig. 1E).
Quadriceps enzyme activities
Very few changes were seen in quadriceps enzyme activity with aging. Compared to pectoralis, CI activity in quadriceps was ~22% lower at young age and though there was a slight decline of this activity with age it was not significant (Fig. 1A). In contrast, there was only ~5% decline in CII activity of quadriceps from young to middle age and very little further decrease by old age (Fig. 1B). However, CII enzyme activity was significantly higher (~20–35%) at all ages in quadriceps compared to pectoralis. Furthermore, there is no change in CIII and CIV activity and CIV activity only decreased slightly (~6%) with aging. However, a comparison of CIII activity of pectoralis vs quadriceps reveals that the pectoralis showed a significantly higher activity at young age (~28%) but by middle age this difference disappeared, and by old age its activity is lower. Although no change was noted in CV activity in middle age compared to young age, there was ~14% decline in this enzyme’s activity by old age (Fig. 1E). Thus, the pectoralis showed more overall dramatic changes (declines) with age compared to quadriceps.
Pectoralis and quadriceps coupled activities
Similar to CI enzyme activity, there was a 20% decrease in CI–III coupled activity in pectoralis from young to middle-age and there was no further decline from middle-aged to old mice (Fig. 2A). The coupled CII–III activity of pectoralis also showed an age-associated decline, i.e., 18% decline in middle age and no further decline in old age (Fig. 2B). On the other hand, CI–CIII coupled activity decreased continuously by ~9% in the quadriceps (Fig. 2A) while the CII–CIII coupled activity showed a slight increase (Fig. 2B). Interestingly, the overall coupled CI–CIII and CII–CIII activities of the quadriceps are significantly higher than those of the pectoralis, suggesting a tighter coupling in the quadriceps at all ages (Fig. 2B). In addition, in the pectoralis all enzyme activities show an age-associated decline from young to middle age and CI and CIII activity declines further in old age while CII, CIV, and CV show very little further decline by old age. This differs significantly from the quadriceps which shows a minimal level of change in almost all enzyme activities with the exception of CV activity which decreases slightly at old age. Furthermore, CI, CIII, and CIV activities of the pectoralis at young age are at least 30% higher compared to quadriceps and level out by middle age as opposed to CII activity being higher in all ages in quadriceps compared to pectoralis.
Coenzyme Q levels in pectoralis and quadriceps mitochondria
Coenzyme Q (CoQ) levels play a basic role in enzyme function and coupling, and CoQ9 is the predominant form in mouse tissues (Table 1) [33,34]. Since enzyme function and coupling activities differ significantly between pectoralis and quadriceps we measured their CoQ9 levels in young, middle-aged, and aged mice, and used these values as a predictor of total CoQ for muscle mitochondria. Our results show that the pectoralis has 3 times more CoQ9 per milligram of mitochondria compared to quadriceps and that aging does not affect the levels of CoQ9 from either muscle (Table 1). Thus, the loss of enzyme activities of CI, CII, CIII, and CI–CIII plus CII–CIII coupled activities in pectoralis is not due to decreased substrate availability. Furthermore, the enhanced coupling seen in quadriceps cannot be attributed to changes in substrate availability, since quadriceps has 3-fold lower levels of CoQ9. These data suggest that the tighter coupling in quadriceps may facilitate its almost constant level of enzyme activities compared to the primarily aerobic pectoralis muscle.
Table 1.
Coenzyme Q9 levels in mouse skeletal muscle mitochondria with aging
| Age | Pectoralis CoQ9 (nmol/mg) | Quadriceps CoQ9 (nmol/mg) |
|---|---|---|
| Young | 65.7 ± 1.9 | 21.4 ± 0.5‡‡ |
| Middle age | 62.9 ± 0.1 | 22.0 ± 0.1‡‡ |
| Old | 60.5 ± 1.3 | 20.1 ± 1.0‡‡ |
Pooled skeletal muscle mitochondrial samples were used for each age group. Coenzyme Q was extracted and quantified as described under Materials and methods. Data are an average of three experiments±SE. Coefficients of variance for pectoralis and quadriceps CoQ9 were 5.0 and 4.4% (young), 0.4 and 0.7% (middle age), and 3.9 and 8.9% (old), respectively.
P<0.001 compared to pectoralis.
Abundance of ETC complexes in young, middle-aged, and old mitochondria
To determine if the changes in enzyme functions are due to changes in enzyme levels, skeletal muscle mitochondria were isolated, solubilized, and subjected to BN-PAGE to resolve intact complexes I–V. We measured the levels of ETC complexes in young, middle-aged, and aged muscle, using immunoblotting with complex-specific antibodies. In previous studies we established the use of immunobloting of BN-PAGE resolved complexes with complex-specific antibody to determine the abundance of CI–CV and whether there are age-related quantitative differences in individual complexes [22,32]. Thus, using immunoblotting of BN-PAGE-resolved complexes (CI–CV) we analyzed duplicate gels to measure complex abundance [14]. Our results show very little age-related changes in complex levels in both skeletal muscle mitochondria (Fig. 3). CI and CIII levels from pectoralis show a 15–25% increase in middle age (Figs. 3A and 3C) which is not consistent with the dramatic decrease in enzyme activities of both individual complexes and their coupled activity. Though there is a decrease in immunoreactive signal in CI of quadriceps by old age, the background-corrected values do not show any differences when compared to young age. Immunoblotting for CI alone (data not shown) similarly demonstrated that there is very little change. However, we note here that since all ETC complexes are multiprotein complexes, our procedure is not sensitive enough to detect subtle changes in other components of these complexes.
Fig. 3.
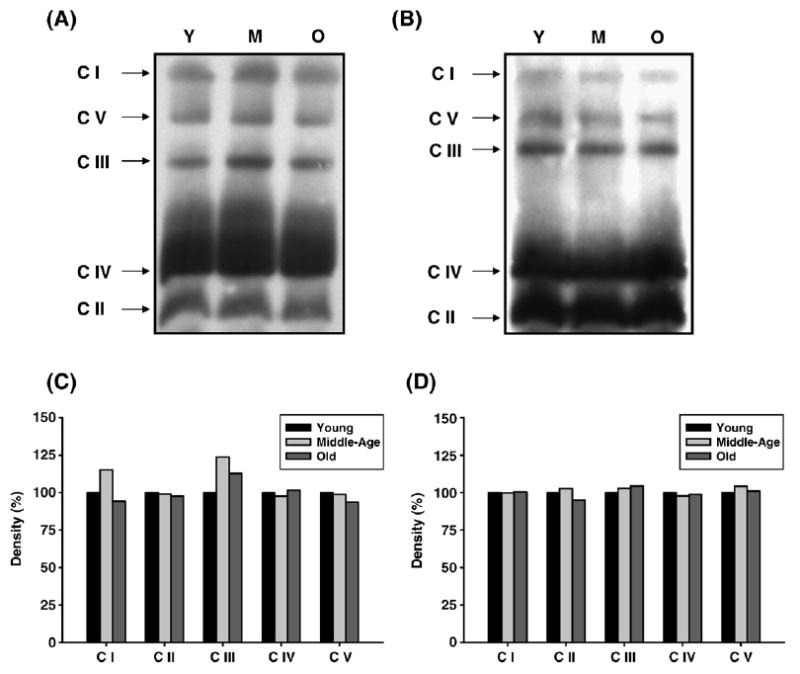
Protein abundance of ETC complexes in young (3–5 months), middle-aged (12–14 months), and old (20–22 months) skeletal muscle mitochondria. Skeletal muscle mitochondria (160 μg) from each age group were solubilized and the ETC complexes were separated on a BN-PAGE as described under Materials and Methods. (A) Immunoblot of pectoralis BN-PAGE using complex-specific antibodies. Lane 1, 2, and 3 represent young, middle-aged, and old pectoralis mitochondrial ETC complexes, respectively. (B) Density values of each pectoralis ETC complex band are plotted as a percentage of young complexes. (C) Immunoblot of quadriceps BN-PAGE using complex-specific antibodies. Lane 1, 2, and 3 represent young, middle-aged, and old quadriceps mitochondrial ETC complexes, respectively. (D) Density values of each quadriceps ETC complex band are plotted as a percentage of young complexes. Y, young mitochondria; M, middle-age mitochondria; and O, old mitochondria.
Oxidative modification of ETC complex subunits with aging
To identify oxidatively modified ETC complex proteins from pectoralis and quadriceps we detected individual carbonylated (Figs. 4A and 5A, respectively), HNE-modified (Figs. 6A and 7A, respectively), nitrotyrosine-modified (Figs. 8A and 9A, respectively), and MDA-modified proteins (Figs. 10A and 11A, respectively) by immunoblotting of second-dimension gels. The corresponding change in percentage density for protein modification is expressed relative to young protein density and is shown in Figs. 4B, 4C, 5B, 6B, 7B, 8B, 9B, 10B, and 11B. Duplicate second-dimension gels were run simultaneously for each immunoblot and used for identification of modified proteins by MALDI-TOF-TOF from pectoralis (Table 2) and from quadriceps (Table 3). All experiments, which were performed twice, showed almost identical results. This along with results from Fig. 3 confirmed that immunoblots used to detect oxidative modifications were valid.
Fig. 4.
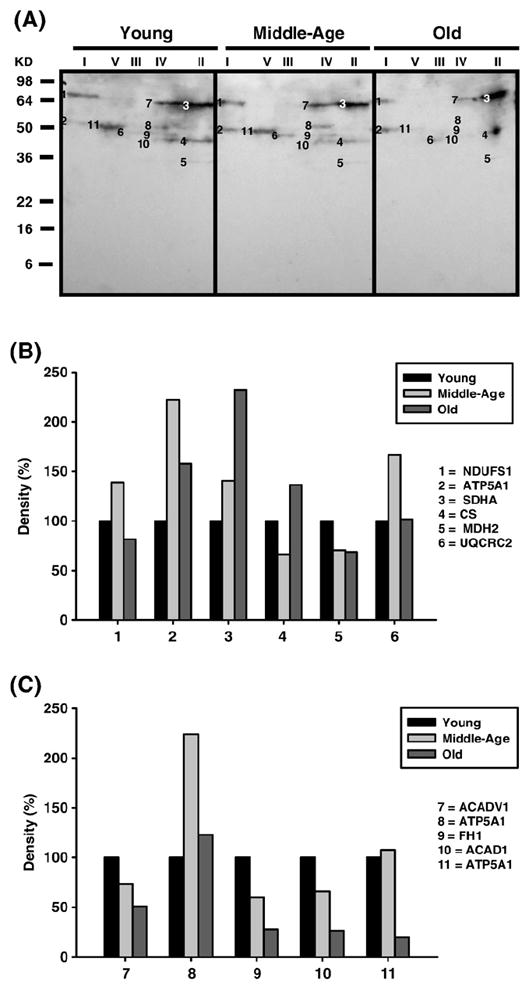
Identification of carbonylated proteins of young (3–5 months), middle-aged (12–14 months), and old (20–22 months) pectoralis mitochondrial ETC complex subunits. Pectoralis mitochondrial ETC complexes were resolved into individual subunits and DNP-derivatized after transfer to PVDF membrane as described under Materials and methods followed by immunoblotting. (A) Immunoblot of young, middle-aged, and old pectoralis mitochondrial ETC complex subunits using anti-DNP antibody. Modified proteins were numbered according to their complex localization followed by the highest to the lowest molecular weight of the proteins. Protein loading was normalized using complex-specific antibodies as described under Materials and methods. Normalized density values of each individual carbonylated protein are plotted as a percentage of the young pectoralis protein density for all five ETC complex subunits. (B) Densitometry for modified proteins found in CI (1 and 2), CII (3–5), and CIII (6). (C) Densitometry for modified proteins found in CIV (7–10) and CV (11). Identification of each numbered band is summarized in Table 2.
Fig. 5.
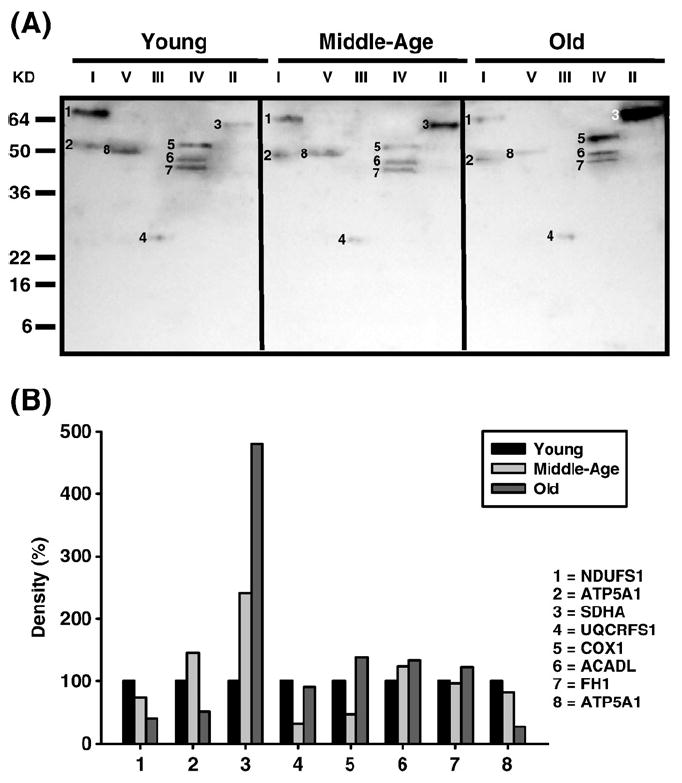
Identification of carbonylated proteins of young (3–5 months), middle-aged (12–14 months), and old (20–22 months) old quadriceps mitochondrial ETC complex subunits. Quadriceps mitochondrial ETC complexes were resolved into individual subunits and DNP-derivatized after transfer to PVDF membrane as described under Materials and methods followed by immunoblotting. (A) Immunoblot of young, middle-aged, and old quadriceps mitochondrial ETC complex subunits using anti-DNP antibody. Modified proteins were numbered according to their complex localization followed by the highest to the lowest molecular weight of the proteins. Protein loading was normalized using complex-specific antibodies as described under Materials and methods. Normalized density values of each individual carbonylated protein are plotted as a percentage of the young quadriceps protein density for all five ETC complex subunits. (B) Densitometry for modified proteins found in CI (1 and 2), CII (3), CIII (4), CIV (5–7), and CV (8). Identification of each numbered band is summarized in Table 3.
Fig. 6.
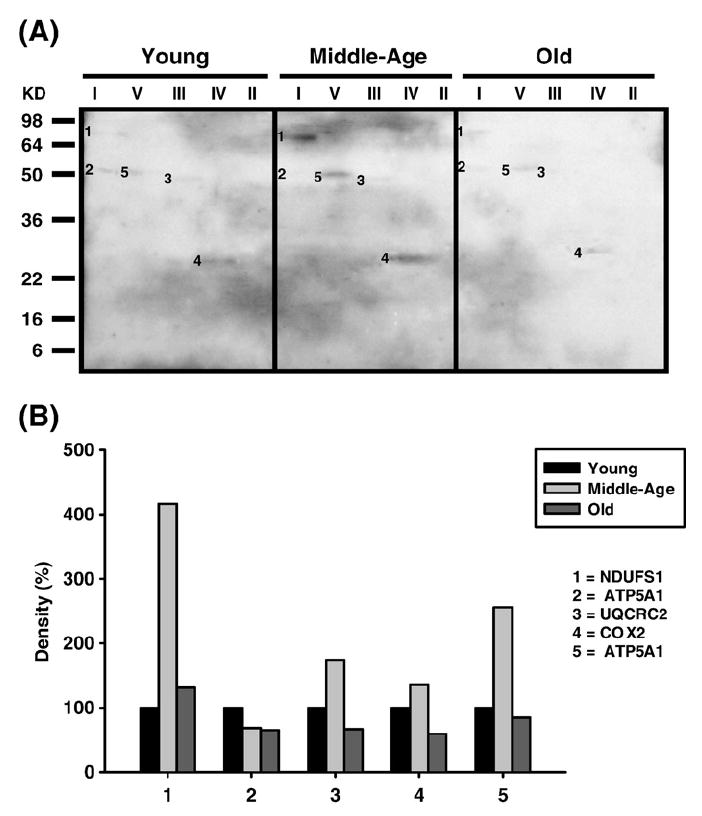
Identification of HNE-modified proteins of young (3–5 months), middle-aged (12–14 months), and old (20–22 months) pectoralis mitochondrial ETC complex subunits. Pectoralis mitochondrial ETC complexes were resolved into individual subunits as described under Materials and methods followed by immunoblotting. (A) Immunoblot of young, middle-aged, and old pectoralis mitochondrial ETC complex subunits using anti-HNE antibody. Modified proteins were numbered according to their complex localization followed by the highest to the lowest molecular weight of the proteins. Protein loading was normalized using complex-specific antibodies as described under Materials and methods. Normalized density values of each individual protein modified by HNE are plotted as a percentage of the young pectoralis protein density for each specific complex subunit. (B) Densitometry for modified proteins found in CI (1 and 2), CIII (3), CIV (4), and CV (5). Identification of each numbered band is summarized in Table 2.
Fig. 7.
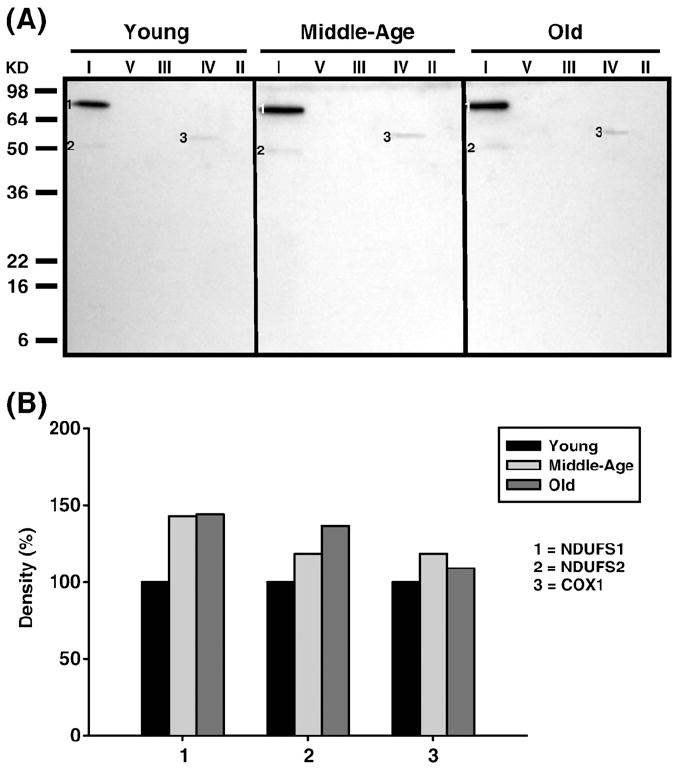
Identification of HNE-modified proteins of young (3–5 months), middle-aged (12–14 months), and old (20–22 months) old quadriceps mitochondrial ETC complex subunits. Quadriceps mitochondrial ETC complexes were resolved into individual subunits as described under Materials and methods followed by immunoblotting. (A) Immunoblot of young, middle-aged, and old quadriceps mitochondrial ETC complex subunits using anti-HNE antibody. Modified proteins were numbered according to their complex localization followed by the highest to the lowest molecular weight of the proteins. Protein loading was normalized using complex-specific antibodies as described under Materials and methods. Normalized density values of each individual protein modified by HNE are plotted as a percentage of the young quadriceps protein density for each specific complex subunit. (B) Densitometry for modified proteins found in CI (1 and 2) and CIV (3). Identification of each numbered band is summarized in Table 3.
Fig. 8.
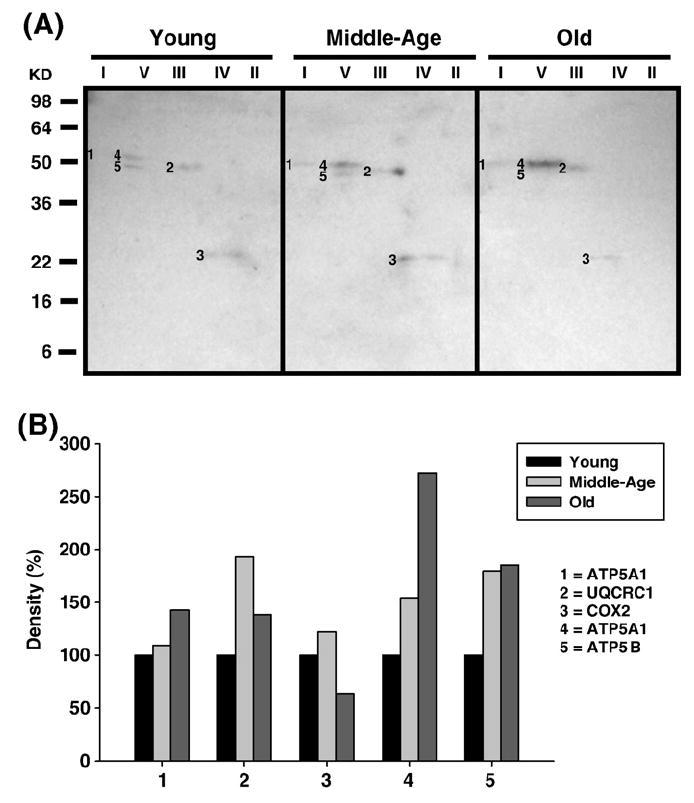
Identification of nitrotyrosine-modified proteins of young (3–5 months), middle-aged (12–14 months), and old (20–22 months) pectoralis mitochondrial ETC complex subunits. Pectoralis mitochondrial ETC complexes were resolved into individual subunits as described under Materials and methods followed by immunoblotting. (A) Immunoblot of young, middle-aged, and old pectoralis mitochondrial ETC complex subunits using anti-nitrotyrosine antibody. Modified proteins were numbered according to their complex localization followed by the highest to the lowest molecular weight of the proteins. Protein loading was normalized using complex-specific antibodies as described under Materials and methods. Normalized density values of each individual protein modified by nitrotyrosine are plotted as a percentage of the young pectoralis protein density for each specific complex subunit. (B) Densitometry for modified proteins found in CI (1), CIII (2), CIV(3), and CV (4 and 5). Identification of both bands is summarized in Table 2.
Fig. 9.
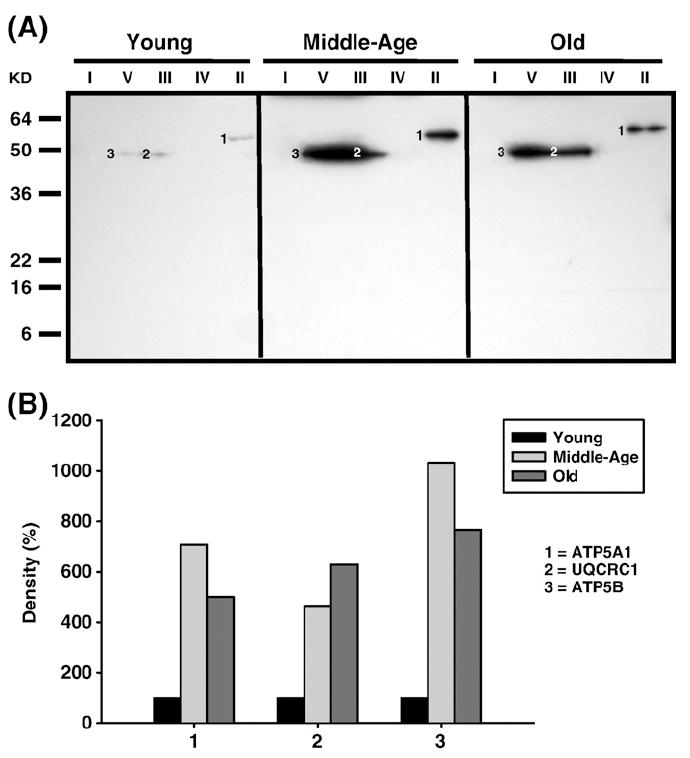
Identification of nitrotyrosine-modified proteins of young (3–5 months), middle-aged (12–14 months), and old (20–22 months) quadriceps mitochondrial ETC complex subunits. Quadriceps mitochondrial ETC complexes were resolved into individual subunits as described under Materials and methods followed by immunoblotting. (A) Immunoblot of young, middle-aged, and old quadriceps mitochondrial ETC complex subunits using anti-nitrotyrosine antibody. Modified proteins were numbered according to their complex localization followed by the highest to the lowest molecular weight of the proteins. Protein loading was normalized using complex-specific antibodies as described under Materials and methods. Normalized density values of each individual protein modified by nitrotyrosine are plotted as a percentage of the young quadriceps protein density for each specific complex subunit. (B) Densitometry for modified proteins found in CII (1), CIII (2), and CV (3). Identification of both bands is summarized in Table 3.
Fig. 10.
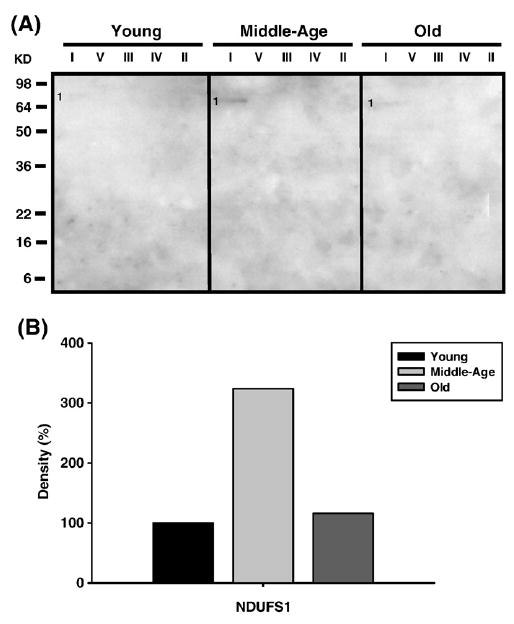
Identification of MDA-modified proteins of young (3–5 months), middle-aged (12–14 months), and old (20–22 months) pectoralis mitochondrial ETC complex subunits. Pectoralis mitochondrial ETC complexes were resolved into individual subunits as described under Materials and methods followed by immunoblotting. (A) Immunoblot of young, middle-aged, and old pectoralis mitochondrial ETC complex subunits using anti-MDA antibody. Protein loading was normalized using complex-specific antibodies as described under Materials and methods. Normalized density values of the individual protein modified by MDA are plotted as a percentage of the young pectoralis protein density for CI subunit. (B) Densitometry for modified protein found in CI. Identification of the CI band is summarized in Table 2.
Fig. 11.
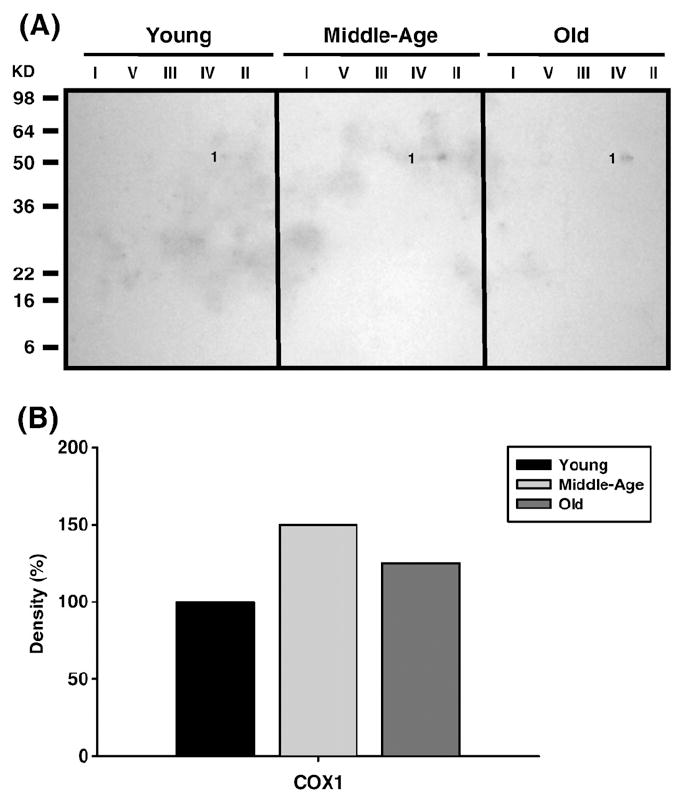
Identification of MDA-modified proteins of young (3–5 months), middle-aged (12–14 months), and old (20–22 months) quadriceps mitochondrial ETC complex subunits. Quadriceps mitochondrial ETC complexes were resolved into individual subunits as described under Materials and methods followed by immunoblotting. (A) Immunoblot of young, middle-aged, and old quadriceps mitochondrial ETC complex subunits using anti-MDA antibody. Protein loading was normalized using complex-specific antibodies as described under Materials and methods. Normalized density values of the individual protein modified by MDA are plotted as a percentage of the young quadriceps protein density for CIV subunit. (B) Densitometry for modified protein found in CIV. Identification of the CIV band is summarized in Table 3.
Table 2.
Carbonylated and HNE-, nitrotyrosine-, and MDA-modified protein subunits of mouse pectoralis mitochondrial electron transport chain complexes
| Band no. | Gene name | ProFound protein ID (MW) | E valuea | Localization |
|---|---|---|---|---|
| Carbonylated (Fig. 4A) | ||||
| 1 | NDUFS1s | Fe–S subunit 1 (79.7 kDa) | 2.5×10-59 | Complex I |
| 2 | ATP5A1 | α Chain (59.7 kDa) | 5.0×10-64 | Complex V |
| 3 | SDHA | Succinate dehydrogenase 1 (72.3 kDa) | 1.3×10-9 | Complex II |
| 4 | CS | Citrate synthase (51.7 kDa) | 4.0×10-21 | Mitochondria |
| 5 | MDH2 | Malate dehydrogenase 2 (35.6 kDa) | 5.0×10-31 | Mitochondria |
| 6 | UQCRC2 | Core 2 (48.2 kDa) | 6.3×10-52 | Complex III |
| 7 | ACADV1 | Acyl-CoA dehydrogenase V1 (70.8 kDa) | 3.2×10-9 | Mitochondria |
| 8 | ATP5A1 | α Chain (59.7 kDa) | 2.5×10-26 | Complex V |
| 9 | FH1 | Fumarate hydratase 1(50.9 kDa) | 1.0×10-20 | Mitochondria |
| 10 | ACAD1 | Acyl-CoA dehydrogenase 1 (47.9 kDa) | 1.3×10-17 | Mitochondria |
| 11 | ATP5A1 | α Chain (59.7 kDa) | 1.0×10-76 | Complex V |
| HNE-modified (Fig. 6A) | ||||
| 1 | NDUFS1 | Fe-S subunit 1 (79.7 kDa) | 2.5×10-59 | Complex I |
| 2 | ATP5A1 | α Chain (59.7 kDa) | 5.0×10-64 | Complex V |
| 3 | UQCRC2 | Core 2 (48.2 kDa) | 6.3×10-52 | Complex III |
| 4 | COX2 | Subunit 2 (25.9 kDa) | 3.3×10-9 | Complex IV |
| 5 | ATP5A1 | α Chain (59.7 kDa) | 1.0×10-76 | Complex V |
| Nitrotyrosine-modified (Fig. 8A) | ||||
| 1 | ATP5A1 | α Chain (59.7 kDa) | 5.0×10-64 | Complex V |
| 2 | UQCRC1 | Core 1 (52.7 kDa) | 8.0×10-35 | Complex III |
| 3 | COX2 | Subunit 2 (25.9 kDa) | 3.3×10-9 | Complex IV |
| 4 | ATP5A1 | α Chain (59.7 kDa) | 1.0×10-76 | Complex V |
| 5 | ATP5B | β Chain (56.3 kDa) | 1.3×10-62 | Complex V |
| MDA-modified (Fig. 10A) | ||||
| 1 | NDUFS1 | Fe–S subunit 1 (79.7 kDa) | 2.5×10-59 | Complex I |
Protein E value or expectation value assigned by the Mascot database is the number of matches with equal or better scores that are expected to occur by chance alone. In each MALDI-TOF-TOF run the significance threshold was set at a more stringent value of 1.0 ×10-3 compared to the default value of 0.05. Thus, any number below the threshold was considered significant.
Table 3.
Carbonylated and HNE-, nitrotyrosine-, and MDA-modified protein subunits of mouse quadriceps mitochondrial electron transport chain complexes
| Band no. | Gene name | ProFound protein ID (MW) | E valuea | Localization |
|---|---|---|---|---|
| Carbonylated (Fig. 5A) | ||||
| 1 | NDUFS1 | Fe–S subunit 1 (79.7 kDa) | 2.9×10-34 | Complex I |
| 2 | ATP5A1 | α Chain (59.7 kDa) | 1.5×10-51 | Complex V |
| 3 | SDHA | Succinate dehydrogenase 1 (72.3 kDa) | 5.8×10-24 | Complex II |
| 4 | UQCRFS1 | Rieske iron–sulfur protein (29.3 kDa) | 1.2×10-10 | Complex III |
| 5 | COX1 | Subunit 1 (56.9 kDa) | 9.2×10-17 | Complex IV |
| 6 | ACADL | Long chain (47.9 kDa) | 9.2×10-9 | Mitochondria |
| 7 | FH1 | Fumarate hydratase 1(50.9 kDa) | 8.3×10-8 | Mitochondria |
| 8 | ATP5A1 | α Chain (59.7 kDa) | 2.9×10-42 | Complex V |
| HNE-modified (Fig. 7A) | ||||
| 1 | NDUFS1 | Fe-S subunit 1 (79.7 kDa) | 2.9×10-34 | Complex I |
| 2 | NDUFS2 | Fe-S subunit 2 (52.6 kDa) | 4.6×10-28 | Complex I |
| 3 | COX1 | Subunit 1 (56.9 kDa) | 9.2×10-17 | Complex IV |
| Nitrotyrosine-modified (Fig. 9A) | ||||
| 1 | ATP5A1 | α Chain (59.7 kDa) | 1.5×10-51 | Complex V |
| 2 | UQCRC1 | Core 1 (52.7 kDa) | 1.2×10-12 | Complex III |
| 3 | ATP5B | β Chain (56.3 kDa) | 1.5×10-58 | Complex V |
| MDA-modified (Fig. 11A) | ||||
| 1 | COX1 | Subunit 1 (56.9 kDa) | 9.2×10-17 | Complex IV |
Protein E value or expectation value assigned by the Mascot database is the number of matches with equal or better scores that are expected to occur by chance alone. In each MALDI-TOF-TOF run the significance threshold was set at a more stringent value of 1.0 ×10-3 compared to the default value of 0.05. Thus, any number below the threshold was considered significant.
Oxidatively modified proteins of Complex I
Carbonylated CI proteins from pectoralis are shown in Fig. 4A. One of the proteins, the Fe–S subunit 1 (NDUFS1, band 1), showed an increase in carbonylation at 12–14 months and a subsequent decrease in old age to below young levels (No. 1, Fig. 4B). NDUFS1, also HNE modified, increased 4-fold by middle age and declined back to young levels by old age (band 1, Fig. 6A, and No. 1, Fig. 6B), and modification of MDA which increased by 3-fold in middle age and declined to young levels by old age. Thus, this subunit of the iron–sulfur protein (IP) region is differentially modified with age. Interestingly, the carbony-lated (band 2, Fig. 4A), HNE modified (band 2, Fig. 6A), and nitrated (band 1, Fig. 8A) α chain of CV also comigrated with CI. The CI-associated α chain also showed an age-associated increase in carbonylation (No. 2, Fig. 4B) and nitration (No. 1, Fig. 8B) but no increase in HNE modification (No. 2, Fig. 6B).
Carbonylated CI proteins from quadriceps are shown in Fig. 5A. The modified Fe–S subunit 1 (NDUFS1, band 1) was detected in all age groups, although the level of modification decreased continuously with aging (No. 1, Fig. 5B). Furthermore, HNE-modified NDUFS1 was also detected (band 1, Fig. 7A) and showed a 50% increase by middle age and no further increase in old age (No. 1, Fig. 7B). The HNE modified Fe–S subunit 2 (NDUFS2) (band 2, Fig. 7A) was also detected and shown to increase by 25% at both the middle and old age. Nitrotyrosine or MDA modifications were not detected in CI from quadriceps. Finally, similar to pectoralis, the carbonylated α chain of CV also comigrated with CI (band 2, Fig. 4A) and its carbonylated levels increased at middle age compared to young and declined below young levels by old age (No. 2, Fig. 4B).
Oxidatively modified proteins of Complex II
Subunit 1 (SDHA, band 3, Fig. 4A) is the only protein of CII in pectoralis thatwas carbonylated. This modification, whichwas seen in all ages, increased ~40% at middle age and ~2.5-fold at old age compared to young mice. SDHA catalyzes the conversion of succinate to fumarate coupled with the generation of FADH2 and thus is the catalytic subunit of CII. No lipid peroxidation adducts (HNE and MDA) or nitration were found in CII. Interestingly, two other carbonylated proteins of the citric acid cycle, citrate synthase (CS) and malate dehydrogenase 2 (MDH2), were found to comigrate with CII. Carbonylation of these proteins showed a differential profile such that CS modification increased by ~45% in old age compared to young mice while MDH2 showed an age-related decrease in modification. Both of these enzymes are located in the mitochondrial matrix and associated with the inner mitochondrial membrane.
In quadriceps, carbonylated SDHA (band 3, Fig. 5A) dramatically increased by 2.5-fold and almost 5-fold higher in middle age and old age compared to young, respectively (No. 3, Fig. 5B). No additional modifications, such as lipid peroxidation adducts (HNE and MDA) or nitration, were seen in CII proteins from quadriceps. Interestingly, the nitrotyrosine modified α subunit of CV comigrated with CII (band 1, Fig. 9A), and showed a dramatic increase in modification to over 6-fold in middle age and ~5-fold in old age compared to young mice (No. 1, Fig. 9B).
Oxidatively modified proteins of Complex III
The oxidatively modified CIII proteins include Core 1 (UQCRC1, band 2, Fig. 8A) and Core 2 (UQCRC2, band 6, Fig. 4A and band 3, Fig. 6A). While Core 1 is nitrated, Core 2 is carbonylated and HNE modified. Both proteins show a 2-fold increase in middle age and a decrease to basal levels by old age (No. 2, Fig. 8B, and No. 6, Fig. 4B, as well as No. 3, Fig. 6B, for Core 1 and Core 2, respectively). Both Core 1 and Core 2 proteins are anchored to the inner mitochondrial membrane with most of the protein exposed to the matrix. Since CIII is one of the ROS-generating sites the topographical arrangement of these CIII proteins and the proximity to electron transfer sites may explain the differential modifications for these proteins. No MDA adducts were found in the pectoralis CIII.
Oxidatively modified CIII proteins of the quadriceps include the Rieske Fe–S subunit (ISP) and Core 1 subunit. The ISP was carbonylated (UQCRFS1, band 4, Fig. 5A) and the Core 1 protein was nitrated (UQCRC1, band 2, Fig. 9A). Both proteins showed very different modification profiles such that the ISP modification decreased by 50% in middle age and no change in old age compared to young whereas Core 1 showed a dramatic ~5-fold increase in nitration in middle age and ~6-fold by old age compared to young mice. No lipid peroxidation adducts (HNE and MDA) were seen in CIII from quadriceps.
Oxidatively modified proteins of Complex IV
Subunit 2 (COX2) was the only pectoralis CIV protein found to be modified by both HNE (band 4, Fig. 6A) and nitrotyrosine (band 3, Fig. 8A). Each modification showed a similar pattern such that the modification increased ~50% in middle age and decreased just below basal levels by old age (No. 4, Fig. 6B, and No. 3, Fig. 8B, respectively). No carbonylation or MDA modifications were found in CIV from pectoralis. Interestingly, several other oxidatively modified proteins were also found to comigrate with CIV. These include acyl-CoA dehydrogenase very long chain 1 (ACADV1, band 7, Fig. 4A), α chain of CV (ATP5A1, band 8, Fig. 4A), fumarate hydratase 1 (FH1, band 9, Fig. 4A), and acetyl-CoA dehydrogenase 1 (ACAD1, band 10, Fig. 4A). All of the proteins showed an age-related decrease in carbonylation levels (No. 7, No. 9, and No. 10, Fig. 4B), with the exception of the α chain which increased in modification by ~2.5-fold in middle age and decreased to just above basal levels by old age (No. 8, Fig. 4B).
Subunit 1 (COX1) was the only CIV protein in quadriceps to be modified by carbonylation (band 5, Fig. 5A), HNE (band 3, Fig. 7A), and MDA (band 1, Fig. 11A). While carbonylation levels were lower in middle age and slightly higher in old age (No. 5, Fig. 5B) both lipid peroxidation adducts showed a modest increase only in middle age compared to young mice (No. 3, Fig. 7B, and Fig. 11B). No nitrationwas found in CIV, although similar to pectoralis, additional proteins were found to comigrate with CIV that were carbonylated. These proteins, which include acetyl-CoA dehydrogenase long chain (ACADL, band 6, Fig. 5A) and fumarate hydratase 1 (FH1, band 7, Fig. 5A), show very little changes in levels of modification with aging (No. 6 and No. 7, Fig. 5B, respectively).
Oxidatively modified proteins of Complex V
The α and β chains of CV are differentially modified in aged pectoralis. The α chain was carbonylated (ATP5A1, band 11, Fig. 4A), HNE (band 5, Fig. 6A), and nitrotyrosine modified (band 4, Fig. 8A) while the β chainwas only nitrated (band 5, Fig. 8A). All modifications of the α chain show different patterns such that there is no change in carbonylation in middle age and lower levels are seen in old age (No. 11, Fig. 4B) whereas HNE modification increased to ~2.5-fold in middle age and reverts to basal levels by old age (No. 5, Fig. 6B), and nitrotyrosine modification continues to rise with aging and is ~3-fold higher (No. 4, Fig. 8B) compared to young age. These differentially modified forms of the α chain also comigrate with other ETC complexes as described above. The β chain, on the other hand, shows a 2-fold increase in nitration and no further modification by old age compared to young mice (No. 5, Fig. 8B). No MDA adducts were found in quadriceps muscle ETC complexes.
Both the α and β chains of quadriceps CV were also found to be differentially modified with aging. The α chain carbonylation (band 8, Fig. 5A) decreased with age (No. 8, Fig. 5B). In addition, the β chain is heavily nitrated, (band 3, Fig. 9A) showing a ~11-fold increase in middle age and ~8-fold increase by old age (No. 3, Fig. 9B) compared to young mice. No lipid peroxidation adducts (HNE and MDA) were found in CV from quadriceps.
Discussion
Our studies show that the levels of oxidatively modified mitochondrial ETC proteins in the mouse pectoralis, whose physiological characteristics include high levels of myoglobin and mitochondria, hence the high oxygen-carrying capacity and aerobic respiration, are significantly lower than those of the quadriceps, a relatively anaerobic muscle, which contains considerably less amounts of myoglobin and mitochondria. We propose that the differences in the levels of their oxidatively modified proteins, both qualitative and quantitative, suggest a pattern of modification that may be due to the organellar microenvironment in these muscles as well as the availability of modifiable amino acids which is dependent on protein structure. Our correlation of the changes in age-related enzyme activities of the modified subunit proteins with their proximity to sites of free radical production provides some indication of the basis of age-associated mitochondrial dysfunction. In the case of carbonylation, the pectoralis and quadriceps show similar levels of this modification, although carbonylation is the only major age-associated increase in oxidative modification in the pectoralis. This suggests a specific physiological environment that favors the other modifications, i.e., HNE and tyrosine nitration in the quadriceps. Furthermore, carbonylation correlates with decreased complex activity in both muscles, although the pectoralis appears to be more severely affected. Surprisingly, however, the endogenous coupled activity of CI→CIII and CII→CIII showed a minimal decrease with age which does not correlate with the decreased complex activities. It has been reported that not all modifications have detrimental or measurable effects on function [35] and that in some cases, the consequences of ETC protein modifications are enhanced under conditions of oxidative stress which may explain the minimal effects of modification we observed in the resting unchallenged muscle [36]. Interestingly, the endogenous coupled activity of the pectoralis is considerably lower than the quadriceps even though the former contains higher numbers of mitochondria and has the capacity for higher oxygen uptake. Thus, it is possible that with the larger mitochondrial population the individual mitochondria experience a lower metabolic demand.
Although the quadriceps is an anaerobic muscle, the significantly higher levels of HNE and nitrotyrosine modifications suggest that the physiological environment of the quadriceps, at each age, favors these modifications and serve as an indication of the physiological differences between these two muscles For example, the higher level of HNE modification suggests differences in lipid peroxidation while the higher level of nitrotyrosine modification suggests differences in peroxynitrite/NO metabolism. Our studies suggest that both lipid peroxidation and NO metabolism (peroxynitrite production) are favored in the hypoxic environment of the resting quadriceps.
Our overall data indicate that many of the same proteins are modified in both muscles, although the level of modification differs significantly. The oxidative modifications of essentially the same ETC proteins, with age, indicate that these modifications are protein specific and, although we predict that most modifications may affect protein structure, we do not know whether only certain sites of modification will affect the function of the complex. We conclude that the physiological characteristics of the pectoralis and quadriceps may play a role in the level and specificity of oxidative activity and modification of the ETC proteins. Our similar observations with kidney and heart mitochondria are consistent with this hypothesis [22,23].
The decrease in complex enzyme activity in the pectoralis correlates with the level of oxidative damage with age [37,38]. However, modification of the same proteins in quadriceps, but at higher levels, did not affect their enzyme function to the extent seen in the pectoralis. These data suggest different levels of sensitivity to these modifications and that different tissues may age at different rates depending upon the physiological characteristics. Thus, we propose that the physiological status of the muscles, aerobic vs anaerobic, may determine the level and rate of modification, when the modified proteins begin to accumulate and whether the modification is damaging or of physiological significance.
Our inhibitor-sensitive enzyme activities show that a dramatic decline in CI–CV enzyme activities in the pectoralis, and that this occurs at middle age. The fact that the pectoralis, whose functions are more severely affected by the oxidative modification, exhibits characteristics of aging at a much earlier age than the quadriceps suggests that the aerobic activity of this posturing muscle may play a role in the early development of aging characteristics and that the minimal change in the ETC enzyme functions in the quadriceps may also be due to the physiological status of this muscle.
Interestingly, NDUFS1, a component of the iron protein (IP) region of CI, located in the mitochondrial matrix, was found to contain multiple modifications. Its carbonylation and HNE and MDA modification in middle age compared to young tissue correlate with the dramatic decline in CI function as well as CI–CIII coupled activity. However, the loss of oxidatively modified species from middle to old age did not affect or improve these functions. Thus, if further oxidation of the carbonyls and lipid peroxidation adducts occurs, this might generate immuno-unrecognizable carboxylate adducts and hence the decline in the immuno-signal as seen in old age.
An interesting difference between the pectoralis and the quadriceps is that the CI enzyme activity of the former is higher whereas the CI–CIII coupled activity is significantly lower. These characteristics suggest a tighter coupled activity in the quadriceps. The loss of CI activity as well as coupled enzyme activity of CI–CIII in pectoralis which occurs between young and middle age is not, however, due to CoQ levels since the availability of CoQ is 3 times higher in the quadriceps, and does not change with age. Thus, a higher coupled activity of the quadriceps would minimize ROS generation due to the loss of electrons from these complexes. Since the ubisemiquinone intermediate formed during the electron transfer process in CI has the propensity to produce superoxide anion [10,15,16,39], the tighter coupling and lower levels of CoQ9 may decrease ROS production in the quadriceps.
The significantly higher level of HNE modification of NDUFS1 (CI) in the quadriceps compared to the pectoralis indicates that there is a muscle-type, specific differential modification and suggests a higher level of lipid peroxidation in the quadriceps. Furthermore, the significantly lower level of modification of NDUFS2, also a Fe–sulfur component of CI, is further indication of the differential susceptibility of these proteins to oxidative modification, although they are components of the same complex. These observations raise the question of whether the relative position of these Fe–sulfur proteins within the complex plays a role in the level of modification, i.e., exposure to ROS, and whether the amino acid residue and/or level of modification may be a factor in the decline of CI function in quadriceps. In addition, specificity and level of residue modification may explain the minimal effect of the HNE modification on enzyme function of CI in the pectoralis. Perhaps identification of themodified sitesmay clarify themechanismof decline in quadriceps enzyme function [40-45]. We propose that the decline of complex function in the mitochondria of the aging quadriceps may be the consequence of a primarily anaerobic skeletal muscle physiology whose increase in oxidative modification may be due to lipid peroxidation. Similarly the negligible level of loss of CI function in mitochondria of aging pectoralis may relate to the aerobic physiological nature of this muscle.
The CII and CII–CIII coupled activities in pectoralis decline dramatically at middle age after which no further decline occurs. Although we do not understand the basis for this mid-aged decline it supports our proposal that the loss of these functions may be the consequences of altered structure of the modified CII subunits. Furthermore, since the loss of CII function is independent of CoQ levels and since there are no significant changes in complex levels suggest that the defect is possibly due to the structural change(s) incurred by subunit modification. This is consistent with the modification to CII protein (SDHA) that may cause increased release of ROS. In fact, the data show that carbonylation of SDHA increases significantly with age. In contrast, the quadriceps CII and CII–CIII coupled activities show very little change with age. The significant differences in CII and CII–CIII activities between these two muscles is clearly a distinguishing characteristic especially since the carbonylation of SDHA in the pectoralis and quadriceps increases progressively with age. Again, we propose that the tighter coupling may mediate efficient electron transfer that would be consistent with lower ROS production.
The levels of carbonylation of SDHA differ significantly in both tissues and the modification increases with aging, more so in quadriceps. However, only the pectoralis shows decreased enzyme activities that correlate with carbonylation, which peaks at middle age, i.e., further modification from middle age to old age does not affect either enzyme function or coupled activity. We reported a similar age-associated pattern of activity vs modification in mouse kidney [23]. This supports the concept that certain proteins are more susceptible to oxidative modification that may affect their structure and function. Our studies suggest, therefore, that increased in vivo oxidative modification of the SDHA subunit directly correlates with an age-associated functional deficiency in CII from pectoralis. Thus, identification of sites of amino acid modification should provide structural information relevant to the effects of the modification on function.
The increasing modification of the pectoralis Core 1 and Core 2 subunits in middle age is likely due to their proximity to the predicted site of ROS generation, i.e., the heme bL of the cytochrome b subunit [46-48]. The oxidative modification of Core 1 and Core 2 correlates directly with the loss of CIII function in pectoralis. However, even though Core 1 is heavily nitrated in the quadriceps, there is no change in CIII function with aging. We speculate that this may be a reflection of the alternative role for the mitochondrial respiratory chain under hypoxic conditions in which mitochondrially produced NO is involved in hypoxic signaling, possibly via a pathway that involves protein tyrosine nitrations [49]. Furthermore, the significantly greater tyrosine nitration of ATP5A and ATP5B which are part of CV may be part of the ATP synthase activity under hypoxic conditions and nitration of UQCRC1 of CIII may involve the transport processing activity of Core I. For example, it has been reported that cytochrome oxidase produces nitric oxide under hypoxic conditions, suggesting that mitochondrially produced NO is involved in hypoxic signaling, possibly through tyrosine nitration [49]. This raises the question of whether the hypoxic environment of the quadriceps favors the level of nitration we observe in these proteins.
The decreased enzyme activity during middle age in CIV also parallels the increased oxidative modification of COX2 in pectoralis. COX2 is the first subunit in the electron transfer pathway of CIV and contains a copper center (CuA) that mediates electron transfer between reduced cytochrome c and heme a in COX1 [49]. We propose that the loss of enzyme activity of modified COX2 may contribute to the increased mitochondrial dysfunction in the pectoralis muscle with aging. Interestingly, in the quadriceps, COX1, but not COX2, shows only a modest increase in modification and decline in CIV enzyme function with aging, further supporting the hypothesis that the modification of specific sites may affect the overall outcome of such damage. These results suggest that under hypoxic conditions these signaling processes in the quadriceps may represent an adaptation to the physiological change.
Although CV or F1F0-ATP synthase is not part of the ETC processes its location within the matrix, as well as its abundance, make it a prime candidate for oxidative modification. The increased oxidative modification of the pectoralis α and β chains directly correlates with the decline in enzyme function, which in turn may result in decreased ATP production, a hallmark of mitochondrial dysfunction with aging. Furthermore, oxidatively modified α chain was also found to comigrate with CI and CIV, suggesting that modification may facilitate its dissociation from the active complex and association with these complexes. Thus, its binding to CI may play a role in increased ROS production at CI and further ETC complex dysfunction. The α and β chains of the quadriceps are more strongly nitrated than in the pectoralis. Despite the dramatic modifications of both α and β chains, there appears to be no effect on CV function which suggests that these modifications may not be damaging. For example, our data show a strong nitration of ATP5B, and ATP5A1 in the quadriceps. Recent studies have shown that rat liver mitochondria produce nitric oxide (NO) under hypoxic conditions. Thus, NO production is nitrite (NO2-) dependent, requires an electron donor, and is carried out by cytochrome c oxidase (COX1, 2) in a pH-dependent manner. These studies have suggested that mitochondrial generated NO and superoxide combine to form peroxynitrite which then tyrosine nitrates protein components of a hypoxic signaling pathway [49]. In addition, Koeck et al. [48] presented evidence that tyrosine nitration may serve as a signaling process under conditions of hypoxia in liver mitochondria. More specifically they showed that induction of hypoxia/anoxia followed by reoxygenation caused the reversible nitration of tyrosines in the same mitochondrial proteins. Our data report endogenous levels of nitration which are highest in the hypoxic quadriceps. Actually, these nitrations if reversible may be associated with signaling [50]. On the other hand, there is also evidence that tyrosine nitration (irreversible) may be a marker of oxidative protein modifications. Interestingly, since the modified α chain comigrates with CI apparently no functional consequences result from this association. However, if the nitrated protein is misfolded, this structural change may solicit an unfolded protein stress response which may affect overall respiration aswell as ROS production from CI.
Our studies suggest that many of the ETC complex subunits may be specific targets of ROS-mediated oxidative modifications. We propose that some of these modifications may lead to mitochondrial dysfunction and an increased state of oxidative stress in aged tissue. Alternatively, nitration, which does not affect the enzyme or coupled function, may be a signaling process. We have identified significant differences between the pectoralis, in which the decreased enzyme activities correlate with modification of complex proteins and loss of function. However, the oxidative modifications had very little effect on ETC function in quadriceps [51]. Although there are age-related increases in modification of essentially the same proteins in both muscles, the quadriceps may adapt to these modifications. We propose that this adaptation may involve nitrotyrosine signaling linked to hypoxia. Finally, our studies show that two different groups of skeletal muscles exhibit unique characteristics of aging which raise questions of the mechanisms of differential muscle aging in humans.
Numerous studies that have examined the effects of aging on enzyme function of ETC complexes in skeletal muscle mitochondria of both rodents and humans have reported conflicting results possibly due to use of various markers for normalization of mitochondrial content and/or failure to include enzyme activities as well as the CI–CIII and CII–III coupled activities [51-57].
Nearly all oxidized proteins detected in these studies are partly or fully exposed to the mitochondrial matrix. The location of these oxidized subunits is consistent with the possibility that ROS generated by dysfunctional ETC complexes may react with the surrounding mitochondrial membranes, thereby causing increased levels of lipid peroxidation products that modify neighboring membrane-associated subunits of various complexes. Clearly, two very different age-associated profiles emerge from pectoralis and quadriceps where proteins are increasingly modified mainly at middle age in pectoralis and with the exception of nitration and SDHA carbonylation very little changes in levels of modification are noted in quadriceps. Thus, the differential targeting of specific ETC subunits in two different muscles may cause either unfavorable physiological effects to the enzyme activities or an alternative signaling mechanism which minimizes deleterious effects.
Acknowledgments
This publication was supported by U.S.P.H.S. grant 1P01 AG021830-04 awarded by the National Institute on Aging, and the National Institute on Aging 1 P30 AG024832-03 Claude D. Pepper Older Americans Independence Center grant and by the Sealy Center on Aging. J.E.N. thanks the Kempner Foundation and the National Institutes of Environmental Health Sciences Training Grant (T32-07254) for additional fellowship support.
Abbreviations
- ACAD1
acetyl-CoA dehydrogenase 1
- ACADV1
acetyl-CoA dehydrogenase V1
- ATP5A1
complex V α chain
- ATP5B
complex V β chain
- BN-PAGE
blue-native polyacrylamide gel electrophoresis
- CI
complex I
- CII
complex II
- CIII
complex III
- CIV
complex IV
- CV
complex V
- CoQ
coenzyme Q
- COX1
cytochrome c oxidase subunit 1
- COX2
cytochrome c oxidase subunit 2
- CS
citrate synthase
- DNP
2,4-dinitrophenylhydrazone
- DNPH
2,4-dinitrophenylhydrazine
- ETC
electron transport chain
- FH1
fumarate hydratase 1
- HNE
4-hydroxynonenal
- ISP
Rieske iron-sulfur protein
- MALDI-TOF-TOF
matrix-assisted laser desorption/ionization–time of flight-time of flight
- MDA
malondialdehyde
- MDH2
malate dehydrogenase 2
- MPP
mitochondrial processing peptidase
- NDUFS1
NADH dehydrogenase Fe–S subunit 1
- NDUFS2
NADH dehydrogenase Fe–S subunit 2
- ROS
reactive oxygen species
- SDHA
succinate dehydrogenase subunit 1
- UQCRC1
Core 1 subunit
- UQCRC2
Core 2 subunit
- UQCRFS1
Rieske iron–sulfur protein
References
- 1.Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. 2000;4:140–142. [PubMed] [Google Scholar]
- 2.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Kamel HK. Sarcopenia and aging. Nutr Rev. 2003;61:157–167. doi: 10.1301/nr.2003.may.157-167. [DOI] [PubMed] [Google Scholar]
- 4.Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004;7:405–410. doi: 10.1097/01.mco.0000134362.76653.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 6.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 7.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 8.Balaban RS, Nemoto S, Finkel T. Review: mitochondria, oxidants and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 10.Lenaz G, Bovina C, D’Aurelio M, Fato R, Formiggini G, Genova ML, Giuliano G, Merlo PM, Paolucci U, Parenti CG, Ventura B. Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 12.Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim Biphys Acta. 1998;1366:53–67. doi: 10.1016/s0005-2728(98)00120-0. [DOI] [PubMed] [Google Scholar]
- 13.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Manton KG. The role of oxidative damage in mitochondria during aging: a review. Front Biosci. 2004;9:1100–1117. doi: 10.2741/1298. [DOI] [PubMed] [Google Scholar]
- 15.Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano T, Magnitsky S, Ohnishi T. Characterization of the complex I-associated ubisemiquinone species: toward the understanding of their functional roles in the electron/proton transfer reaction. Biochim Biophys Acta. 2000;1459:299–304. doi: 10.1016/s0005-2728(00)00164-x. [DOI] [PubMed] [Google Scholar]
- 17.Choksi KB, Boylston WH, Rabek JP, Widger WR, Papaconstantinou J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim Biophys Acta. 2004;1688:95–101. doi: 10.1016/j.bbadis.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 19.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 20.Sohal R. Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 21.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 22.Choksi KB, Nuss JE, Boylston WH, Rabek JP, Papaconstantinou J. Age-related increases in oxidatively damaged proteins of mouse kidney mitochondrial electron transport chain complexes. Free Radic Biol Med. 2007;43:1423–1438. doi: 10.1016/j.freeradbiomed.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choksi K, Papaconstantinou J. Age-related alterations in oxidatively damaged proteins of mouse heart mitochondrial electron transport chain complexes. Free Radic Biol Med. 2008;44:1795–1805. doi: 10.1016/j.freeradbiomed.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabek JP, Boylston WH, III, Papaconstantinou J. Carbonylation of ER chaperone proteins in aged mouse liver. Biochem Biophys Res Commun. 2003;305:566–572. doi: 10.1016/s0006-291x(03)00826-x. [DOI] [PubMed] [Google Scholar]
- 25.Uchida K, Stadtman ER. Modification of histidine-residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci USA. 1992;89:4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchida K, Stadtman ER. Selective cleavage of thioether linkage in proteins modified with 4-hydroxynonenal. Proc Natl Acad Sci USA. 1992;89:5611–5615. doi: 10.1073/pnas.89.12.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarian CS, Rebrin I, Sohal RS. Aconitase and ATP synthase are targets of malondialdehyde modification and undergo an age-related decrease in activity in mouse heart mitochondria. Biochem Biophys Res Commun. 2005;330:151–156. doi: 10.1016/j.bbrc.2005.02.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schagger H. Native electrophoresis for isolation of mitochondrial oxidative phosphorylation protein complexes. Methods Enzymol. 1995;260:190–202. doi: 10.1016/0076-6879(95)60137-6. [DOI] [PubMed] [Google Scholar]
- 29.Rajapakse N, Shimizu K, Payne M, Busija D. Isolation and characterization of intact mitochondria from neonatal rat brain. Brain Res Protoc. 2001;8:176–183. doi: 10.1016/s1385-299x(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 30.Jarreta D, Orus J, Barrientos A, Miro O, Roig E, Heras M, Moraes CT, Cardellach F, Casademont J. Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovasc Res. 2000;45:860–865. doi: 10.1016/s0008-6363(99)00388-0. [DOI] [PubMed] [Google Scholar]
- 31.Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys. 2000;373:16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- 32.Venkatraman A, Landar A, Davis AJ, Chamlee L, Sanderson T, Kim H, Page G, Pompilius M, Ballinger S, Darley-Usmar V, Bailey SM. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. J Biol Chem. 2004;279:22092–22101. doi: 10.1074/jbc.M402245200. [DOI] [PubMed] [Google Scholar]
- 33.Boitier E, Degoul F, Desguerre I, Charpentier C, Francois D, Ponsot G, Diry M, Rustin P, Marsac C. A case of mitochondrial encephalomyopathy associated with a muscle coenzyme Q10 deficiency. J Neurol Sci. 1998;156:41–46. doi: 10.1016/s0022-510x(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 34.Duncan AJ, Heales SJ, Mills K, Eaton S, Land JM, Hargreaves IP. Determination of coenzyme Q10 status in blood mononuclear cells, skeletal muscle, and plasma by HPLC with di-propoxy-coenzyme Q10 as an internal standard. Clin Chem. 2005;51:2380–2382. doi: 10.1373/clinchem.2005.054643. [DOI] [PubMed] [Google Scholar]
- 35.Moreau R, Heath SH, Doneanu CE, Lindsey JB, Hagen TM. Age-related increase in 4-hydroxynonenal adduction to rat heart alpha-ketoglutarate dehydrogenase does not cuase loss of its catalytic activity. Antioxid Redox Signal. 2003;5:517–527. doi: 10.1089/152308603770310167. [DOI] [PubMed] [Google Scholar]
- 36.Winger AM, Taylor NA, Heazlewood JL, Day DA, Millar AH. The cytotoxic lipid peroxidation product 4-hydroxy-2-nonenal covalently modifies a selective range of proteins linked to respiratory function in plant mitochondria. J Biol Chem. 2007;282:37436–37447. doi: 10.1074/jbc.M702385200. [DOI] [PubMed] [Google Scholar]
- 37.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidativelymodified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 38.Wei YH. Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med. 1998;217:53–63. doi: 10.3181/00379727-217-44205. [DOI] [PubMed] [Google Scholar]
- 39.Johnson JE, Jr, Choksi K, Widger WR. NADH-Ubiquinone oxidoreductase: substrate-dependent oxygen turnover to superoxide anion as a function of flavin mononucleotide. Mitochondrion. 2003;3:97–110. doi: 10.1016/S1567-7249(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 40.Bautista J, Corpas R, Ramos R, Cremades O, Gutierrez JF, Alegre S. Brain mitochondrial complex I inactivation by oxidative modification. Biochem Biophys Res Commun. 2000;275:890–894. doi: 10.1006/bbrc.2000.3388. [DOI] [PubMed] [Google Scholar]
- 41.Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA. Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. J Biol Chem. 2003;278:37223–37230. doi: 10.1074/jbc.M305694200. [DOI] [PubMed] [Google Scholar]
- 42.Lashin OM, Szweda PA, Szweda LI, Romani AM. Decreased complex II respiration and HNE-modified SDH subunit in diabetic heart. Free Radic Biol Med. 2006;40:886–896. doi: 10.1016/j.freeradbiomed.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, Schenker S, Frosto TA, Henderson GI. Inhibition of cytochrome c oxidase activity by 4-hydroxynonenal (HNE). Role of HNE adduct formation with the enzyme subunits. Biochim Biophys Acta. 1998;1380:336–344. doi: 10.1016/s0304-4165(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Henderson GI, Freeman GL. Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. J Mol Cell Cardiol. 2001;33:1919–1927. doi: 10.1006/jmcc.2001.1454. [DOI] [PubMed] [Google Scholar]
- 45.Picklo MJ, Amarnath V, McIntyre JO, Graham DG, Montine TJ. 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J Neurochem. 1999;72:1617–1624. doi: 10.1046/j.1471-4159.1999.721617.x. [DOI] [PubMed] [Google Scholar]
- 46.Deng K, Shenoy SK, Tso SC, Yu L, Yu CA. Reconstitution of mitochondrial processing peptidase from the core proteins (subunits I and II) of bovine heart mitochondrial cytochrome bc(1) complex. J Biol Chem. 2001;276:6499–6505. doi: 10.1074/jbc.M007128200. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Yu L, Yu CA. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J Biol Chem. 1998;273:33972–33976. doi: 10.1074/jbc.273.51.33972. [DOI] [PubMed] [Google Scholar]
- 48.Michel H, Behr J, Harrenga A, Kannt A. Cytochrome c oxidase: structure and spectroscopy. Annu Rev Biophys Biomol Struct. 1998;27:329–356. doi: 10.1146/annurev.biophys.27.1.329. [DOI] [PubMed] [Google Scholar]
- 49.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;4:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Koeck T, Fu X, Hazen SL, Crabb JW, Stuehr DJ, Aulak KS. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J Biol Chem. 2004;279:27257–27262. doi: 10.1074/jbc.M401586200. [DOI] [PubMed] [Google Scholar]
- 51.Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Experimental evidence against the mitochondrial theory of aging. A study of isolated human skeletal muscle mitochondria. Exp Gerontol. 2003;38:877–886. doi: 10.1016/s0531-5565(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 52.Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1:637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- 53.Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci. 1992;113:91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- 54.Boffoli D, Scacco SC, Vergari R, Solarino G, Santacroce G, Papa S. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim Biophys Acta. 1994;1226:73–82. doi: 10.1016/0925-4439(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 55.Torii K, Sugiyama S, Takagi K, Satake T, Ozawa T. Age-related decrease in respiratory muscle mitochondrial function in rats. Am J Respir Cell Mol Biol. 1992;6:88–92. doi: 10.1165/ajrcmb/6.1.88. [DOI] [PubMed] [Google Scholar]
- 56.Sugiyama S, Takasawa M, Hayakawa M, Ozawa T. Changes in skeletal muscle, heart and liver mitochondrial electron transport activities in rats and dogs of various ages. Biochem Mol Biol Int. 1993;30:937–944. [PubMed] [Google Scholar]
- 57.Lenaz G, Bovina C, Castelluccio C, Fato R, Formiggini G, Genova ML, Marchetti M, Pich MM, Pallotti F, Parenti CG, Biagini G. Mitochondrial complex I defects in aging. Mol Cell Biochem. 1997;174:329–333. [PubMed] [Google Scholar]


