Abstract
Reversal learning has been shown to require intact serotonergic innervation of the forebrain neocortex. Whether dopamine acting through D2 receptors plays a complementary role in this anatomic area is still unclear. Here we show that mice lacking dopamine D2 receptors exhibited significantly impaired performance in the reversal learning phase of an attention-set-shifting task (ASST) and that wild type mice treated chronically with the D2-like receptor antagonist haloperidol exhibited the same cognitive deficit. The test-phase-specific deficits of D2 mutants and haloperidol-treated mice were also accompanied by deficits in the induction of expression of early growth response gene 2 (egr-2), a regulatory transcription factor previously shown to be selectively induced in the ventrolateral orbital frontal cortex and the pre-and infralimbic medial prefrontal cortex of ASST-tested mice. D2-receptor knockout mice and haloperidol-treated wild type, however, exhibited lower egr-2 expression in these anatomic regions after completion of an ASST-test phase that required reversal learning but not after completion of set-shifting phases without rule reversals. In contrast, mice treated chronically with clozapine, an atypical neuroleptic drug with lower D2-receptor affinity and broader pharmacological effects, had deficits in compound discrimination phases of the ASST, but also these deficits were accompanied by lower egr-2 expression in the same anatomic subregions. Thus, the findings indicate that egr-2 expression is a sensitive indicator of test-phase-specific performance in the ASST and that normal function of D2 receptors in subregions of the orbital frontal and the medial prefrontal cortex is required for cognitive flexibility in tests involving rule reversals.
Keywords: dopamine D2 receptor, early growth response gene 2, reversal learning, mouse, orbital frontal cortex, medial prefrontal cortex
Attention is governed by anatomical areas that support three control systems: alerting, orienting, and selecting (Posner and Petersen, 1990). These functions are modulated by different neurotransmitters: Cholinergic systems originating in the basal forebrain play an important role in orienting, the norepinephrine system originating in the locus coeruleus plays a role in alerting, and the mesocortical dopamine system targets prefrontal cortical and anterior cingulate (AC) areas involved in executive control (Posner and Petersen, 1990; Sarter et al., 2001; Raz and Buhle, 2006). Moreover, even within different attention-set-shifting paradigm phases that tax different domains of attentional control function, different neurotransmitter systems exert different effects. Depletion of prefrontal cortical dopamine disrupts the formation of attentional sets and depletion of norepinephrine affects set-shifting, but acetylcholine depletion has no effect on either function (Robbins and Roberts, 2007). Furthermore, intact serotonergic innervation of the forebrain neocortex is essential for cognitive flexibility in tests involving rule reversals. For example, in visual discrimination reversal tasks, depletion of 5-HT in the orbital frontal cortex (OFC) and medial prefrontal cortex (mPFC) of marmoset monkeys elicited perseverative responding to the previously rewarded stimulus (Clarke et al., 2004), but extradimensional set-shifting was unimpaired (Clarke et al., 2005). This effect was 5-HT-specific and not detected after depletion of dopamine in the OFC (Clarke et al., 2007), a region thought to be critical for reversing response selections (Rolls et al., 1994; Hornak et al., 2004). Nevertheless, studies on human, monkey and rodents have also implicated a role of dopamine, and in particular the dopamine D2 receptor, in reversal learning (Ridley et al., 1981; Mehta et al., 2001; Kruzich and Grandy, 2004; Lee et al., 2007). Although it is argued that this effect may be mediated at the level of the striatum (Dodds et al. (2008), there is also evidence for a role of orbital frontal dopamine D1 and D2 receptors in guiding instrumental behavior of the rat under reversal conditions (Calaminus and Hauber, 2008), and of OFC and mPFC D1 and D2 receptors in a operant task of behavioral flexibility (Winter et al., 2009). Moreover, a recent study suggests that not only striatal but also cortical D2 receptors are critical for reversal learning in human since carriers of the A1 allele of the D2 receptor gene (that leads to reduced D2 receptor expression) had reversal learning deficits along with task-related impaired recruitment of the right ventral striatum and the right OFC (Jocham et al., 2009).
In view of these new findings, we examined whether normal expression of D2 receptors is also required for optimal neuronal activation in the mPFC and OFC during a reversal learning phase of an attention-set-shifting task (ASST) designed for rodents. In the present study, we analyzed the expression of early growth response gene 2 (egr-2), a rapidly inducible transcription factor (Herdegen and Leah, 1998; O’Donovan et al., 1999), that has recently been shown to be robustly downregulated in the cortex of subjects with autism and Rett syndrome, i.e. disorders with impaired executive functioning (Swanberg et al., 2009). In a previous study (DeSteno and Schmauss, 2008) we have shown that the expression of egr-2 is induced in the OFC and mPFC of mice performing the ASST, and that the magnitude of egr-2 induction correlates with the magnitude of cognitive control. Moreover, different ASST phases led to egr-2 induction in different anatomic subregions: in the ventrolateral orbital frontal cortex (vlOFC) and in prelimbic (PrL) and infralimbic (IL) subregions of the mPFC, task-evoked egr-2 induction occurred after completion of the compound discrimination (CD) phases of the ASST and, in the IL, further egr-2 induction occurred during set-shifting and/or reversal learning phases (DeSteno and Schmauss, 2008).
Here we used mice lacking D2 receptors and wild type mice treated chronically with the D2-like receptor blocker haloperidol to examine whether their deficits in the reversal phase of the ASST are also accompanied by decreased egr-2 expression in these anatomic subregions.
EXPERIMENTAL PROCEDURES
Animals
All procedures involving animals were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Columbia University and the New York State Psychiatric Institute. During the course of the study, the number of animals was kept to a minimum to ensure statistical validity and no procedures were performed that caused pain or severe discomfort. Adult male congenic C57BL/6J mice lacking D2 receptors (Jung et al., 1999) and their wild type littermates (postnatal ages 60–90 days) were used in this study. These congenic lines resulted from 20 back-crosses, and lines of wild type and lines of homozygous mutants were maintained by breeding wild type to wild type and mutant to mutant. All animals were group-housed in a standard animal care facility with a 12-h light/dark cycle (lights on at 6:00 AM) where they had free access to food and water.
Drug administration
All drugs were administered via the drinking for 24 days. Haloperidol and clozapine were purchased from Sigma (St. Louis, MO, USA). Stock solutions of 1 or 5 mg/ml were prepared in 100% ethanol and diluted in 100 ml of water. During the treatment period, the body weights of the animals and the amount of drinking water consumed were monitored daily. These measurements revealed that, within a 24-h period, mice received 0.2 mg/kg of haloperidol and 0.5 mg/kg of clozapine. At the concentrations administered, none of the drugs elicited obvious motor effects.
ASST
The ASST is the rodent version of the Wisconsin Card Sorting Task in which animals learn to discriminate between two perceptual dimensions, odor and texture (Birrell and Brown, 2000). In the ASST, animals complete a series of CDs in which one of the two stimulus dimensions is associated with the food reward. In our experiments, the ASST is composed of five discrimination phases performed in the order of simple discrimination (SD), CD, intradimensional shift of attention (IDS), extradimensional shift of attention (EDS) and reversal of the EDS (EDS-Rev), and animals complete the entire ASST in a single test session (DeSteno and Schmauss, 2008).
Briefly, two terra cotta pots were placed adjacent to each other in the test box. In these pots, food pellets were deeply buried under bedding media. Odor and bedding media employed in this study are specified in Glickstein et al. (2005). After a 30-min habituation to the test chamber, mice were first trained in an SD of either two odors or two different textures of digging media to a criterion of six consecutive correct trials. The use of odor or texture as the relevant stimulus dimension was randomized. After successful completion of the SD, mice performed the series of discriminations described by Birrell and Brown (2000). Hence, after an SD between two odors or two digging media, a CD followed in which a new dimension that was not a reliable indicator of the food location was added to the stimuli presented in the initial SD. In the IDS, mice have to maintain an attentional set related to the same perceptual dimension that guided correct response selection in the SD and CD, but they must respond to new stimulus properties. In the EDS, mice must shift attention to the previously irrelevant perceptual dimension. In the EDS-Rev phase, the relevant stimulus property of the previous EDS becomes irrelevant and the previously irrelevant stimulus property guides correct response selection. The different phases of the ASST tax different attentional functions: associative learning (SD, CD), set-shifting (IDS, EDS), and reversal learning (EDS-Rev).
Prior to ASST testing, food availability was restricted so that the body weights of the animals were gradually reduced (over a period of 7–10 days) to 85% of their starting weight. Body weights were measured daily (which also served as a habituation period to daily handling prior to testing). Control animals were also food-restricted and received either drug-containing water or regular drinking water. Additional control animals were also exposed to odors but not tested. For these animals, the types of odor and the time of odor exposure were identical to the corresponding exposure of an ASST-tested animal. ASST testing was conducted between 1:00 and 4:00 PM. In each phase of the test, animals had to reach a criterion of six consecutive correct trials. In contrast to our previous study (Glickstein et al., 2005), in the present study only animals that reach criterion in the SD and CD within 25 min were selected for further testing. This eliminated animals with poor SD and CD performance and resulted in a large percentage of animals that were able to finish the entire test that ended with the reversal learning phase. The number of trials to criterion and the mean response latencies per trial were computed and compared by repeated measures ANOVA (threshold of significance, α = 0.05). Significant differences were resolved post hoc using the Tukey–Kramer multiple comparisons test.
Egr-2 mRNA expression
For these experiments, animals were killed by rapid decapitation and their brains were removed. To ensure reproducible sectioning, mouse brains were placed onto an acrylic brain matrix (Stoelting, Wood Dale, IL, USA) that allows coronal sectioning at 1 mm intervals. RNA was extracted from the frontal 5-mm brain sections, and cDNA was synthesized from 5 μg of total RNA using Moloney murine leukemia virus reverse transcriptase (USB, Cleveland, OH, USA). In real-time PCR experiments, performed using the iQ5 Thermal Cycler (Bio-Rad, Hercules, CA, USA) in conjunction with iQSYBRgreen as previously described (Bhansali et al., 2007), egr-2 cDNA was amplified using the primer pair 5′-ATGAACGGAGTGGCGGGA-3′/5′-AGTAGAGGTGGTCCAGTT-3′, which directs amplification of a 346 bp fragment of mouse egr-2 (nucleotides 393–738). In these experiments, measurements were made of the number of cycles required to reach threshold fluorescence intensity (cycle threshold, ct). ct Values for each reaction were normalized to those obtained for amplifications of β-actin cDNA. Differences between normalized ct values were determined using the 2ΔΔCt method, and the results were expressed as fold change over baseline.
Immunocytochemistry and stereology
One hour after completion of the ASST-test phase under study or 1 h after odor exposure alone, mice were deeply anesthetized with ketamine (100 mg/kg) and xylazine (15 mg/kg) and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were postfixed for 1 h (in the same fixative) and cryoprotected overnight (30% sucrose in 0.1 M phosphate buffer).
Egr-2 protein expression was analyzed using immunocytochemical and two-dimensional stereological methods as previously described (DeSteno and Schmauss, 2008). Briefly, a series of coronal 40-μm-thick microtome sections, collected in 200 μm intervals, was incubated overnight with a rabbit polyclonal antibody directed against egr-2 (Covance, Berkeley, CA, USA, 1:1000). After incubation with primary antibody, sections were incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA, 1:400), followed by incubation in avidin–biotin–peroxidase complex (Vectastain Elite Kit; 1:100 in phosphate buffered saline (PB); Vector Laboratories). To visualize bound immunoperoxidase, sections were incubated in 0.022% 3,3′-diaminobenzidine (Aldrich, Milwaukee, WI, USA) and 0.003% hydrogen peroxide in PB. All sections were rinsed in PB and mounted onto gelatin-coated slides. In all experiments, sections obtained from ASST-tested mice were processed in parallel with their respective controls. A representative example of immunolabeled sections processed in parallel is shown in Fig. 1.
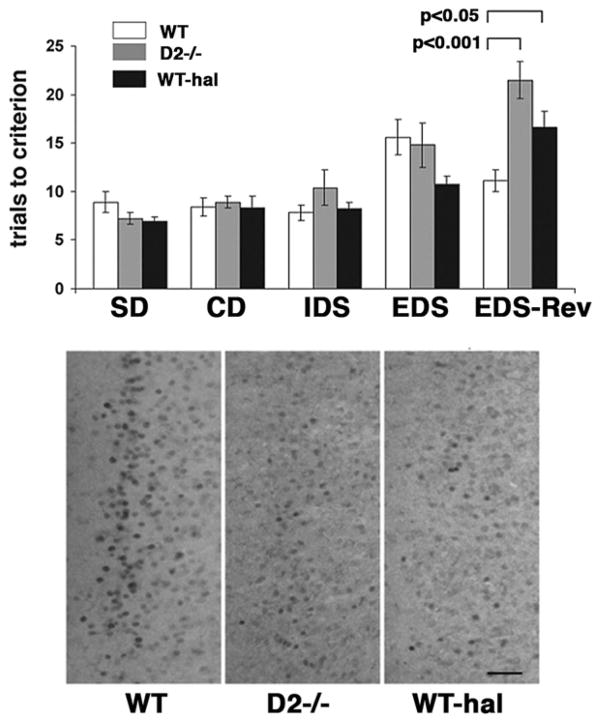
Fig. 1.
ASST performance of wild type (WT), D2 mutants (D2−/−) and haloperidol (hal)-treated wild type. Top: The individual test phases of the ASST are indicated. For each test phase, data are means ± SEM of trials to criterion obtained from 11 animals per genotype or treatment group. Statistical differences revealed by ANOVA were resolved post hoc (Tukey–Kramer multiple comparisons test) as indicated. Despite differences in response accuracy, there were no differences in response latencies between the three groups of animals (SD: 1.03 ± 0.06 min (WT), 0.88 ± 0.06 min (D2−/−), 1.06 ± 0.13 min (hal); CD: 0.87 ± 0.07 min (WT), 0.77 ± 0.08 min (D2−/−), 1.08 ± 0.06 min (hal); IDS: 1.25 ± 0.07 min (WT), 0.97 ± 0.22 min (D2−/−), 1.29 ± 0.04 min (hal); EDS: 1.35 ± 0.07 min (WT), 0.97 ± 0.33 min (D2−/−), 1.57 ± 0.22 min (hal); EDS-Rev: 1.13 ± 0.09 min (WT), 1.00 ± 0.09 min (D2−/−), 1.24 ± 0.26 min (hal)). Bottom: Representative examples of egr-2-labeled sections comprising the IL subregion of the mPFC of WT, D2 mutants (D2−/−) and haloperidol-treated wild type (WT-hal) after EDS-Rev testing. Scale bar = 0.05 mm.
Quantitative estimates of the numbers of egr-2-labeled nuclei in the mPFC and OFC were obtained using a 2D stereological counting method as previously described (DeSteno and Schmauss, 2008). Briefly, sections were viewed at 20× magnification using a Zeiss Axioskop 2 microscope (Oberkochen, Germany) interfaced with an Apple computer and Improvision Open-lab 3.1 software (Coventry, UK). The total number of egr-2-labeled nuclei was determined in three subregions of the mPFC (IL, PrL, and AC) and in the medial and vlOFC. Counting grids were created using the coordinates from the mouse brain atlas (Hof et al., 2000). Counting grid dimensions (0.08×0.3 mm for layers II/III of AC, 0.08×0.4 mm for layers II/III of IL or PrL, 0.15×0.3 mm for the medial orbital frontal cortex (mOFC), and 0.3×0.3 mm for the vlOFC) were selected to ensure adequate coverage of the areas of egr-2 expression at baseline. Measurements were taken from one (medial and vlOFC) or three (mPFC) sections from the series, beginning at bregma 2.0 and extending caudally in 200 μm intervals. All cells with clearly detectable nuclear expression of egr-2 immunoreactivity were counted regardless of relative differences in the intensity of egr-2 immunoreactivity. Cell counts were normalized by the area counted and expressed as number of cells/mm2 (density). Thus, quantitative differences in egr-2 expression reported here reflect differences in the number of cells expressing egr-2. Results were either compared by one-way ANOVA (threshold of significance, α = 0.05) and statistical differences were resolved post hoc using Tukey–Kramer multiple comparisons test or, when only two groups were compared, by two-tailed t-tests.
RESULTS
ASST performance of D2 mutants and haloperidolor clozapine-treated wild type
Fig. 1 illustrates the response accuracy (number of trials to criterion) of wild type, D2 mutants, and haloperidol-treated wild type. In wild type mice, a repeated measures ANOVA revealed significant differences when the number of trials to criterion was compared between test phases (F(4,40) = 3.276; P = 0.02). A post hoc Tukey–Kramer multiple comparisons test revealed that the number of trials to criterion needed to complete the EDS phase was significantly higher than that needed for either SD or IDS phases (P<0.05). Such differences were also evident in D2 mutants (F(4,40) = 11,526; P<0.0001). In these animals, the number of trials to criterion was significantly higher in the EDS phase compared with the SD phase (P<0.05), but the number of trials to criterion in the EDS-Rev phase was also significantly higher compared with either the SD, CD or IDS phases (P<0.05). In mice treated chronically with haloperidol (F(4,40) = 11.126; P<0.0001), the number of trials to criterion in the EDS phase was also higher compared with the SD phase. While this difference was only marginally significant (P = 0.05), the number of trials to criterion in the EDS-Rev phase was significantly higher than corresponding numbers for SD, CD, IDS, or EDS phases (P<0.05).
A further comparison between the three groups of mice (ANOVA; F(2,30) = 10.083; P = 0.0004) revealed that the number of trials to criterion of wild type, D2 mutants and haloperidol-treated wild type differed only for the EDS-Rev phase. In this phase, both D2 mutants and haloperidol-treated wild type exhibited significantly lower response accuracies relative to wild type (wild type versus D2 mutants: P<0.001, wild type versus haloperidol-treated wild type: P<0.05; D2 mutants versus haloperidol treated wild type: ns) (Fig. 1). Since the mean response latencies (time between release from the holding box and digging for food) in the different test phases were not significantly different between wild type, D2 mutants, and haloperidol-treated wild type (see legend to Fig. 1), the deficit in the reversal phase of the ASST is unlikely due to increased error rates made by increasingly satiated D2 mutants or drug-treated wild type.
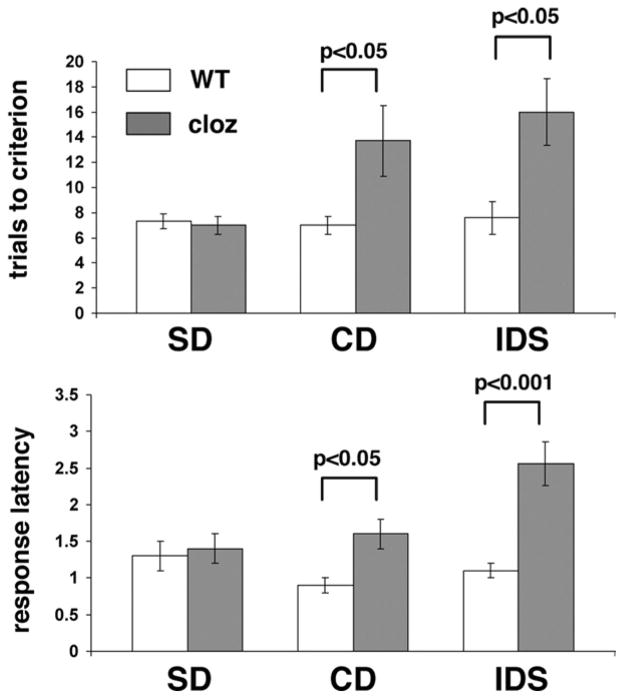
Haloperidol is a prototypical example of typical neuroleptic drugs that predominantly block D2-like receptors. In contrast, the newer generation of atypical neuroleptics has lower affinity for D2 receptors and broader pharmacological actions affecting also serotonergic and cholinergic neurotransmission (Seeman et al., 1997; Reynold, 2004). We therefore tested whether treatment with a prominent representative of the class of atypical neuroleptics would also affect reversal learning. Thus, we treated wild type mice chronically with the atypical neuroleptic drug clozapine (0.5 mg/kg/day for 24 days in drinking water). Unlike haloperidol-treated wild type, clozapine-treated mice already exhibited deficits in the first two compound discrimination phases of the ASST (CD, IDS), only five of nine animals were able to complete the IDS, and none of these animals completed the EDS. A repeated measures ANOVA on test phases (F(2,12) = 7.427; P = 0.008) revealed that the numbers of trials to criterion of clozapine-treated mice were significantly higher for the IDS phase when compared to the SD phase (P<0.05) (Fig. 2). Moreover, a comparison between wild type and clozapine-treated animals revealed significantly lower response accuracy of clozapine-treated mice in the CD (P<0.05) and IDS phase (P<0.05) of the ASST (Fig. 2).
Fig. 2.
Comparison of the performance of non-treated wild type (WT) and clozapine (cloz)-treated wild type in the ASST. Trials to criterion and mean response latencies (in minutes) per trial and test phase are shown for the first three test phases (SD, CD, IDS) that clozapine-treated animals were able to complete. Data represent means ± SEM of determinations made from seven animals per treatment group with the exception of IDS-tested clozapine treated mice where n = 5. Statistical differences revealed by ANOVA were resolved post hoc (Tukey–Kramer multiple comparisons) as indicated.
Clozapine-treated mice also exhibited prolonged response latencies in the ASST (repeated measures ANOVA; F(2,12) = 8.785; P = 0.045). Their response latencies in the IDS phase of the ASST were significantly longer than corresponding measures of SD- (P<0.001) and CD-tested animals (P<0.01). Moreover, compared with wild type, clozapine-treated mice had significantly longer response latencies in the CD (P<0.05) and IDS phases (P<0.001) of the ASST (Fig. 2).
In summary, mice lacking dopamine D2 receptors, like mice treated chronically with the D2-receptor antagonist, have reversal learning deficits in the ASST. In contrast to haloperidol, chronic treatment with the atypical neuroleptic drug clozapine led to a different behavioral phenotype with impaired set-shifting performance and prolonged response latencies in all compound discrimination phases, a phenotype that did not permit testing of reversal learning deficits with the present test paradigm.
Egr-2 expression in wild type, D2 mutants, and haloperidol- and clozapine-treated wild type
We have previously shown that ASST-exposure leads to induction of egr-2 expression in the OFC and mPFC (DeSteno and Schmauss, 2008). We therefore tested whether the decrease in ASST performance of D2 mutants and haloperidol-treated wild type during the reversal phase was also accompanied by decreased egr-2 expression in these anatomic regions.
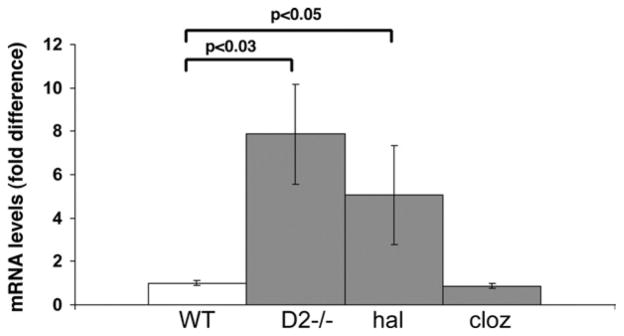
Since it has also been shown that D2-receptor blockade by haloperidol led to increased egr-2 expression levels in the striatum (McGibbon et al., 1995), we first compared basal egr-2 mRNA levels in the forebrain of wild-type, D2-mutants, and haloperidol-treated wild type. As shown in Fig. 3, both D2 mutants and haloperidol-treated wild type had increased basal erg-2 mRNA levels compared with wild type.
Fig. 3.
Comparison of basal egr-2 mRNA levels in the forebrain of non-tested wild type (WT), D2 mutants (D2−/−) and haloperidol (hal)- and clozapine (cloz)-treated wild type. Data represent means ± SEM of determinations made from five animals per group and are expressed as fold difference relative to non-treated wild type. Statistical differences were determined using two-tailed t-tests (with Welch correction).
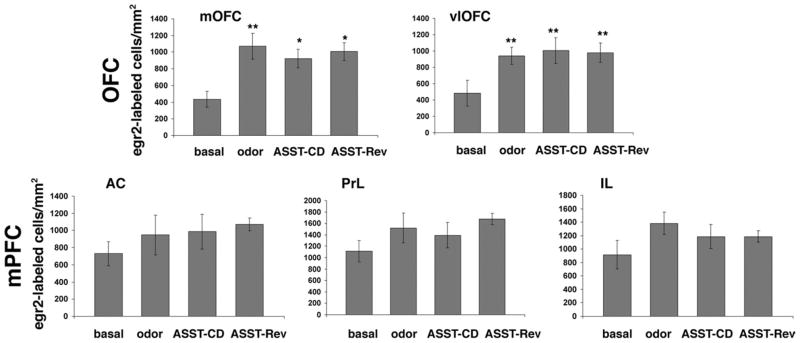
We then performed stereological counts of egr-2-labeled nuclei in the OFC and mPFC of D2 mutants at baseline and after completion of either the CD phase of the ASST or the entire test ending with the EDS-Rev phase. Moreover, since our previous study showed that, in wild type mice, odor-exposure alone led to robust egr-2 induction in the mOFC and the AC subregion of the mPFC (DeSteno and Schmauss, 2008), we also tested whether odor exposure alone leads to egr-2-induction in D2 mutants. Indeed, as shown in Fig. 4, when D2 mutants were exposed to odor (but not tested), egr-2 expression was significantly increased in the mOFC (ANOVA, F(3,17) = 4.643; P<0.02). In the vlOFC of D2 mutants, odor exposure also led to increased densities of egr-2-labeled cells. This difference, however, did not reach significance in the ANOVA (P = 0.08). Hence, in the OFC, D2 mutants exhibited increased egr-2 expression in response to odor exposure alone. However, in contrast to results obtained with wild type (DeSteno and Schmauss, 2008), no further induction of egr-2 expression occurred in ASST-tested D2 mutants. In fact, in both mOFC and vlOFC, the density of egr-2-labeled cells did not differ significantly between ASST-tested mice and odor-exposed controls.
Fig. 4.
Densities of egr-2-labeled cells in the OFC and mPFC of D2 mutants at baseline, after odor exposure alone, or after completion of the CD phase or the EDS-Rev phase of the ASST. Data represent means ± SEM of determinations made from seven (baseline), five (odor controls), seven (CD-tested) and six (EDS-Rev-tested) animals. For the mOFC, statistical differences revealed by one-way ANOVA were resolved post hoc (Tukey–Kramer multiple comparisons) with * P<0.05 and ** P<0.001 compared with the corresponding basal measures. For the vlOFC, statistical differences revealed by two-tailed Student’s t-test are indicated with ** P<0.04 compared with basal.
In the mPFC of D2 mutants, odor-exposure alone also increased egr-2 expression in all three subregions (AC, PrL, IL), but no increase reached significance (Fig. 4). Importantly, however, the density of egr-2-labeled nuclei in ASST-tested D2 mutants did not differ from corresponding densities measured after odor exposure alone (Fig. 4). Thus, although odor-evoked egr-2 expression was intact in D2 mutants, no test-evoked induction was observed in either the OFC or in the mPFC. Nevertheless, D2 mutants proceeded through the CD and set-shifting phases of the ASST just like wild type mice (Fig. 1). To test whether their increased basal levels of egr-2 are comparable to those achieved in ASST-tested wild type, and since our previous stereological studies (using the optical fractionator) revealed no differences in the regional neocortical volumes as well as number of neurons and glia between these (congenic) mutants and their wild type littermates (Glickstein et al., 2005), we compared the density of egr-2-labeled nuclei between ASST-tested wild type and D2 mutants.
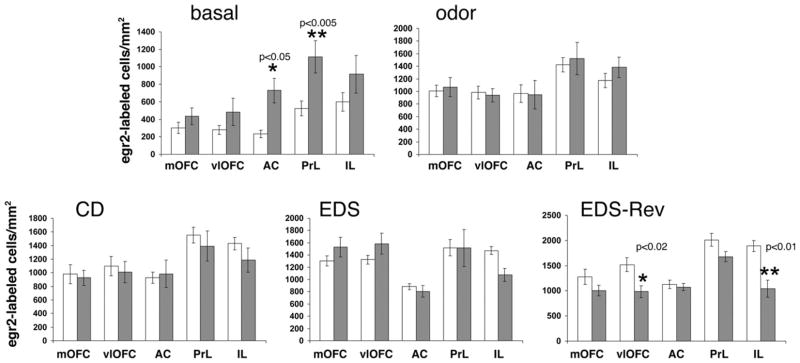
We first compared basal egr-2 expression between the two genotypes. As shown in Fig. 5 and consistent with the results shown in Fig. 3, D2 mutants exhibited higher basal egr-2 expression in all five anatomic subregions studied here. This increase was significant for the AC (two-tailed t-test; P<0.05) and PrL (two-tailed t-test; P<0.005) subregions of the mPFC. As expected from results shown in Fig. 4, when both genotypes were exposed to odor (but not tested), no difference in the densities of egr-2-labeled nuclei was detected. Moreover, the densities of egr-2-labeled nuclei of CD- and EDS-tested wild type and D2 mutants did also not differ significantly. However, in EDS-Rev-tested D2 mutants, the densities of egr-2-expressing cells were lower in all five subregions relative to wild type, and these differences were significant in the vlOFC (two-tailed t-test; P<0.02) and the IL subregion (two-tailed t-test; P<0.005) of the mPFC (Fig. 5).
Fig. 5.
Comparison of the densities of egr-2-labeled cells in the OFC and mPFC of wild type and D2 mutants at baseline, after odor exposure alone, and after completion of the CD, EDS, or EDS-Rev phases of the ASST. White bars: wild type. Gray bars: D2 mutants. The individual subregions of the OFC (mOFC, vlOFC) and the mPFC (AC, PrL, IL (layers II/III)) are indicated. Data are means ± SEM of determinations made from five to seven animals per group and genotype. egr-2 Densities for D2 mutants at baseline, after odor exposure, and after CD- and EDS-Rev-testing are those also shown in Fig. 4. The P-values indicated reflect the differences between measures of D2 mutants and wild type in the same anatomic region under the same test condition (two-tailed t-tests).
Additional measures of the densities of egr-2-labeled nuclei in haloperidol-treated wild type yielded results similar to those obtained from D2 mutants. Table 1 compares the results obtained from wild type that are graphically illustrated in Fig. 5 to corresponding results obtained from haloperidol-treated wild type. Like D2 mutants, haloperidol-treated wild type had significantly higher basal egr-2 expression in the AC and PrL subregions of the mPFC (two-tailed t-test; P<0.0001 and P<0.04, respectively) (Table 1). As further shown in Table 2, they also exhibited significantly lower egr-2 expression in the vlOFC and IL subregion of the mPFC after ASST-Rev-testing (two-tailed t-test; P<0.02 and P<0.0004, respectively). In addition, lower egr-2 expression was also detected in the PrL sub-region of the mPFC (two-tailed t-test; P<0.02) of haloperidol-treated, ASST-Rev-tested wild type (Table 2).
Table 1.
Densities of egr-2-labeled cells/mm2 in wild type, and haloperidol (hal)-or clozapine (cloz)-treated wild type at baselinea
| Wild type | hal | cloz | |
|---|---|---|---|
| mOFC | 300.0 ± 63.9 | 666.6 ± 54.4 | 416.5 ± 32.0 |
| vlOFC | 279.0 ± 51.1 | 597.8 ± 54.7 | 389.0 ± 64.7 |
| AC | 231.7 ± 43.5 | 677.4 ± 58.9*** | 196.3 ± 18.5 |
| PrL | 525.0 ± 84.4 | 882.0 ± 118.3** | 305.8 ± 24.8 |
| IL | 599.0 ± 105.9 | 622.2 ± 73.1 | 305.5 ± 48.3 |
Means ± SEM of determinations made from five animals per group, genotype, and treatment. Between the three groups of mice, there is a significant effect of treatment in the AC (ANOVA; F(2,19) = 16.35; P<0.0001) and PrL subregion of the mPFC (ANOVA; F(2,19) = 6.717). Results of post hoc Tukey–Kramer multiple comparisons tests are indicated:
P<0.001 and
P<0.01 compared with wild type.
Table 2.
Densities of egr-2-labeled cells/mm2 in EDS-Rev-tested wild type and haloperidol (hal)-treated wild typea
| Wild type | hal | |
|---|---|---|
| mOFC | 1275.0 ± 150.7 | 1039.8 ± 67.2 |
| vlOFC | 1519.3 ± 141.2 | 994.6 ± 98.5* |
| AC | 1125.3 ± 83.45 | 917.6 ± 59.4 |
| PrL | 2007.7 ± 127.5 | 1452.4 ± 140.2* |
| IL | 1892.2 ± 110.7 | 1071.8 ± 89.6** |
Means ± SEM of determinations made from five animals per group, genotype, and treatment. A comparison between wild type, D2 mutants, and haloperidol-treated wild type by one-way ANOVA revealed significant differences in the vlOFC (F(2,16) = 6.53; P = 0.0008); PrL (F(2,17) = 4.867; P = 0.023) and IL (F(2,17) = 19.6; P<0.001). Results of post hoc Tukey–Kramer multiple comparisons tests are indicated:
P<0.001 and
P<0.05 compared with wild type.
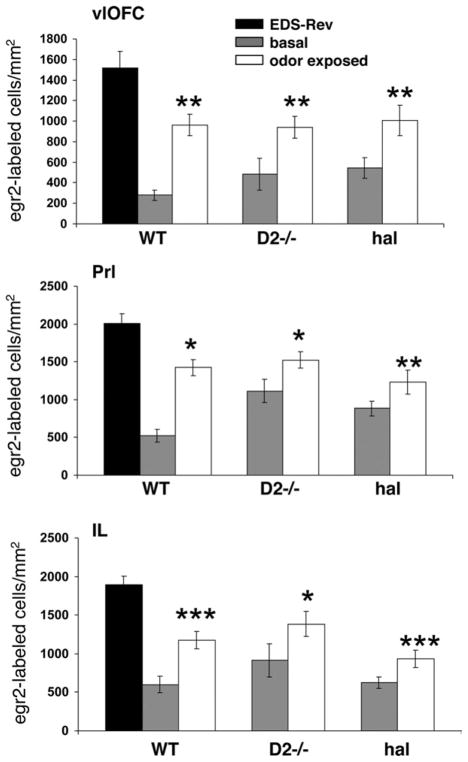
The present data indicate that chronic inactivation of dopamine D2 receptors and the resulting deficit in the EDS-Rev phase of the ASST are also accompanied by lower egr-2 expression in the IL/PrL subregions of the mPFC and vlOFC after completion of the EDS-Rev. Fig. 6 further illustrates that the lack of task-evoked egr-2 induction in these subregions is not due to a ceiling effect achieved with the higher basal or odor-evoked egr-2 expression in D2 mutants and haloperidol-treated wild type. In fact, compared with the densities of egr-2-expressing cells in EDS-Rev-tested wild type, the densities of egr2- labeled cells at baseline and after odor exposure of D2 mutants and haloperidol-treated wild type are still significantly lower in the vlOFC (ANOVA, F(3,22) = 10.5; P = 0.0002), and IL (ANOVA, F(3,22) = 19.69; P<0.0001) and PrL (ANOVA, F(3,22) = 9.44; P = 0.0003) subregions of the mPFC (Fig. 6).
Fig. 6.
Densities of egr-2-labeled cells in the vlOFC and IL/PrL subregions of the mPFC in EDS-Rev-tested wild type compared with the densities of egr-2-labeled cells in these regions in D2 mutants and haloperidol-treated wild type at baseline and after odor exposure. Data are means ± SEM of determinations made from five to seven animals per group. Statistical differences revealed by ANOVA were resolved post hoc (Tukey–Kramer multiple comparisons) as indicated. * P<0.05, ** P<0.01, ***P<0.001.
Or finding of reduced egr-2 expression detected only after completion of a test phase in which mice exhibited deficits suggests that egr-2 expression levels are critical for optimal ASST performance. To further investigate the relationship between test-phase-specific deficits in the ASST and egr-2 expression levels in the OFC and mPFC, we also measured the densities of egr-2-labeled nuclei in clozapine-treated mice that had deficits in the CD and IDS phases of the ASST (Fig. 2) and normal basal egr-2 expression levels (Fig. 3, Table 1). Table 3 compares the results obtained from CD-tested wild type to corresponding results obtained from clozapine-treated wild type. In contrast to D2 mutants and haloperidol-treated wild type, the density of egr-2-labeled nuclei was already lower in clozapine-treated wild type that completed the CD-phase of the ASST, and this decrease was significant in all anatomic regions implicated in task-evoked egr-2 induction, namely the vlOFC and PrL/IL subregions of the mPFC (see Table 3). This further supports the hypothesis that egr-2 expression levels in these three subregions are critical for optimal ASST performance regardless of which test phase is being investigated.
Table 3.
Densities of egr-2-labeled cells/mm2 in CD-tested wild type and clozapine (cloz)-treated wild typea
| Wild type | cloz | |
|---|---|---|
| mOFC | 1083.8 ± 103.5 | 928.6 ± 110.7 |
| vlOFC | 1202.3 ± 106.4 | 793.4 ± 48.6* |
| AC | 986.5 ± 64.5 | 888.2 ± 87.6 |
| PrL | 1641.5 ± 84.7 | 1227.4 ± 34.9** |
| IL | 1498.7 ± 70.1 | 1029.8 ± 61.4*** |
Means ± SEM of determinations made from five animals per group. Statistical differences (two-tailed t-tests) are indicated:
P<0.02;
P<0.007;
P<0.001.
DISCUSSION
The present study illustrates that knockout mice lacking dopamine D2 receptors and wild type mice treated chronically with the D2-like receptor blocker haloperidol have a deficit in the reversal phase of the ASST. In addition, both groups of mice exhibited decreased test-evoked expression of the transcription factor egr-2 in the vlOFC and IL/PrL subregions of the mPFC.
We show here that the reversal learning deficits of D2 mutants, their higher basal levels of egr-2 expression, and their decreased egr-2 expression levels after completion of the reversal learning phase of the ASST are precisely mimicked in wild type mice treated chronically with the D2-like receptor blocker haloperidol. This supports the conclusion that the inactivation of D2 receptors (rather then other compensatory changes in brains of D2 mutants) underlies the observed phenotypes. Moreover, haloperidol was administered via the drinking water for 24 days, i.e. a route of administration that is devoid of stressful environmental influences and that eliminates behavioral consequences due to daily handling. In addition, chronic administration of neuroleptic drugs via the drinking water has been shown to yield and maintain steady state D2-receptor occupancy levels in rodents that are similar to those achieved in humans, in particular for haloperidol (Perez-Costas et al., 2008). Hence, the dose of haloperidol (0.2 mg/kg/day) is predicted to lead to a D2-receptor occupancy of ~77% (Perez-Costas et al., 2008). Indeed, the present study shows that chronic treatment with this dose of haloperidol elicited an effect on ASST performance that mimics the effect of a D2-receptor knockout.
We have previously reported that D2 mutants also have deficits in the CD phase of the ASST (Glickstein et al., 2005). Thus, since we were particularly interested in studying the role of D2 receptors in reversal learning, in the present study we introduced two important changes to our design of the ASST paradigm. First, we selected for this study only mice that completed the CD phase of the ASST within 25 min. This omitted mice with deficits in the associative learning phase. Second, in contrast to our previous study in which an IDS reversal was placed prior to the EDS phase (Glickstein et al., 2005) we now placed the reversal phase of the ASST after the EDS phase so that deficits in set-shifting and reversal learning could be more clearly distinguished from one another. These changes not only allowed us to selectively examine set-shifting and reversal learning abilities of D2 mutants with no potential carryover effect of significant deficits in the early CD phase, but also uncovered a reversal learning deficit that was not apparent prior to the establishment of extradimensional sets (Glickstein et al., 2005).
It should also be stressed that the D2-mutant phenotype described here is not due to impaired processing of (or responding to) olfactory information. Like wild type mice, D2 mutants exhibited robust egr-2 induction in the OFC (the secondary olfactory association cortex; Rolls and Baylis, 1994) in response to odor alone. However, although no test-evoked egr-2 induction was detected in these mutants, either in the OFC or the mPFC, they proceeded through the CD and set-shifting phases of the ASST in a manner indistinguishable from wild type. Yet, D2 mutants express higher levels of egr-2 at baseline, and a comparison between egr-2 expression of ASST-tested wild type and D2 mutants revealed similar densities of egr-2-labeled cells after odor exposure alone and after CD and EDS test-phase completion. However, the density of egr-2-labeled cells of EDS-Rev-tested animals was significantly lower in D2 mutants with reversal learning deficits, and this difference was largest in the vlOFC and the IL subregion of the OFC, i.e. subregions with truly task-evoked (and not just odor-evoked) egr-2 induction (DeSteno and Schmauss, 2008). Importantly, the most salient findings obtained from D2 mutants, namely higher basal egr-2 expression and lower egr-2 expression after EDS-Rev testing, were also found in mice treated chronically with haloperidol.
Unlike the effect of chronic D2 receptor inactivation on reversal learning, set-shifting was unimpaired in D2 mutants and haloperidol-treated wild type. This contrasts with the results of Floresco et al. (2006) who found set-shifting impairments in rats after an acute infusion of a D2-receptor antagonist into the mPFC. Thus, while there may be an effect of acute D2 receptor blockade on flexible response selection, the clinically more relevant scenario of compromised D2 receptor expression (due to the A1 genetic polymorphism of the D2 gene) or function (due to chronic neuroleptic drug treatment) does not recapitulate the effect observed after acute drug administration.
One pharmacological property shared by all neuroleptic drugs is their ability to block dopamine D2 receptors. However, atypical neuroleptics such as clozapine have lower affinity for the D2 receptor and thus, they are thought to be more readily displaced from the receptor by endogenous dopamine (Seeman et al., 1997; Reynold, 2004). We show here that, unlike chronic treatment with haloperidol, chronic treatment with clozapine did not mimic the D2-mutant phenotype but resulted in significantly decreased response accuracy in the early CD phases of the ASST along with significantly prolonged response latencies in these test phases. This effect is likely due to the anticholinergic property of the drug since a similar phenotype was also observed in mice treated chronically with the M1/M2 muscarinic acetylcholine receptor antagonist scopolamine (D.A.D. and C.S., unpublished observations). Importantly, however, clozapine-treated mice that had normal basal egr-2 expression levels failed to exhibit increased egr-2 expression after completion of the CD phase of the ASST. Thus, although the deficits of clozapine-treated mice in the early ASST test phases did not allow us to test whether chronic clozapine treatment also results in reversal learning deficits, the test-phase specific deficits detected in clozapine-treated mice did allow us to further investigate whether a positive correlation exists between egr-2 expression levels and ASST performance across the different test phases. Indeed, clozapine-treated wild type had lower egr-2 expression after completion of the CD phase of the ASST and, just like in mice with reversal learning deficit, lower egr-2 expression was detected in the vlOFC and IL/PrL subregions of the mPFC.
It is well established that neurons respond to increased activity by changing levels of gene expression and that this process is triggered by the upregulation of specific regulatory transcription factors (Ferguson et al., 2001; Frankland et al., 2004; Boehm et al., 2005; Choi et al., 2005; Glickstein et al., 2005). However, it is presently unresolved whether induction of egr-2 expression triggers the transcription of genes that, in turn, promote plasticity needed to support distinct cognitive functions or whether egr-2 stimulates transcription of genes that restore or maintain a metabolic homeostasis of neurons that are challenged during cognitive task performance. Notably, egr-1 and egr-2 transcription-factor binding sites, for example, are found in a number of neuronal genes that are known to play a role in mediating plastic changes in response to cognitive demand (Thiel et al., 1994; Petersohn et al., 1995; Archer et al., 1990; Özçelik et al., 1990; Nikam et al., 1995; Li et al., 1993) suggesting that these transcription factors are indeed plasticity-inducible transcriptional regulators. Moreover, studies on knockout mice indicate different and non-overlapping cognitive functions of different early growth response gene proteins in the adult brain. For example, although both egr-1 and egr-3 mutants have learning and memory deficits, egr-1 mutants have deficits in hippocampal late-phase LTP and long-term memory (Jones et al., 2001) and egr-3 mutants have deficits in early phases of hippocampal LTP and short-term-memory (Li et al., 2007). In contrast, no spatial learning deficits were detected in conditional egr-2 mutants and these mutants also exhibited superior memory in an object recognition task (Poirier et al., 2007). It is, however, presently unknown whether these egr-2 mutants have deficits in other cognitive functions such as attention and working memory. We have previously shown that egr-2 (but not egr-1 and egr-3) expression is induced in the IL and PrL subregions of the mPFC and the vlOFC of mice exposed to the ASST but not in mice that performed a spatial working memory task suggesting that egr-2 induction is a not a promiscuous response to a variety of different cognitive challenges. Moreover, we found that the magnitude of egr-2 induction of ASST-tested mice positively correlated with the magnitude of attentional demand (DeSteno and Schmauss, 2008). Here we show that mice with impaired ASST performance exhibited decreased egr-2 expression in the same anatomic subregions. Together, these findings suggest a functional link between egr-2 expression levels in the vlOFC and IL and PrL subregions of the mPFC and optimal ASST performance and thus, they begin to shed light onto the physiological relevance of decreased egr-2 expression in the cortex of subjects with autism and Rett syndrome (Swanberg et al., 2009).
Acknowledgments
This work was supported by grants from the National Institutes of Health MH56123 and MH062185.
Abbreviations
- AC
anterior cingulate
- ASST
attention-set-shifting task
- CD
compound discrimination
- ct
cycle threshold
- EDS
extradimensional set-shift
- EDS-Rev
EDS-reversal
- egr-2
early growth response gene 2
- IDS
intradimensional set-shift
- IL
infralimbic
- mOFC
medial orbital frontal cortex
- mPFC
medial prefrontal cortex
- OFC
orbital frontal cortex
- PB
phosphate buffered saline
- PrL
prelimbic
- SD
simple discrimination
- vlOFC
ventrolateral orbital frontal cortex
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- Archer BT, Özçelik T, Jahn R, Francke U, Südhof TC. Structures and chromosomal localization of two human genes encoding synaptobrevins 1 and 2. J Biol Chem. 1990;265:17267–17273. [PubMed] [Google Scholar]
- Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G protein Gq. J Neurosci. 2007;27:1467–1473. doi: 10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attention set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm U, Zhou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Calaminus C, Hauber W. Guidance of instrumental behavior under reversal conditions requires dopamine D1 and D2 receptor activation in the orbital frontal cortex. Neuroscience. 2008;154:1195–1204. doi: 10.1016/j.neuroscience.2008.04.046. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong H-W, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- DeSteno DA, Schmauss C. Induction of early growth response gene 2 expression in the forebrain of mice performing an attention-set-shifting task. Neuroscience. 2008;152:417–428. doi: 10.1016/j.neuroscience.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds CM, Müller U, Clark L, van Loon A, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008;28:5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MTL. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, DeSteno DA, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cereb Cortex. 2005;15:1016–1024. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos, and Krox, and CREB/AFT. Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hof PR, Youngm WG, Bloom FE, Belichenko PV, Celio MR. Comparative atlas of the C57BL/6 and 129/SV mouse brains. Amsterdam: Elsevier; 2000. [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excision in orbito-frontal and dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J Neurosci. 2009;29:3695–3704. doi: 10.1523/JNEUROSCI.5195-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TVP, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and consolidation of long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Jung M-Y, Skryabin BV, Arai M, Abbondanzo S, Fu D, Robakis NK, Polites HG, Pintar JE, Schmauss C. Potentiation of the D2-mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Grandy DK. Dopamine D2 receptors mediate two-odor discrimination and reversal learning in C57BL/6 mice. BMC Neurosci. 2004;5:12. doi: 10.1186/1471-2202-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Li Y, Camp S, Rachinsky TL, Bongiorno C, Taylor P. Promotor elements and transcriptional control of the mouse acetylcholinesterase gene. J Biol Chem. 1993;268:3563–3572. [PubMed] [Google Scholar]
- Li L, Yun SH, Keblesh J, Trommer BL, Xiong H, Radulovic J, Tourtellotte WG. Erg3, a synaptic activity regulated transcription factor that is essential for learning and memory. Mol Cell Neurosci. 2007;35:76–88. doi: 10.1016/j.mcn.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGibbon GA, Lawler PA, Hughes P, Young D, Dragunow M. Differential expression of inducible transcription factors in basal ganglia neurons. Mol Brain Res. 1995;34:294–302. doi: 10.1016/0169-328x(95)00184-t. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology (Berl) 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- Nikam SS, Tennekoon GI, Christy BA, Yoshino JE, Rutkowski JL. The zinc finger transcription factor zif268/Egr-1 is essential for Schwann cell expression of the p75 NGF receptor. Mol Cell Neurosci. 1995;6:337–348. doi: 10.1006/mcne.1995.1026. [DOI] [PubMed] [Google Scholar]
- O’Donovan KJ, Tourtellotte WG, Milbrandt J, Baraban JM. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999;22:167–173. doi: 10.1016/s0166-2236(98)01343-5. [DOI] [PubMed] [Google Scholar]
- Özçelik T, Lafreniere RG, Archer BT, Johnston PA, Willard HF, Francke U, Südhof TC. Synaptophysin: structure of the human gene and assignment to the X chromosome in man and mouse. Am J Hum Genet. 1990;47:551–561. [PMC free article] [PubMed] [Google Scholar]
- Perez-Costas E, Guidetti P, Melendez-Ferro M, Kelley JJ, Roberts RC. Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J Neural Transm. 2008;115:745–753. doi: 10.1007/s00702-007-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersohn D, Schoch S, Brinkmann DR, Thiel G. The human synapsin II gene promotor. J Biol Chem. 1995;270:24361–24369. doi: 10.1074/jbc.270.41.24361. [DOI] [PubMed] [Google Scholar]
- Poirier R, Cheval H, Mailhes C, Charnay P, Davis S, Laroche S. Paradoxical role of an Egr transcription factor family member, Egr2/Krox20, in learning and memory. Front Behav Neurosci. 2007;1:6. doi: 10.3389/neuro.08.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–32. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nat Rev Neurosci. 2006;7:367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Reynold GP. Receptor mechanisms in the treatment of schizophrenia. J Psychopharmacol. 2004;18:340–345. doi: 10.1177/026988110401800303. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Haystead TA, Baker HF. An analysis of visual object reversal learning in the marmoset after amphetamine and haloperidol. Pharmacol Biochem Behav. 1981;14:345–351. doi: 10.1016/0091-3057(81)90401-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Roberts AC. Differential regulation of fronto-executive function by monoamines and acetylcholine. Cereb Cortex. 2007;17:i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Seeman P, Corbett R, Van Tool HHM. Atypical neuroleptics have low affinity for dopamine D2 receptors or are selective for D4 receptors. Neuropsychopharmacology. 1997;16:93–110. doi: 10.1016/S0893-133X(96)00187-X. [DOI] [PubMed] [Google Scholar]
- Swanberg SE, Nagarajan RP, Peddada S, Yasui DH, LaSalle JM. Reciprocal co-regulation of EGR2 and MECP2 is disrupted in Rett syndrome and autism. Hum Mol Genet. 2009;18:525–534. doi: 10.1093/hmg/ddn380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G, Schoch S, Petersohn D. Regulation of synapsin I gene expression by the zinc finger transcription factor zif268/egr-1. J Biol Chem. 1994;269:15294–15301. [PubMed] [Google Scholar]
- Winter S, Dieckman M, Schwabe K. Dopamine in the prefrontal cortex regulates behavioral flexibility to changing reward value. Behav Brain Res. 2009;198:206–213. doi: 10.1016/j.bbr.2008.10.040. [DOI] [PubMed] [Google Scholar]