Abstract
Corticotrophin-releasing factor (CRF) is expressed in the central nucleus of the amygdala (CeA), where the CRF receptor (CRFr) plays an important role in anxiety- and stress-related behaviors. To determine the subcellular sites of CRFr activation in this region, we examined the electron microscopic immunolabeling of antisera recognizing CRF or CRFr. The ultrastructural analysis was principally conducted in the lateral subdivision of the rat CeA, with comparisons being made in mice so as to optimally utilize mutant mice in control experiments. The CRFr labeling was seen in many small dendrites and dendritic spines as well as in a few somata, large dendrites, axons, and axon terminals or more rarely in glial processes. Approximately 35% of the CRFr-labeled dendrites contained CRF immunoreactivity, which was distributed diffusely throughout the cytoplasm, or specifically affiliated with either endomembranes or large dense-core vesicles. The CRF-immunoreactive vesicles also were present in somata and axon terminals with or without CRFr labeling. The CRF immunoreactivity was usually absent from both terminals and dendrites joined by asymmetric, excitatory-type synapses, where a postsynaptic location of the CRFr was commonly observed. Numerous terminals containing both CRF and CRFr were seen, however, within the neuropil and sometimes apposing the excitatory synapses. These results provide ultrastructural evidence for a primary involvement of CRF receptors in modulation of the postsynaptic excitability of CeA neurons, an effect that may be limited by the availability of CRF. The findings have important implications for understanding CRF mediation of rapid responses to stress.
Indexing terms: autoregulation, stress, autonomic, limbic, drug addiction
The central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST) are critical components of the glucocorticoid-sensitive extrahypothalamic circuit involved in fear and anxiety as well as stress and addictive disorders (Gray and Bingaman, 1996; Aston-Jones et al., 1999; Curtis et al., 2002; Cook, 2004; Santibanez et al., 2005). Both regions contain neurons that express CRF (Swanson et al., 1983; Bale and Vale, 2004; Asan et al., 2005), and have extensive bidirectional connections with each other (Erb et al., 2001). The CRF-containing neurons are mainly located in the CeA lateral subdivision (CeL) that 1) receives substantial input from midline thalamic nuclei implicated in arousal and attention (Li and Kirouac, 2008), and 2) projects extensively to hypothalamic and brainstem regions involved in neuroendocrine and autonomic responses (Veening et al., 1984; Moga and Gray, 1985; Sakanaka et al., 1986; Van Bockstaele et al., 1998; Wu et al., 1999; Curtis et al., 2002). The involvement of these neurons in drug addiction is strongly supported by the marked elevation of CRF peptide and mRNA in the CeA of rats receiving chronic morphine or cocaine administration (Maj et al., 2003; Erb et al., 2005; Wang et al., 2006). In addition, there is accumulating evidence for CRF-mediated activation of hypocretin neurons and hypocretin signaling back to the amygdala, which may play a role in arousal states associated with the stress response and addiction (Winsky-Sommerer et al., 2004, 2005; Paneda et al., 2005). The precipitous withdrawal from several addictive drugs also evokes the release of CRF in the CeA and associated brain regions (Heinrichs et al., 1995; Iredale et al., 2000; George et al., 2007). This CRF release contributes to anxiogenic and autonomic withdrawal symptoms as well as stress-evoked reinstatement of drug-seeking behavior (Shaham et al., 1997; Maj et al., 2003; Contarino and Papaleo, 2005).
The CRF receptors are a family consisting of at least two major subtypes (type 1 and type 2) and several different isoformes (Perrin and Vale, 1999; Lewis et al., 2001), which include the corticotropin-releasing factor receptor (CRFr), referred to as CRF2alpha-tr, whose cDNA was cloned from the rat amygdala library (Miyata et al., 1999). The CRF type 1 receptor (CRFr1) is the primary receptor subtype activated by CRF (Perrin and Vale, 1999; Lewis et al., 2001), and is thought to play the greatest role in anxiety-like behavior mediated in part through the CeA (Heinrichs et al., 1997; Ji et al., 2007; Zhao et al., 2007). This region, however, shows low mRNA expression of CRFr1 (Radulovic et al., 1998; Van Pett et al., 2000) as well as CRF receptor-2 (CRFr2), a subtype principally activated by urocortin (Sanchez et al., 1999; Bittencourt and Sawchenko, 2000). In addition, CRFr immunolabeling is slightly more prevalent in the medial CeA (CeM), a region that contains less CRF immunoreactivity than the CeL (Chen et al., 2000). The low expression levels of CRFr in brain regions where there are large numbers of CRF-containing neurons has been suggested to reflect a mismatch between the receptor and its endogenous ligand (Chen et al., 2000). In addition, however, the discrepancy between CRF and CRF receptor in the CeL also may reflect CRF-induced receptor downregulation comparable to that reported in the rat cerebral cortex and in pituitary derived cell lines (Iredale et al., 1996; Brunson et al., 2002). Such regulation has been implicated in the known decrease in the stress response to repeated stressors (Bale and Vale, 2004).
The CRFr binding sites and CRF binding protein are identified in the CRF-enriched CeL as well as other CeA subdivisions in rat and most other species thus far examined (De Souza et al., 1985; Behan et al., 1995). High-resolution electron microscopy has a considerable advantage over the lower-resolution light microscopic methods used in these earlier studies in being able to 1) define potentially small quantities of CRFr immunoreactivity in pre- and postsynaptic neuronal compartments, and 2) determine synaptic and/or intracellular associations between CRF receptors and CRF. Thus, to determine these functionally relevant sites we used electron microscopy to examine the subcellular distribution of CRFr and CRF immunolabeling in single sections through the CeA. The location of CRF-immunoreactive neurons was used as a guide for selecting the CeL for ultrastructural analysis in both rat and mouse, the latter of which were included in the study largely for purposes of defining the specificity of the labeling in mutant mice. The primary goal was to test the hypothesis that the CeL is one of the autonomic brain regions in which the CRFr is targeted to pre- and/or postsynaptic sites in neurons that either express CRF or have synaptic associations with CRF-containing neurons.
Materials and Methods
Animals
All experiments were performed in keeping with the National Institutes of Health (NIH) regulations of animal care and were reviewed by the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University. Male Sprague–Dawley rats (200–250 g) were obtained from Taconic Farms (Germantown, NY). Adult C57BL/6J mice and both CRF (B6;129S2-Crhtm1Maj/J, stock #002783) and CRFr1 (B6;129-Crhr1tm1Klee/J, stock #004454) and heterozygous (+/−) knockout mice were purchased from the Jackson Laboratories (Bar Harbor, ME). These mouse lines were originally developed by Smith et al. (1998) and Muglia et al. (1995), respectively. To generate the homozygous mice, the heterozygous littermates were crossed and the litters were genotyped by standard polymerase chain reaction (PCR) procedures with tail DNA. As expected, three types of littermates were obtained in this type of breeding: homozygous (−/−), heterozygous (+/−), and wildtype (+/+). In all the experiments the wildtype (+/+) littermates for either CRF or CRFr1 were used as controls. The CRF homozygous mice were given 30 μg/L corticosterone (Calbiochem, La Jolla, CA) in their drinking water to replace the missing hormone. This was necessary to ensure adequate lung development and survival of the pups (Keck et al., 2005).
CRF antiserum
A guinea pig polyclonal antiserum raised against human/rat CRF (Bachem Peninsula, San Carlos, CA) was used for immunolabeling CRF. The 41 amino acid CRF antigenic peptide was derived from the rat sequence: H-Ser-Glu-Glu-Pro-Pro-Ile-Ser-Leu-Asp-Leu-Thr-Phe-His-Leu-Leu-Arg-Glu-Val-Leu-Glu-Met-Ala-Arg-Ala-Glu-Gln-Leu-Ala-Gln-Gln-Ala-His-Ser-Asn-Arg-Lys-Leu-Met-Glu-Ile-Ile-NH2 (Rivier et al., 1983). This amino acid peptide has 100% homology with CRF and CRF precursor and 47% homology with urocortin (GenBank search). The guinea pig CRF antiserum was tested for specificity by comparatively examining the immunoperoxidase labeling in CRF (+/+) and CRF (−/−) mice.
The distribution in both wildtype and mutant mice was compared with that seen after preadsorption of the CRF antiserum with a brain lysate prepared from CRF (−/−) mouse brain. To prepare the lysate, fresh forebrain tissue of one adult, male CRF (−/−) mouse was gently homogenized in detergent lysis buffer (25 mM HEPES, pH 7.4, 50 mM NaCl, 1 mM EDTA, 1% Triton X-100, protease inhibitor cocktail) and incubated on ice for 10 minutes (Zhou et al., 2001). The lysate was subsequently centrifuged at 3,500 rpm for 3 minutes and the protein concentration was determined for the supernatant using an HTS 7000 Plus bioassay reader. The CRF antiserum (1:4,000) was then adsorbed against the lysate (16.6 mg/mL) for 24 hours at 4°C and centrifuged. The supernatant containing the adsorbed antiserum was subsequently used for immunoperoxidase labeling in brain sections from CRF (+/+) and CRF (−/−) mice. The optimal concentration of the CRF antiserum and lysate was selected by light microscopic analysis of CRF immunoperoxidase labeling in the CeA over a range of dilutions.
CRFr antiserum
A rabbit polyclonal antiserum raised against amino acids 230-444 mapping at the C-terminus of the CRFr1 of human origin (CRF-R1/2 (H-215): sc-5543; Santa Cruz Biotechnology, Santa Cruz, CA) was used for CRFr immunolabeling. Adsorption of the CRFr antiserum with the antigenic peptide in the C terminal of the receptor was used as one of the criteria of specificity. The peptide adsorption was prepared by incubation of the CRFr1 antiserum at the working dilution of 1:1,000 for 24 hours with 1 mg/mL of the antigenic peptide before use for immunolabeling with the avidin-biotin peroxidase complex (ABC) method.
The CRFr antiserum used in our study is known to recognize CRFr1 and CRFr2 in Western blots, but the CRFr1 predominates in many brain regions such as the locus coeruleus, where CRFr1 (+/+) mice are without detectable CRFr immunoreactivity (Reyes et al., 2008). We examined the CRFr immunoperoxidase labeling in the CeA of CRFr1 (−/−) to assess the specificity of the CRFr antiserum in this brain region. We also examined the CRFr immunolabeling following adsorption of the antiserum with brain lysate from CRFr1 (−/−) mice. The CRFr1 (−/−) brain lysate was prepared as described above for the CRF. The CRFr1 antiserum (1:375) was adsorbed against the lysate (4.9 mg/mL) for 24 hours at 4°C and centrifuged. As for CRF, the optimum antibody and lysate concentrations for CRFr1 were determined based on serial dilution tests. The immunolabeling with the lysate preadsorbed antiserum was examined in brain sections from CRFr1 (+/+) and (−/−) mice.
Fixation and immunolabeling
Six rats and four each of 1) CRF (+/+) and CRF (−/−) mice and 2) CRF-R1 (+/+) and CRF-R1 (−/−) mice were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.). For light microscopy, the animals were perfused via the ascending aorta (rats) or left ventricle of the heart (mice) with heparin (1,000 U/mL) saline, which was followed by 200 mL (rats) or 50 mL (mice) of 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). For electron microscopy, the heparin-saline in rats was followed by 60 mL of 3.75% acrolein and 2% paraformaldehyde in 0.1 M PB) and 200 mL of 2% paraformaldehyde in PB. In mice, the respective volumes were reduced to 5 mL heparin-saline, 30 mL acrolein, and 100 mL paraformaldehyde. The brains from all animals were removed from the cranium and thick slices through the CeA region were placed in 2% paraformaldehyde in 0.1 M PB for 30 minutes at 4°C. These were then transferred to PB and cut into 40-μm coronal sections on a Vibratome (Leica, Deerfield, IL). Sections containing the CeA in rat and mouse were identified based on the respective atlas descriptions (Franklin and Paxinos, 1997; Paxinos and Watson, 1998).
Immunocytochemical labeling
The paraformaldehyde-fixed brain tissue used for light microscopy was processed for immunolabeling with inclusion of 0.3% Triton X-100 in the primary antiserum. The acrolein-fixed brain sections used for electron microscopy were placed in 1% sodium borohydride in 0.1 M PB for 30 minutes to remove excess aldehydes, then washed thoroughly in 0.1 M PB. To enhance penetration, these sections of tissue were placed in a cryprotectant solution (25% sucrose and 3.5% glycerol in 0.05 M PB) for 15 minutes, then rapidly freeze-thawing by immersion in liquid chlorodifluoromethane (Freon, Refron, NY) followed by liquid nitrogen and lastly in room temperature 0.1 M PB. Nonspecific binding of the antisera to the sections of tissue was minimized by incubation of the sections in 0.5% bovine serum albumin (BSA) in 0.1 M TBS, pH 7.6, for 30 minutes before placement in the primary antisera.
The sections were rinsed in 0.1 M Tris-buffered saline (TBS) and incubated for 24 hours at room temperature (about 21°C) followed by an additional 24 hours incubation at 4°C in a solution containing one or both primary antisera. For dual labeling, we used a 1:500 rabbit anti-CRFr for immunoperoxidase and 1:500 guinea pig anti-CRF for immunogold-silver. For the reverse labeling scheme, the primary antisera solution consisted of 1:100 rabbit anti-CRFr for immunogold-silver labeling and 1:2,000 guinea pig anti-CRF for immunoperoxidase labeling. All antisera dilutions were prepared in 0.1% BSA in 0.1 M TBS. These were subsequently processed for dual immunoperoxidase and immunogold-silver labeling using the method of Chan et al. (1990). For immunoperoxidase localization of the primary antisera, the sections were rinsed in 0.1 M TBS and then placed for 30 minutes in a 1:400 dilution of biotinylated antirabbit CRFr or antiguinea pig (CRF). These were then washed in 0.1 M TBS and incubated in avidin-biotin peroxidase complex (Vectastain Elite Kit, Vector Labs, Burlingame, CA) for 30 minutes. This incubation was followed by a wash in 0.1 M TBS and reaction for 6 minutes in 0.022% 3,3′-diaminobenzidine (DAB, Aldrich, Milwaukee, WI) and 0.003% hydrogen peroxide in 0.1 M TBS.
For immunogold-silver labeling the sections were rinsed in 0.01 M PBS, pH 7.4, blocked in 0.8% BSA and 0.1% gelatin in PBS for 10 minutes, and subsequently incubated for 2 hours at room temperature with shaking in a 1:50 dilution of antirabbit (for CRFr) or antiguinea pig (for CRF) IgG conjugated with 1 nm colloidal gold (Amersham, Arlington Heights, IL). The sections were washed several times with 0.1 M TBS. To aid in the binding of gold particles conjugated to IgG, the sections were incubated in 2% glutaraldehyde for 10 minutes. Silver intensification was performed using the IntenS-EM kit (Amersham) for 7 minutes at room temperature. The sections were fixed in 2% osmium tetroxide in 0.1 M PB for 60 minutes, washed in PB, and dehydrated by passing through increasingly concentrated solutions up to 100% ethanol, followed by 100% propylene oxide. The sections were incubated overnight in a 1:1 mixture of propylene oxide and Epon (Electron Microscopy Science, Fort Washington, PA), and then transferred to 100% Epon for 2-3 hours before flat-embedding between sheets of Aclar plastic. These sections were viewed with a light microscope to select immunolabeled portions of the CeL at Bregma −1.8 to −2.8 mm (Franklin and Paxinos, 1997; Paxinos and Watson, 1998). The ultrathin (60 nm) sections were cut from the superficial surface of the section using a diamond knife (Diatome, Fort Washington, PA) on an ultratome (NOVA LKV-Productor AB, Bromma, Sweden). The thin sections were collected on 400-mesh copper grids (Electron Microscopy Sciences), then counterstained with uranyl acetate and lead citrate (Reynolds, 1963). The counterstained sections on grids were rinsed and allowed to air dry before viewing at 60 kV with a Philips CM10 transmission electron microscope (FEI, Hillsboro, OR). The thin sections were initially examined at low (8-9K) magnification to identify the surface of the tissue, and those regions showing immunolabeling of both CRF and CRFr. These were then magnified and captured as digital images using AMT Advantage HR/HR-B CCD Camera System (Advanced Microscopy Techniques, Danvers, MA).
Electron microscopic analysis
Profiles immunolabeled for CRF and/or CRFr were classified based on conventional nomenclature as neuronal somata, and either neuronal (dendrites, dendritic spines, axons, and axon terminals) or glial processes (Peters et al., 1991). Immunoperoxidase labeling was regarded as positive when the electron-dense reaction product in individual profiles was greater than that seen in other morphologically similar profiles in the neuropil. Profiles were considered immunogold-labeled when they contained two gold particles, or one membrane-associated gold particle, a criterion that was most often applied to small structures having a mean diameter of less than 0.05 μm. The analysis was done exclusively in thin sections at the surface of the tissue, where there were few spurious gold particles (i.e., less than 0.10% of the gold particles overlying myelin or other portions of the tissue or plastic not known to possess CRF or CRFr). The intracellular locations of immuno-gold particles was examined to determine their location near (<50 nm distant) the plasma membrane or in association with endomembranes or vesicular organelles.
Thin sections were examined from the outer surface of one vibratome section in each of three rats, two CRFr1 (+/+) and two CRFr (−/−) mice. The tissue sections used for electron microscopic analysis were taken from those animals that showed qualitatively similar distributions and intensities of CRF and CRFr immunoreactivity. This similarity enabled pooling of the data from the three rats used for quantitative ultra-structural analysis. All electron microscope images were imported into an IBM computer, where Adobe Photoshop (v. 7.0.1, Adobe Systems, Mountain View, CA) was used to sharpen and enhance contrast as needed. These images were then imported into PowerPoint, Microsoft Office, 2003, which was used to assemble figures and add lettering.
Results
Immunoperoxidase labeling for CRF was seen by light microscopy in isolated perikarya and varicose processes that were most prevalent in the CeL (Fig. 1A). There were no species-specific differences between rats and mice in the CRF labeling pattern, although the density of immunoreactivity was usually greater in the rat compared with mouse CeA. The CRF immunoreactivity in the CeA and in the hypothalamic median eminence, which was examined because of the known high density of CRF-immunoreactive processes (Henry et al., 2005), was, with one exception, absent in CRF (−/−) mice (Figs. 1, 2). The exception was the immunoreactivity seen in a few punctate processes in the CeA of the CRF (−/−) mice (Fig. 1B). Prior adsorption of the CRF antiserum with a fresh brain lysate prepared from CRF (−/−) mice slightly improved the CRF labeling in CRF (+/+) mice (Fig. 1C). The enhanced clarity of the labeling after lysate adsorption appeared to reflect a loss of the diffuse background staining. The residual peroxidase labeling in the CeL of the CRF (−/−) mouse (Fig. 1B) is attenuated, but not completely removed by the lysate adsorption (Fig. 1D), as might be expected from the homologies between CRF and CRF-related peptides (see CRF antiserum characterization in Materials and Methods).
Figure 1.
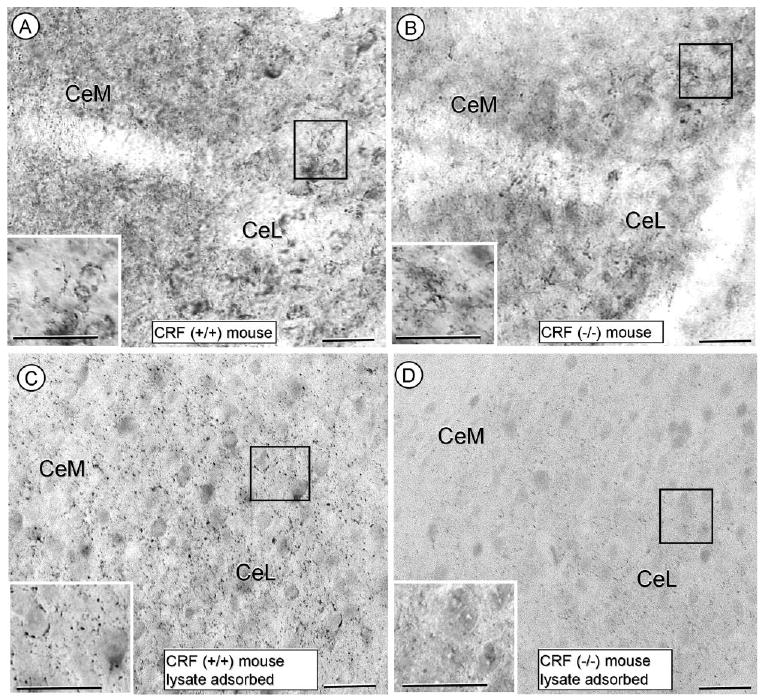
Photomicrographs showing the immunoperoxidase labeling for CRF in the CeA using either nonadsorbed (A,B) or CRF (−/−) mouse brainlysate-adsorbed (C,D) CRF antiserum. In A, the CRF-immunoperoxidase reaction product is seen in the cytoplasm of isolated somata and many varicose processes in the CeA of a CRF (+/+) mouse. The varicose CRF-immunoreactive processes also surround unlabeled soma (insert from boxed region in A). In B, isolated processes (insert from boxed region) show immunoperoxidase reaction product for the CRF antiserum in the CeA of a CRF (−/−) mouse. These processes are not well organized and do not have the perisomatic distribution seen in the wildtype mouse. In C and D, respectively, the differential labeling between CRF (+/+) and CRF (−/−) mice is seen in tissue processed with antiserum adsorbed with a brain lysate from CRF (−/−) mouse. CeM, central amygdala, medial subdivision; CeL, central amygdala, lateral subdivision. Scale bars = 12.5 and 25.0 (insert) μm.
Figure 2.
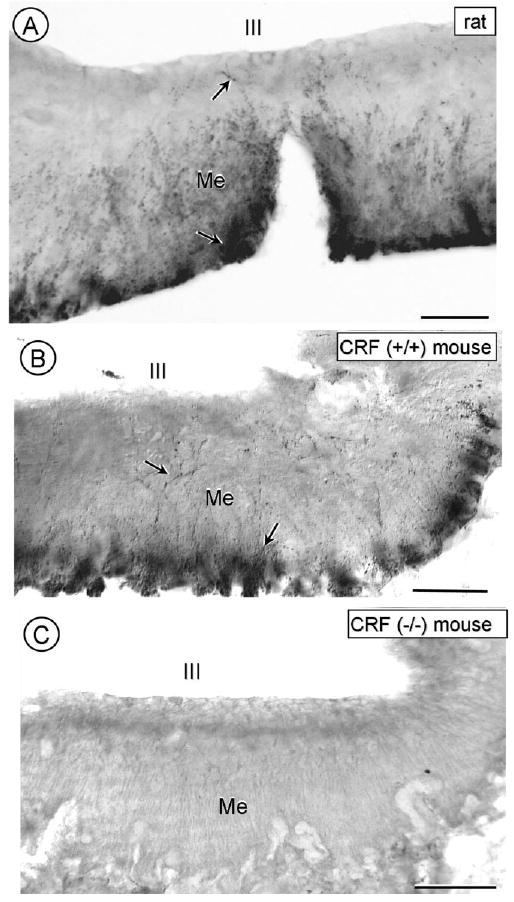
Light microscopic images showing immunoperoxidase labeling for CRF in the hypothalamic median eminence of a rat (A), a CRF (+/+) mouse (B), and a CRF (−/−) mouse (C). The CRF-immunoreactivity is seen in varicose processes (arrows) mainly in the outer but also inner layer of the median eminence (Me) below the third ventricle (III). No similar labeling is seen in a CRF (−/−) mouse. Scale bars = 80 mM.
As compared with CRF, the CRFr immunoperoxidase labeling was substantially less prevalent and more commonly seen within the cytoplasm of isolated somata and processes in the CeA of rat (not shown) and wildtype mice (Fig. 3A). No similarly CRFr-labeled neurons were evident in CRFr1 KO (Fig. 3B) mice. Preadsorption of the CRFr antisera with brain lysate from a CRFr1 KO mouse minimally affected the CRFr immunoreactivity in either the wildtype (Fig. 3C) or KO (Fig. 3D) mice.
Figure 3.
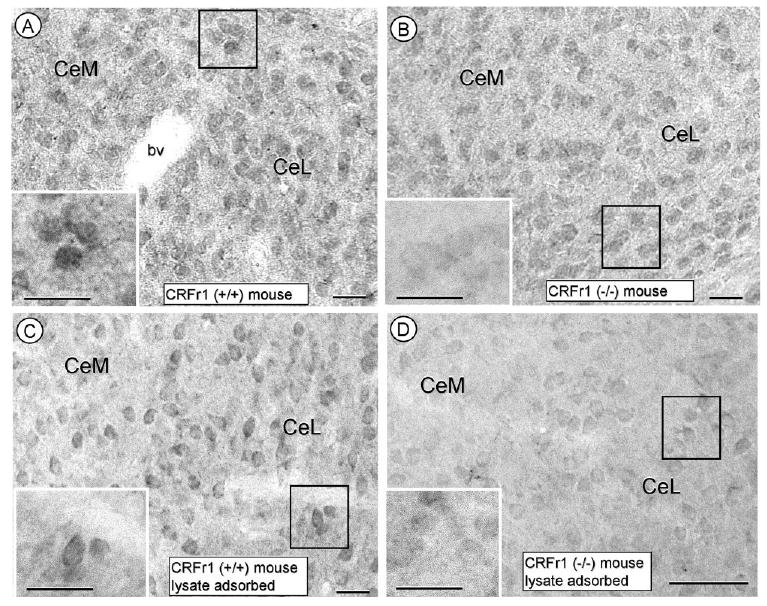
Photomicrographs showing the immunoperoxidase labeling for CRF receptor in the CeA using either nonadsorbed (A,B) or CRF (−/−) mouse brain-lysate-adsorbed (C,D) CRF antiserum. The immunoperoxidase reaction product is intensely distributed within the cytoplasm of somata (inserts of A and B) within the CeA of a CRFr (+/+) mouse without (A) or with (B) lysate adsorption. No comparable labeling is seen in the CRFr (−/−) mouse in tissue processed with CRFr antiserum without (C) or with (D) lysate adsorption. CeM, central amygdala, medial subdivision; CeL, central amygdala, lateral subdivision. Scale bars = 12.5 and 25.0 (insert) μm.
Electron microscopy confirmed the predominant presynaptic (axonal location of CRF and the postsynaptic (somatodendritic) distribution of CRFr in both rat and mouse CeA. There were no qualitative species-specific differences in the subcellular location of CRFr immunoreactivity. In rat, the only species quantitatively examined, ≈35% of the CRFr-labeled profiles in CeL were dendritic, as seen using immunoperoxidase (n = 2,196) or immunogold (n = 1,054) labeling methods. The remainder included a few somata and many small axons and axon terminals, as well as a few glial processes. Because of the similarity between rat and mouse, these subcellular locations are described jointly with reference to figures in which representative micrographs are shown from each species.
Dendritic and somatic CRFr immunolabeling and relation to CRF
In tissue processed for single CRFr labeling, the immunoperoxidase reaction product was intensely localized within asymmetric excitatory-type synapses on small dendritic spines (Fig. 4A). The CRFr immunoreactivity was also seen at postsynaptic specializations formed by en passant axons on small dendrites (Fig. 4B). In addition, the CRFr labeling was prominently distributed at perisynaptic sites near symmetric synapses or appositional contacts with small unmyelinated axons or glial processes (Fig. 4C). Immunogold confirmed the location of CRFr at asymmetric synapses on dendritic spines (Fig. 5A) as well as on small (Fig. 5B), or sometimes large dendrites (Fig. 5C). Similarly, the CRFr-immunogold particles were located on portions of dendritic plasma membrane contacted by unlabeled terminals that formed a symmetric synapse (Fig. 5D). These distributions were characteristically seen in dendritic profiles containing little, if any, CRF immunoreactivity in tissue processed for immunogold-detection of anti-CRFr and immunoperoxidase labeling for anti-CRF (Fig. 5).
Figure 4.
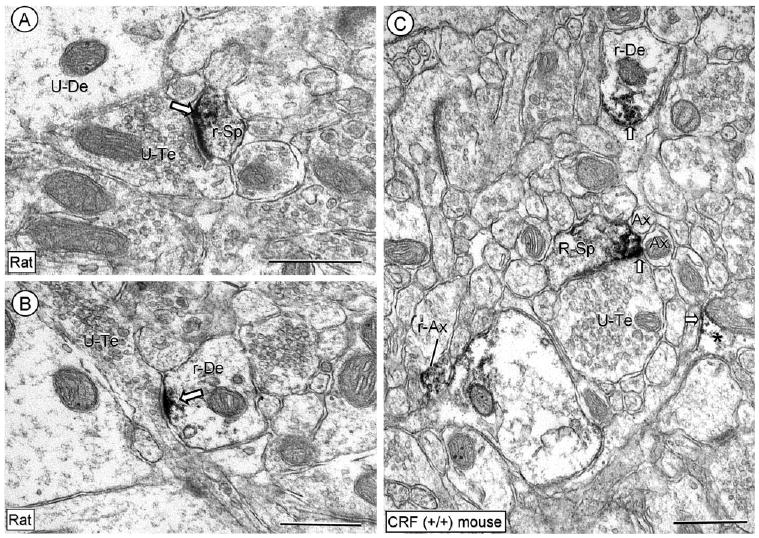
Electron micrographs showing the predominant dendritic immunoperoxidase labeling for CRFr in the CeL. The peroxidase reaction product is seen in the region of the postsynaptic membrane specialization in a dendritic spine (r-Sp) and small dendrite (r-De) in A and B, respectively. Unlabeled dendrites (U-De) are seen in the neuropil. In C, aggregates of immunoreactivity are seen on and near a restricted portion of the plasma membrane in a CRFr-labeled dendrite (r-De) and in a dendritic spine (r-Sp). Immunolabeling is also seen in small axons (I-Ax) and a glial process (*). Block arrow, plasmalemmal labeling in dendritic and glial profiles; U-Te, unlabeled terminal; U-Sp, unlabeled spine; Ax, unlabeled axon. Scale bars = 0.5 μm.
Figure 5.
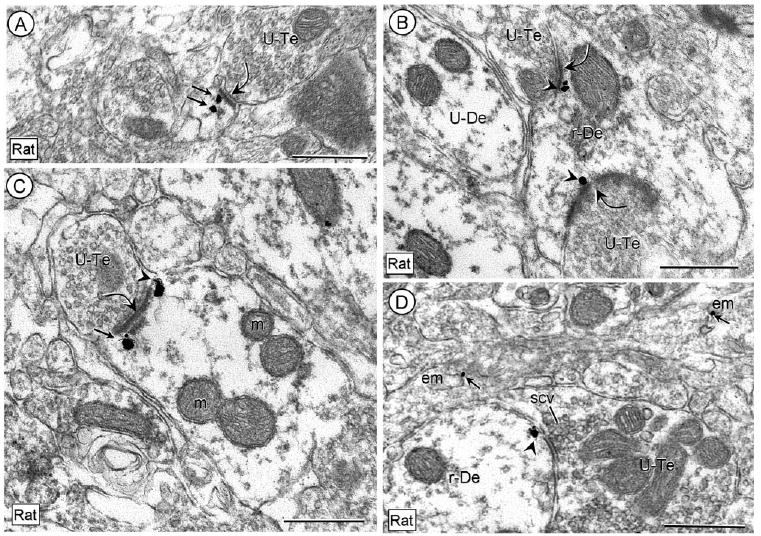
Subsynaptic distribution of CRFr immunogold on plasma membranes (arrowheads) in dendritic profiles without immunoperoxidase labeling for CRF in tissue from the rat CeL processed for dual labeling. The immunogold is located near excitatory-type synapses (curved arrows) in a dendritic spine (A) and in both small (B) and large (C) dendrites. In D, an immunogold particle (arrowhead) is also located beneath an appositional or symmetric membrane specialization. Other immunogold particles (straight arrows) are located beneath the excitatory synapse in the labeled dendritic profiles in A and C or in association with endomembranes (em) of other neuronal processes in C. U-Te, unlabeled terminal; scv, small synaptic vesicle. Scale bars = 0.5 μm.
In contrast to the dendritic profiles without CRF-immunoreactivity, those containing CRF appeared to have a less consistent distribution of CRFr immunogold within and near synapses on either small (Fig. 6A) or large (Fig. 6B) dendrites. Instead the CRFr labeling in these profiles had a mainly diffuse cytoplasmic or endomembrane distribution (Fig. 6A,B). When all sampled dendrites in the rat CeL were quantitatively examined without regard to their content of CRF, 86% (n = 794) of the CRFr-immunogold particles were localized to cytoplasmic sites, while the remainder were on synaptic or nonsynaptic plasma membranes. This is consistent with our observation that over half (190/370) of the CRFr-immunogold labeled somatodendritic profiles contained CRF, and 80% (154/196) of the CRF-immunoperoxidase labeled dendritic profiles contained CRFr immunogold. Reversal of the markers using immunogold for CRF and immunoperoxidase for detection of CRFr showed less extensive coexpression. This may reflect the limited immunogold penetration in large dense core vesicles (dcv's), the organelles most commonly showing CRF immunoperoxidase reaction product in axon terminals.
Figure 6.

Immunogold labeling for CRFr and CRF-immunoperoxidase reaction product in dendrites (A,B) and somata (C,D) in rat CeL In A, the immunogold CRFr labeling is located on an endomembrane (em; arrow) and on the plasmamembrane (arrowhead). In the large dendrite of B as well as somata in C and D the gold particles (arrows) are located in the cytoplasm amid endomembranes and dense core vesicles (dcv) showing peroxidase labeling for CRF. CRF immunoperoxidase reaction product is also more diffusely distributed in the cytoplasm and extends into a small dendrite (De) and initial segment of an axon (I-Ax) that emerge from the somata in C and D, respectively. The plasmalemmal gold particle in A is located near an appositional contact from an unlabeled terminal (U-Te-1), but absent from the asymmetric synapse formed by U-Te2. Du-De, dual labeled dendrite; Du-soma, dual labeled soma; curved arrows, asymmetric synapse; U-Te, unlabeled terminal; Ax, small unlabeled axon; m, mitochondrion. Scale bars = 0.5 μm.
In small dendrites the CRFr-labeled endomembranes showed both CRF peroxidase immunoreactivity and immuno-gold labeling for the CRFr (Fig. 6A). In larger dendrites (Fig. 6B) and somata (Fig. 6C,D) the CRF-immunoperoxidase reaction product was seen throughout the cytoplasm and more rarely associated with dcv's (Fig. 6D).
Axonal distribution of CRF and CRFr labeling
Peroxidase reaction product for CRF was diffusely distributed throughout many axon terminals containing few, if any, large dcv's (Fig. 7A,B). These terminals were apposed to dendrites in which there were either no detectable, or almost exclusively cytoplasmic, CRFr immunogold (Fig. 7A,B). More rarely (2/794 gold particles), a single CRFr immunogold particle was seen on the dendritic plasma membrane in contact with a CRF-labeled axon terminal (Fig. 7B).
Figure 7.
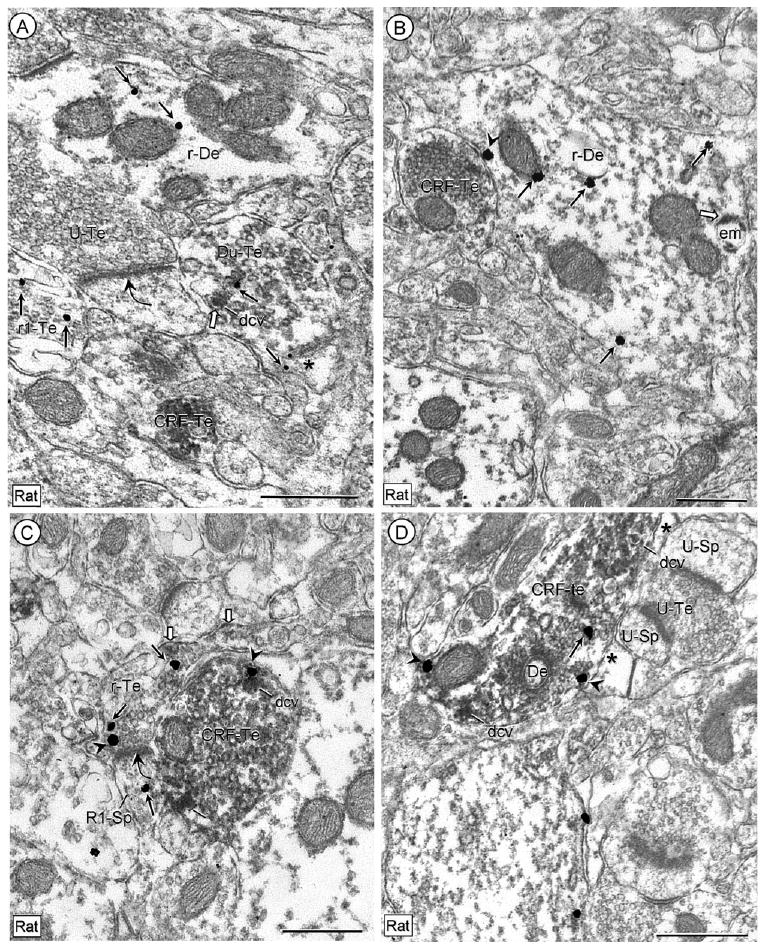
Electron micrographs showing the axonal location of CRF-immunoperoxidase labeling in axon terminals that appose unlabeled or CRFr-immunogold labeled dendrites in rat CeL. In A, the immunogold particles (arrows) are seen near a large dense core vesicle (dcv) in a dually labeled terminal (Du-Te). This terminal is substantially less immunoreactive for CRF than another small terminal (CRF-Te) within the neuropil. Cytoplasmic immunogold (thin arrows) is also seen in a dendrite (r1-De), an axon terminal (R1-Te), and a glial process (asterisk) within the neuropil. The dually labeled terminal is apposed to an unlabeled spine that receives an asymmetric synapse (curved arrow) from an unlabeled terminal (U-Te). In B, plasmalemmal (arrowhead) and cytoplasmic (arrows) immunogold labeling for CRF-R1 is seen in a dendrite (r1-De) that shows diffuse and endomembrane (block arrow) distributions of CRF-immunoperoxidase labeling and input from a CRF-immunoreactive terminal (CRF-Re). In C, CRFr immunogold is seen on the plasma membrane (arrowhead) near an asymmetric synapse (curved arrow) onto a small dendritic spine that also contains cytoplasmic (arrow) immunogold. This terminal shows a diffuse, mainly plasmalemmal (block arrows) distribution of peroxidase labeling for CRF that is in marked distinction from the dense accumulation of CRF peroxidase reaction product in an apposed axon terminal (CRF-Te). In this terminal, a CRFr-immunogold particle (arrowhead) is seen on portions of the plasma membrane near an intensely peroxidase labeled dense core vesicle (dcv). In D, the CRFr-immunogold (arrowheads) is seen on the nonsynaptic plasma membrane (arrowheads) of a large terminal that contains dense immunoperoxidase labeling for CRF (CRF-Te) and dense core vesicles (dcv). This terminal forms what appears to be a symmetric synapse with a small unlabeled dendritic profile (De). The terminal is separated from adjacent unlabeled dendritic spines (U-Sp) and an unlabeled terminal (U-Te) by an intervening astrocytic process (*). Scale bars = 0.5 μm.
Intensely CRF-labeled terminals were apposed to asymmetric axospinous synapses in which the axon terminals and/or dendritic spine showed CRFr immunoreactivity, but only faint and largely plasmalemmal distributions of CRF (Fig. 7C). Rarely, an apparent symmetric synapse was seen between an intensely CRF-labeled terminal and a small dendritic profile (Fig. 7D).
Discussion
We have shown that CRFr immunoreactivity is present in somata and many dendrites as well as large nonsynaptic and synaptic terminals in the CeA of normal rats and mice. The somatodendritic CRFr labeling was most often localized to endomembrane structures, but was also seen on segments of the plasma membrane contacted by terminals containing CRF and/or CRFr. These terminals formed asymmetric excitatory-type synapses. The CRFr labeling, however, was predominantly seen within and near the postsynaptic membrane at excitatory-type synapses on neurons containing little, if any, CRF immunoreactivity (Fig. 8). These observations contribute to understanding the involvement of CRF receptors in modulation of the 1) postsynaptic excitability of CeA neurons, and 2) availability of CRF. Together, our results have direct implications for CRF-mediation of rapid responses to environmental challenge as well as in CRF autoregulation in the induction of anxiety-like states during chronic stress (Gehlert et al., 2005).
Figure 8.
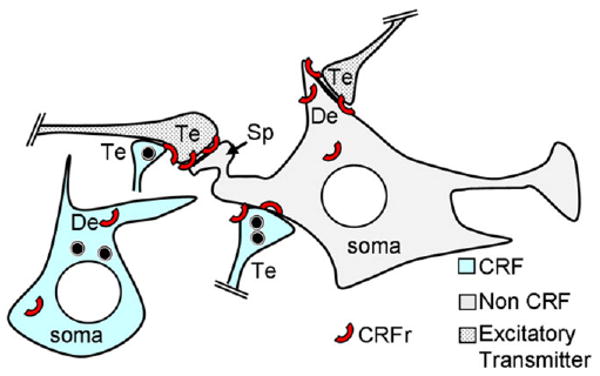
Schematic diagram showing the predominant, but not exclusive, postsynaptic distribution of CRFr (red arc) at asymmetric excitatory-type synapses on the spine (sp) and dendrite (De) of a non-CRF-containing neuron in the CeA. The asymmetric synapses are typical of afferent inputs from terminals that contain excitatory transmitters. The CRF labeling is depicted in a separate neuron, whose soma, dendrite (De), and terminals (Te) show a more sparing distribution of the CRFr. The black circular symbols in the CRF De and Te are peptide-storage vesicles.
Methodological considerations
The light microscopic distribution of peroxidase immunolabeling of the guinea pig antiserum against the CRF peptide is comparable to that seen previously using a well-characterized rabbit antiserum recognizing the same CRF peptide sequence (Bachem Peninsula; Asan et al., 2005). Here we have established in CRF (−/−) mice that the guinea pig CRF antiserum recognizes CRF in the median eminence and CeA. Thus, in the CeA, urocortin and possibly other peptides having sequence homologies with CRF are also identified when using this antiserum, making it necessary to consider all labeling using this antiserum as CRF-like immunoreactivity. In contrast, our light microscopic data provide evidence from CRFr1 (−/−) mice that the rabbit polyclonal antiserum raised against the C-terminal peptide of CRFr1 (Chen et al., 2000) recognizes only this receptor in the CeA. The scarcity of detected immunoreactivity using the rabbit CRFr antiserum is consistent with known low levels of CRFr mRNA in the CeA (Chen et al., 2000; Bale and Vale, 2004). Our results confirm and extend the recent evidence that the rabbit CRFr antiserum (Santa Cruz), which is known to recognize both CRFr1 and CRFr2, identifies CRFr1 in the locus coeruleus (Reyes et al., 2008). In other brain regions, however, this antiserum may also recognize CRFr2, which leads us to maintain the more general term of CRF receptor without reference to the subtypes throughout this manuscript.
We considered a single membrane-associated immunogold particle to be evidence of immunolabeling in sections where such particles were rarely seen over tissue components such as myelin. Even using this relatively low stringency criterion, substantially fewer profiles were detectably labeled with immunogold compared with the high sensitivity and diffusible immunoperoxidase method (Leranth and Pickel, 1989). The methods, however, showed remarkable similarity in determining the relative proportion of CRFr immunoreactivity in dendritic versus axonal compartments, thus suggesting the identity of many of the same neuronal profiles. Thus, our results are consistent with immunochemical identification of both CRF and CRFr in the lateral CeA, but do not entirely exclude the possibility of either 1) false-positive results ascribed to recognition of closely related antigens, or 2) false-negative labeling due to a sparse distribution of CRF and/or CRFr in small profiles.
The cellular and subcellular distributions of CRF and CRFr were similar in rat and mouse CeA, although that seen in the mouse was substantially less abundant. This would suggest that the quantitative assessment of the frequencies of codistribution and cellular associations reported in the rat may not be representative of the mouse. Thus, the quantitative data should be considered only as a relative index of distributions and the extent of coexistence between CRF and CRFr in neuronal and glial profiles in the CeL.
Somatodendritic CRFr distribution
Somata and dendrites within the CeA showed extensive cytoplasmic and circumscribed plasmalemmal or perisynaptic distributions of CRFr immunoreactivity. In these profiles the CRFr immunogold labeling was most often located near endomembranes resembling smooth endoplasmic reticulum, which is involved in both calcium storage and intracellular trafficking of receptors and other surface proteins (Lee et al., 2002). The prominent association of CRFr immunoreactivity with somatodendritic endomembranes suggests that under resting conditions the CRFr is present in reserve, but largely unavailable for activation until mobilized to the cell surface. This cytoplasmic distribution was particularly evident in somata and dendrites that contained CRF immunoreactivity. The cytoplasmic location of the CRFr in the CeA neurons is consistent with a rapid adaptation of the CRF system to novel or repeated stressors (Reyes et al., 2008). Such plasticity is thought to be necessary for the development of chronic anxiety states or stress disorders (Cook, 2004; Gehlert et al., 2005).
CRF and CRF receptor distributions
CRF immunoreactivity was seen in somata and dendrites as well as in many axon terminals in the CeL. These terminals apposed or formed symmetric synapses, which are typical of terminals containing inhibitory amino acids (Nitecka and Frotscher, 1989). This is consistent with prior studies showing the presence of CRF in many of the GABAergic neurons within the CeA stress network (Cook, 2004). The comparatively low abundance of CRFr immunoreactivity in CeL neurons expressing CRF is also consistent with the known ability of CRF to affect the transcriptional regulation of CRFr1 in a variety of neuronal and nonneuronal cell-types (Iredale et al., 1996). Such regulation could account for the known high levels of CRF and low levels of CRF-R1 mRNA in the CeA (Sawchenko et al., 1993; Potter et al., 1994), as well as the lack of correspondence between CRF-R1 mRNA and CRF-induced expression of the c-fos gene in the CeA and other central autonomic nuclei (Bittencourt and Sawchenko, 2000).
Predominant postsynaptic CRFr labeling at excitatory-type synapses
In dendrites and dendritic spines, CRFr immunoreactivity was principally seen within and near asymmetric, excitatory-type synapses from axon terminals that were largely devoid of CRF, but apposed intensely CRF-immunoreactive terminals. These observations suggest that the CRF active at these pre- and postsynaptic receptors is delivered by a relatively short range volume transmission, a classic mechanism for CRF and other peptide modulators (Swinny et al., 2003; Agnati et al., 2004). Our results also suggest, however, that this receptor is integrated with the postsynaptic specializations at excitatory-type synapses in the CeL. The perisynaptic and subsynaptic distributions of CRFr labeling at excitatory synapses suggests that the activation of the CRF receptors affects excitatory postsynaptic currents. This modulation may reflect, in part, the activation of cAMP response element-binding protein (CREB; Kasagi et al., 2002). In other limbic brain regions, CREB is actively involved in the regulation of the expression of AMPA GluR1 subunits that are essential for AMPA receptor-mediated glutamatergic transmission (Olson et al., 2005). The results have important implications for stress-evoked changes in the postsynaptic excitability of amygdala neurons (Liu et al., 2004; Cook, 2004).
Presynaptic CRFr immunoreactivity
The present detection of CRFr immunoreactivity in axon terminals forming asymmetric synapses typical of glutamatergic terminals (Farb et al., 1992) suggests that this receptor mediates at least some of the presynaptic effects of CRF on the release of excitatory transmitters. The proximity of these terminals to apposed CRF-labeled terminals would facilitate local activation that may potently affect glutamate release through multiple intracellular signaling mechanisms (Blank et al., 2003). The vast majority of the neurons in the CeA are GABAergic (Pitkanen and Amaral, 1994; Saha et al., 2000), suggesting that the excitatory-type terminals immunolabeled for the CRF-R1 are extrinsic in origin. These may derive from the midline thalamus (Li et al., 2008) as well as the insular cortex and/or the basolateral amygdala (BLA), all of which provide major glutamatergic input to the CeA (Sun et al., 1994; Quirk et al., 2003).
In contrast to the terminals forming excitatory-type synapses, which were either without CRF or showed faint plasmalemmal and vesicular labeling for CRF, other CRFr-labeled terminals that were nonsynaptic or formed inhibitory-type synapses often contained abundant CRF-immunoreactive vesicles. This distribution is in agreement with the recent in vitro evidence that activation of the amygdala CRF-type 1 receptor inhibits the release of both GABA and CRF (Bagosi et al., 2008). The more limited distribution of CRFr immunoreactivity in axon terminals as compared to dendrites, where the labeling is prominently associated with excitatory-type synapses, is most consistent, however, with physiological evidence that CRF receptors in the CeA are principally involved in modulation of the postsynaptic excitability of CeA neurons (Liu et al., 2004; Daniels et al., 2004).
Implications
The synthesis of CRF in CeA neurons is supported by the expression of CRF mRNA in this region and by the pivotal role of CeA neurons in the CRF circuitry integrating autonomic and behavioral responses to stress (Gray and Magnuson, 1992; Gray, 1993; Gray and Bingaman, 1996). An autoregulatory role for the CRFr1 is strongly supported by the upregulation of CRF mRNA in the hypothalamic regions of CRFr1 (−/−) mice (Lee et al., 2001). Similarly, preexposure to cocaine has been shown to induce long-lasting changes in responsivity of the CRF system in the CeA (Erb et al., 2005). Upon further CRF stimulation, animals preexposed to drugs show increased drug-seeking, elevated levels of c-fos mRNA associated with neuronal activation, and heightened CRF-induced locomotor activity. Prior CRF activation in the CeA appears to prime future CRF signaling, possibly through autoregulation within the CRF system. Many of the CRF neurons in the CeA, like those in the paraventricular hypothalamic nuclei, may co-store GABA and CRF (Meister et al., 1988). The activation of CRF receptors in GABAergic neurons of the CeA may potently affect GABAergic transmission contributing to anxiety disorders (Quirk and Gehlert, 2003; Nie et al., 2004). CRF-expressing neurons are known to participate in a feedback circuit that regulates stress-induced arousal (Winsky-Sommerer et al., 2005). Teasing apart the interplay between the CRF and other functionally related peptides may characterize the specific mechanisms leading to the modulation of stress, arousal, motivation, and addiction.
In addition to the CRF location in peptide-like storage vesicles, CRF immunoreactivity was also seen in a diffuse plasmalemmal or endomembrane distribution in many of the somatodendritic as well as axonal profiles expressing the CRFr. This distribution suggests that CRF bound to CRF receptors in CeA neurons may be internalized in a manner analogous to that seen in pituitary corticotropes (Leroux and Pelletier, 1984; Childs et al., 1986, 1991). Activation of CRF receptors within CeA terminals that express CRF-like peptides and their postsynaptic targets as well as in glutamatergic inputs to the CeA may dynamically affect anxietylike states (Smith et al., 1998; Contarino et al., 1999). These actions could contribute significantly to autonomic withdrawal symptoms associated with addictive diseases (Shaham et al., 1997; Erb et al., 2001).
Acknowledgments
National Institute on Drug Abuse (NIDA); Grant numbers: DA005130 and DA 04600; National Institute of Mental Health (NIMH); Grant number: MH40342; Grant sponsor: National Institutes of Health (NIH); Grant number: HL 18974.
Literature Cited
- Agnati LF, Bjelke B, Fuxe K. Volume transmission in the brain. Am Sci. 2004;80:362–374. [Google Scholar]
- Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Bagosi Z, Jaszberenyi M, Szabo G, Telegdy G. The effects of CRF and the urocortins on [3H]GABA release from the rat amygdala—an in vitro superfusion study. Brain Res Bull. 2008;75:15–17. doi: 10.1016/j.brainresbull.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko PE, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors? An analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Grigoriadis DE, Lorang MT, Baram TZ. Corticotropin-releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of the immature rat brain. Exp Neurol. 2002;176:75–86. doi: 10.1006/exnr.2002.7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocy-tochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1)-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs GV, Morell JL, Niendorf A, Aguilera G. Cytochemical studies of corticotropin-releasing factor (CRF) receptors in anterior lobe corticotropes: binding, glucocorticoid regulation, and endocytosis of [biotinyl-Ser1]CRF. Endocrinology. 1986;119:2129–2142. doi: 10.1210/endo-119-5-2129. [DOI] [PubMed] [Google Scholar]
- Childs GV, Westlund KN, Tibolt RE, Lloyd JM. Hypothalamic regulatory peptides and their receptors: cytochemical studies of their role in regulation at the adenohypophyseal level. J Electron Microsc Tech. 1991;19:21–41. doi: 10.1002/jemt.1060190104. [DOI] [PubMed] [Google Scholar]
- Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci U S A. 2005;102:18649–18654. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale W, Gold LH. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav. 2004;82:751–762. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Connolly KR, Valentino RJ. Corticotropin-releasing factor neurones of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. J Neuroendocrinol. 2002;14:667–682. doi: 10.1046/j.1365-2826.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- Daniels WM, Richter L, Stein DJ. The effects of repeated intraamygdala CRF injections on rat behavior and HPA axis function after stress. Metab Brain Dis. 2004;19:15–23. doi: 10.1023/b:mebr.0000027413.42946.61. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Funk D, Le AD. Cocaine pre-exposure enhances CRF-induced expression of c-fos mRNA in the central nucleus of the amygdala: an effect that parallels the effects of cocaine pre-exposure on CRF-induced locomotor activity. Neurosci Lett. 2005;383:209–214. doi: 10.1016/j.neulet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Farb CR, Aoki C, Milner T, Kaneko T, LeDoux JE. Glutamate immunoreactive terminals in the lateral amygdaloid nucleus: a possible substrate for emotional memory. Brain Res. 1992;593:145–158. doi: 10.1016/0006-8993(92)91303-v. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, Shaw J, Fitz SD, Sajdyk TJ. Stress and central urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol. 2005;509:145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroen-docrine, and behavioral responses to stress. Ann N Y Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Heinrichs SS, Menzaghi FF, Schulteis GG, Koob GG, Stinus LL. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Henry BA, Lightman SL, Lowry CA. Distribution of corticotropin-releasing factor binding protein-immunoreactivity in the rat hypothalamus: association with corticotropin-releasing factor-, urocortin 1- and vimentin-immunoreactive fibres. J Neuroendocrinol. 2005;17:135–144. doi: 10.1111/j.1365-2826.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- Iredale PA, Terwilliger R, Widnell KL, Nestler EJ, Duman RS. Differential regulation of corticotropin-releasing factor1 receptor expression by stress and agonist treatments in brain and cultured cells. Mol Pharmacol. 1996;50:1103–1110. [PubMed] [Google Scholar]
- Iredale PA, Alvaro JD, Lee Y, Terwilliger R, Chen YL, Duman RS. Role of corticotropin-releasing factor receptor-1 in opiate withdrawal. J Neurochem. 2000;74:199–208. doi: 10.1046/j.1471-4159.2000.0740199.x. [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain. 2007;3:13. doi: 10.1186/1744-8069-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasagi Y, Horiba N, Sakai K, Fukuda Y, Suda T. Involvement of cAMP-response element binding protein in corticotropin-releasing factor (CRF)-induced down-regulation of CRF receptor 1 gene expression in rat anterior pituitary cells. J Neuroendocrinol. 2002;14:587–592. doi: 10.1046/j.1365-2826.2002.00816.x. [DOI] [PubMed] [Google Scholar]
- Keck ME, Ohl F, Holsboer F, Muller MB. Listening to mutant mice: a spotlight on the role of CRF/CRF receptor systems in affective disorders. Neurosci Biobehav Rev. 2005;29:867–889. doi: 10.1016/j.neubiorev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Lee S, Smith GW, Vale W, Lee KF, Rivier C. Mice that lack corticotropin-releasing factor (CRF) receptors type 1 show a blunted ACTH response to acute alcohol despite up-regulated constitutive hypothalamic CRF gene expression. Alcohol Clin Exp Res. 2001;25:427–433. [PubMed] [Google Scholar]
- Lee MC, Cahill CM, Vincent JP, Beaudet A. Internalization and trafficking of opioid receptor ligands in rat cortical neurons. Synapse. 2002;43:102–111. doi: 10.1002/syn.10014. [DOI] [PubMed] [Google Scholar]
- Leranth C, Pickel VM. Electron microscopic pre-embedding double immunostaining methods. In: Heimer L, Zaborszky L, editors. Neuroanatomical tract-tracing methods 2: recent progress. 1st. New York: Plenum Publishing; 1989. pp. 129–172. [Google Scholar]
- Leroux P, Pelletier G. Radioautographic study of binding and internalization of corticotropin-releasing factor by rat anterior pituitary corticotrophs. Endocrinology. 1984;114:14–21. doi: 10.1210/endo-114-1-14. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24:4020–409. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M, Turchan J, Smialowska M, Przewlocka B. Morphine and cocaine influence on CRF biosynthesis in the rat central nucleus of amygdala. Neuropeptides. 2003;37:105–110. doi: 10.1016/s0143-4179(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Meister B, Hokfelt T, Geffard M, Oertel W. Glutamic acid decarboxylase- and gamma-aminobutyric acid-like immunoreactivities in corticotropin-releasing factor-containing parvocellular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1988;48:516–526. doi: 10.1159/000125058. [DOI] [PubMed] [Google Scholar]
- Miyata I, Shiota C, Ikeda Y, Oshida Y, Chaki S, Okuyama S, Inagami T. Cloning and characterization of a short variant of the corticotropin-releasing factor receptor subtype from rat amygdala. Biochem Biophys Res Commun. 1999;256:692–696. doi: 10.1006/bbrc.1999.0392. [DOI] [PubMed] [Google Scholar]
- Moga MM, Gray TS. Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J Comp Neurol. 1985;241:275–284. doi: 10.1002/cne.902410304. [DOI] [PubMed] [Google Scholar]
- Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nitecka L, Frotscher M. Cholinergic-GABAergic synaptic interconnections in the rat amygdaloid complex: an electron microscopic double immunostaining study. EXS. 1989;57:42–49. doi: 10.1007/978-3-0348-9138-7_4. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneda C, Winsky-Sommerer R, Boutrel B, De Lecea L. The corticotropin-releasing factor-hypocretin connection: implications in stress response and addiction. Drug News Perspect. 2005;18:250–255. doi: 10.1358/dnp.2005.18.4.908659. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat brain in stereotaxic coordinates. 2nd. San Diego: Academic Press; 1998. [Google Scholar]
- Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. The fine structure of the nervous system. 3rd. New York: Oxford University Press; 1991. [Google Scholar]
- Pitkanen A, Amaral DG. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: an immunohisto-chemical and in situ hybridization study. J Neurosci. 1994;14:2200–2224. doi: 10.1523/JNEUROSCI.14-04-02200.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Sydow S, Spiess J. Characterization of native corticotropin-releasing factor receptor type 1 (CRFR1) in the rat and mouse central nervous system. J Neurosci Res. 1998;54:507–521. doi: 10.1002/(SICI)1097-4547(19981115)54:4<507::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intra-cellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J, Spiess J, Vale W. Characterization of rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci U S A. 1983;80:4851–4855. doi: 10.1073/pnas.80.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Batten TFC, Henderson Z. A gabaergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohis-tochemical study. Neuroscience. 2000;99:613–626. doi: 10.1016/s0306-4522(00)00240-2. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Santibanez M, Gysling K, Forray MI. Adrenalectomy decreases corticotropin-releasing hormone gene expression and increases nor-adrenaline and dopamine extracellular levels in the rat lateral bed nucleus of the stria terminalis. J Neurosci Res. 2005;81:140–152. doi: 10.1002/jnr.20538. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Sun N, Yi H, Cassell MD. Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J Comp Neurol. 1994;340:43–64. doi: 10.1002/cne.903400105. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swinny JD, Kalicharan D, Blaauw EH, IJkema-Paassen J, Shi F, Grams-bergen A, Van der Want JJ. Corticotropin-releasing factor receptor types 1 and 2 are differentially expressed in pre- and post-synaptic elements in the post-natal developing rat cerebellum. Eur J Neurosci. 2003;18:549–562. doi: 10.1046/j.1460-9568.2003.02776.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, De Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, De Lecea L. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 2005;32:285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- Wu JS, Ku YH, Li LS, Lu YC, Ding X, Wang YG. Corticotropin releasing factor and substance P mediate the nucleus amygdaloideus centralis-nucleus ventromedialis-nucleus dorsomedialis pressor system. Brain Res. 1999;842:392–398. doi: 10.1016/s0006-8993(99)01862-4. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther. 2007;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- Zhou P, Qian L, Glickstein SB, Golanov EV, Pickel VM, Reis DJ. Electrical stimulation of cerebellar fastigial nucleus protects rat brain, in vitro, from staurosporine-induced apoptosis. J Neurochem. 2001;79:328–338. doi: 10.1046/j.1471-4159.2001.00585.x. [DOI] [PubMed] [Google Scholar]


