Abstract
Zearalenone (ZEA), a nonsteroidal estrogenic mycotoxin, is known to cause testicular toxicity in animals. In the present study, the effects of ZEA on spermatogenesis and possible mechanisms involved in germ cell injury were examined in rats. Ten-week-old Sprague-Dawley rats were treated with 5 mg/kg i.p. of ZEA and euthanized 3, 6, 12, 24 or 48 h after treatment. Histopathologically, spermatogonia and spermatocytes were found to be affected selectively. They were TUNEL-positive and found to be primarily in spermatogenic stages I-VI tubules from 6 h after dosing, increasing gradually until 12 h and then gradually decreasing. Western blot analysis revealed an increase in Fas and Fas ligand (Fas-L) protein levels in the ZEA-treated rats. However, the estrogen receptor (ER)α expression was not changed during the study. Collectively, our data suggest that acute exposure of ZEA induces apoptosis in germ cells of male rats and that this toxicity of ZEA is partially mediated through modulation of Fas and Fas-L systems, though ERα may not play a significant role.
Keywords: apoptosis, estrogen receptor, Fas ligand, testis, zearalenone
Introduction
Zearalenone (ZEA) is a naturally occurring estrogenic mycotoxin produced by numerous species of Fusarium(F.) (including F. roseum, F. tricinctum, F. sporotrichioides, F. oxysporum, and F. moniliforme) growing on grains, mainly corn and hay exposed to high moisture during storage [1]. ZEA causes alterations in the reproductive tract of animals such as decreased fertility, increased fetal resorption, reduced litter size, changed weight of endocrine glands and changed serum hormone levels [16,21]. In an NTP cancer bioassay a dose-related incidence of hepatocellular adenomas and endometrial adenocarcinomas were seen in female mice [18]. Despite the potential toxicity of ZEA, only limited information is currently available in regard to the spermatogenesis impairment effects of ZEA.
In our previous study, ZEA induced apoptosis of germ cells in rats [10]. Testicular germ cell apoptosis occurs normally and continuously throughout life [25] and when the testicular environment cannot support spermatogenesis, e.g. during alterations of hormonal support, heat exposure, radiation, or exposure to environmental and chemotherapeutic compounds. Though many different apoptosis-related elements have been identified in the testis, the molecular and cellular mechanisms by which testicular toxicants induce germ cell apoptosis are not thoroughly understood.
The Fas signaling system is composed of the interacting proteins Fas (CD95, APO-1) and Fas ligand (Fas-L; CD95L, APO-1L) [12]. In the testis, the Fas system plays a role in germ cell apoptosis following ischemia- reperfusion, stress, and exposure to testicular toxicants [3,11]. However, the Fas signaling system may be dispensable for nitrobenzene [19] or heat-induced germ cell apoptosis in the testis [9].
Cellular homeostasis in higher organisms is maintained by balancing cell growth, differentiation, and death. Estrogen receptors (ERs) induce expression of genes that control cell fates, including proliferation and cell death via apoptosis [2]. The high expression of ERs in the testis has been demonstrated by Heikinheimo et al. [8], suggesting that these receptors may have some important direct roles in modulating the spermatogenic process by estrogenic chemicals. However, possible roles of ERs underlying the spermatogenesis impairment by estrogenic chemicals are still largely unclear.
In our previous study, ZEA induced apoptosis of germ cells in rats [10]. It is likely that apoptosis by ZEA is controlled in a cell type specific and spermatogenic stage specific fashion, but the basic elements of the death machinery are not thoroughly understood. In the present study, we assessed germ cell apoptosis in rats treated with ZEA in the short term, and possible involvement of Fas, Fas-L, and ERα.
Materials and Methods
Animals and treatments
Nine-week-old male Sprague-Dawley rats (Samtako, Korea) were used after 1-week of acclimation. Rats were randomized by body weight and housed 3 per plastic cage at 23 ± 1℃, 55 ± 5% of humidity and a 12 h light/12 h dark cycle, and given a standard laboratory rodent diet (Jeil Feed, Korea) with sterilized water available ad libitum. The study protocol was approved by the Institutional Animal Ethical Committee of Chungnam National University.
The animals were given a single i.p. dose of 5 mg/kg of ZEA (Sigma-Aldrich, USA) in corn oil (Sigma-Aldrich, USA) at a total volume of 1 mL/kg of body weight and euthanized under CO2 anesthesia after 3, 6, 12, 24, or 48 h after dosing (n = 3, at each time point). Control rats (n = 3) were given an equivalent volume of vehicle and were euthanized at each time point.
Immediately after sacrifice, both testes from each animal were removed, one of which was immersed in Bouin's fixative for histopathological and TUNEL examination. The contralateral testis was decapsulated, snap frozen in liquid nitrogen and stored at -70℃ for immunoblot assay.
Histopathological examination
For light microscopy, testes fixed in Bouin's fixative were processed by conventional methods and stained with hematoxylin and eosin (H&E). The seminiferous tubules were divided into four groups identified by H&E stain (stages I-VI, VII-VIII, IX-XI, and XII-XIV) based on the germ cell types of tubules, in accordance with previous literature [22]. The cell types distinguished here were spermatogonia, preleptotene spermatocytes, leptotene spermatocytes, zygotene spermatocytes, pachytene spermatocytes, round spermatids, elongate spermatids, and Sertoli cells.
In situ TUNEL analysis
Apoptotic cells in the testicular tissue were identified by the TUNEL method using ApopTag Peroxidase Kits (Intergen, USA) according to the manufacturers' instructions. The quantification of TUNEL-labeled germ cells was assessed on 100 sectioned seminiferous tubules from each rat and expressed as numbers of TUNEL-labeled germ cells per 20 tubules in each group of stages.
Immunoblot assay
Fas, Fas-L, and ERα expression was measured in whole lysates of tissue homogenates. At each time point, tests were examined twice. Briefly, the frozen testes were homogenized with a homogenizer in ice-cold lysis buffer (40 mM Tris, 120 mM NaCl, 0.1% NP40, 100 µM PMSF, 5 µg/mL leupeptin, 5 µg/mL aprotinin) and were incubated on ice for 30 min and centrifuged at 15,000 × g for 20 min at 4℃ and the supernatant collected. Protein concentration was determined by the Bradford method using a kit from Bio-Rad (USA) with bovine serum albumin as a standard. Total lysate was separated on SDS-polyacrylamide gel electrophoresis, and then transferred to PVDF-plus membranes (Osmonic, USA) or polyvinylidene difluoride membranes. Nonspecific binding was blocked with 5% non-fat milk in TTBS (20 mM Tris, 0.5 M NaCl, 0.2% Tween 20, pH 7.4) for 1.5 h at room temperature. The membranes were incubated at 4℃, overnight with rat monoclonal antibody against Fas, Fas-L (BD Biosciences, USA), and ERα (SantaCruz, USA), followed by HRP-conjugated secondary antibody for 1 h. After each incubation, blots were washed with TBS containing 0.2% Tween 20. Immunoreactive bands were visualized by an ECL detection system (Amersham, USA).
Statistical analysis
Results were presented as mean ± SD. Statistical analysis was performed with Student's t-test and the level of significance was taken as p < 0.05 or p < 0.01 compared with the control group values.
Results
Testis and epididymis weight
There were no differences in the testis and epididymis weights between control and ZEA-treated rats (data not shown).
Histopathological examination
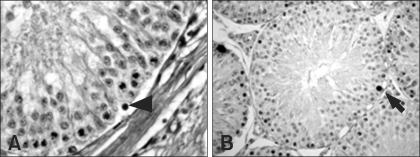
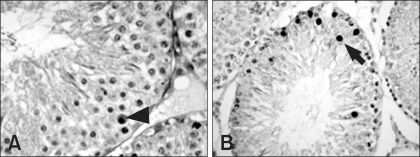
Degeneration of germ cells was first found in seminiferous tubules classified in stages I-VI at 6 h after ZEA treatment, characterized by pyknotic nuclei an eosinophilic cytoplasm (Fig. 1). Spermatogonia and spermatocytes were found to be affected selectively. These changes were observed until the end of study.
Fig. 1.
Seminiferous tubules from control rats. Seminiferous tubules in stages I-VI have degenerating germ cells with pyknotic nuclei and eosinophilic cytoplasm (arrowhead) and TUNEL-labeled germ cells (arrow). A: H&E stain, ×400, B: TUNEL, ×400.
In situ TUNEL analysis
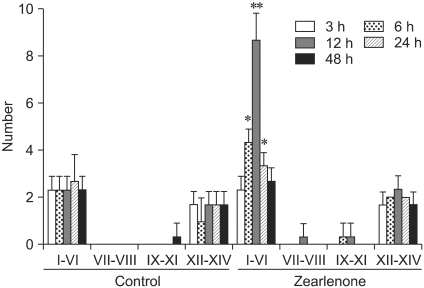
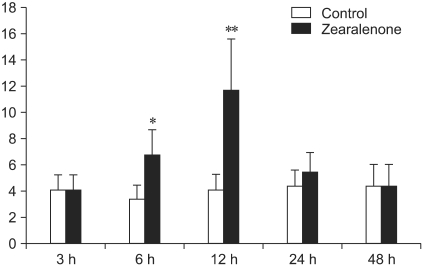
The number of TUNEL-labeled germ cells after a single dose of ZEA was increased in stage-specific and time-dependent manners (Figs. 3 and 4). TUNEL-labeled germ cells were observed mainly in stage I-VI seminiferous tubules and spermatogonia and spermatocytes were labeled selectively (Figs. 1-3). The number of TUNEL-labeled germ cells increased progressively, peaked at 12 h at about four times the control levels, and then gradually decreased until 48 h after dosing (Fig. 4).
Fig. 3.
Stage-specific quantification of TUNEL-labeled germ cells per 20 seminiferous tubules in each time point. TUNEL-labeled germ cells were observed mainly in stages I-VI seminiferous tubules. Values are mean ± SD. Statistical significance was denoted at p < 0.05 (*) and p < 0.01 (**) as compared to control group values.
Fig. 4.
Total quantification of TUNEL-labeled germ cells per 100 seminiferous tubules in each time point. The number of TUNEL-labeled germ cells increased progressively, peaked at 12 h at about four times the control levels, and then gradually decreased. Statistical significance was denoted at p < 0.05 (*) and p < 0.01 (**) as compared to control group values.
Immunoblot assay
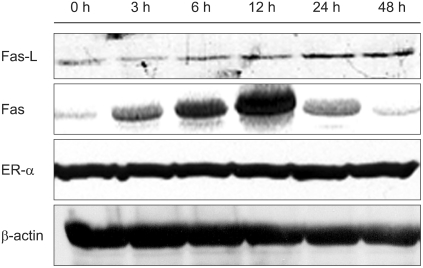
To investigate the underlying mechanisms involved in the spermatogenesis impairment by ZEA, we examined the expression of Fas, Fas-L, and ERα in the testis. The expression levels of the Fas protein were increased at 3 h, peaked at 12 h and then gradually decreased to very low levels at 48 h in the ZEA-treated rats. Fas-L protein, meanwhile, showed a stable increase up to 48 h. In contrast, Fas and Fas-L proteins were detectable at very small levels in the control rats. The ERα expression, however, was not affected by ZEA (Fig. 5).
Fig. 5.
Immunoblot analysis of Fas, Fas ligand (Fas-L), and estrogen receptor (ER)α protein levels. Note the Fas-L protein levels increased compared with the control group values in a time-dependent manner. Fas protein levels were increased from 3 h, peaked at 12 h and then recovered at 48 h after treatment as compared to control samples. However, ERα protein expression levels were not affected by zearalenone treatment.
Discussion
ZEA is a naturally occurring mycotoxin and a potent estrogenic. It is quickly transformed within the liver to α- and β-zearalenol by 3 α-hydroxysteroid dehydrogenase. These products are comparatively much more estrogenic than ZEA [26]. In this study, apoptotic cells were detected maximally at 12 h after ZEA-treatment, and then decreased gradually; these findings were similar to those observed in a previous study [10]. ZEA may have exerted its cytotoxity on target germ cells within only a short period immediately after dosing. In stage specific analysis of apoptosis by ZEA, apoptotic cells were increased in stages I-VI following ZEA-treatment; however, no significant difference was observed between the control and ZEA-treated groups in stage VII-XIV seminiferous tubules. Stages IV-VI correspond to meiotic divisions of primary spermatocytes and mitotic proliferation of B1 and B2 spermatogonia [22]. In contrast, stages V-VI and most importantly VII-VIII (androgen-dependent stages) have been shown to be relatively protected from programmed cell death [15]. Administration of exogenous estradiol affected the hypothalamus pituitary gonadal axis with a dose-dependent concomitant increase in intratesticular estrogen levels and reduced serum gonadotropins and intratesticular testosterone [6]. Selective deprivation of gonadotropins and testicular testosterone by gonadotropin-releasing hormone agonist treatment is followed by a stage-specific increase in germ cell apoptosis in stages VII-VIII [15]. Thus, of the 14 stages in the seminiferous epithelial cycle, stages VII-VIII are the most sensitive to estrogen treatment.
ZEA is a xenoestrogen and binds to ERs, generating an oestrogen-like response [13]. It passively crosses the cell membrane to the cytosolic ER. The receptor-ZEA complex is rapidly transferred to the nucleus, where it binds to the oestrogen-responsive element, thereby activating gene transcription [20]. Two subtypes of ER exist, namely ERα and ERβ, which are distributed differently in the body. An in vitro study demonstrated that 17 β-oestradiol and ZEA acted as competitive agonists for ERα and mixed agonist-antagonists for ERβ [13]. Endogenous estrogens physiologically inhibit steroidogenesis via ERα by acting directly on the testis early in fetal and neonatal development [5]. Also, neonatal administration of the weak estrogenic compound, genistein, affects male reproductive organs at the molecular levels of ERα and AR in adulthood [24]. In the present study, ZEA did not change ERα levels in the testis. This result indicates that spermatogenesis impairments by ZEA in short-term exposure are not closely associated with estrogenic activity of ZEA. Meanwhile, ERα and ERβ levels in testes of mice exposed to tributyltin for 30 days were decreased in one study [4]. However, in another study, long-term treatment of cimetidine induced ERβ overexpression and apoptosis in the rat testis [23]. Clearly, a more thorough understanding of the molecular changes of ERs induced by estrogenic chemicals is needed to understand the pathophysiology of impairment of spermatogenesis.
The mechanisms of how ZEA induces germ cell apoptosis are not well understood. The Fas system was originally characterized as a key mechanism for inducing apoptosis in immune cells [12]. As an immune privileged organ, the testis is known to express Fas. In spermatogenic cell death, it is generally accepted that a direct interaction between Sertoli cell-expressed Fas-L and germ cell-Fas led to the elimination of injured germ cells via apoptosis [27]. In the present study, we confirmed the expression of Fas and Fas-L in testes treated with ZEA as well as the frequency of apoptosis in germ cells. One pathway of testis toxicity may therefore be through apoptosis involving the Fas/Fas-L system. However, while a maximum number of apoptotic cells and a peak in Fas expression were seen at 12 h, Fas-L expression levels increased in a time-dependent manner. In another study, apoptosis and Fas/Fas-L expression levels were not matched [7]. One explanation for this may lie in differences in other molecular mechanisms involved in germ cell apoptosis. In the testis, p21, p53, cytochrome C and the bcl-2 family of proteins, which contains both proapoptotic (such as Bax) and antiapoptotic (such as Bcl-2) family members, play a critical role in regulating the process of apoptosis [11,14,17]. Further studies should be done to determine the precise mechanism of this derangement.
In conclusion, acute exposure of ZEA induces apoptosis in germ cells of male rats and this toxicity is partially mediated through modulation of Fas and Fas-L systems though ERα may not play a significant role.
Fig. 2.
Seminiferous tubules from zearalenone treated rats (12 h after dosing). Seminiferous tubules in stages I-VI show increased degenerating (arrowhead) and TUNEL-labeled germ cells (arrows). A: H&E stain, ×400, B: TUNEL, ×400.
Acknowledgments
This work was supported by Grant 013-E00038 from the Korea Research Foundation, Korea.
References
- 1.Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD. Crossroads of estrogen receptor and NF-kappaB signaling. Sci STKE. 2005;288:pe27. doi: 10.1126/stke.2882005pe27. [DOI] [PubMed] [Google Scholar]
- 3.Boekelheide K. Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr. 2005;34:6–8. doi: 10.1093/jncimonographs/lgi006. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Zuo Z, Chen S, Yan F, Chen Y, Yang Z, Wang C. Reduction of spermatogenesis in mice after tributyltin administration. Toxicology. 2008;251:21–27. doi: 10.1016/j.tox.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Delbès G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R. Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor alpha. Endocrinology. 2005;146:2454–2461. doi: 10.1210/en.2004-1540. [DOI] [PubMed] [Google Scholar]
- 6.D'Souza R, Gill-Sharma MK, Pathak S, Kedia N, Kumar R, Balasinor N. Effect of high intratesticular estrogen on the seminiferous epithelium in adult male rats. Mol Cell Endocrinol. 2005;241:41–48. doi: 10.1016/j.mce.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Geng J, Fan J, Jiang HW, Fang ZJ, Wang X, Sun JL, Ding Q, Chen G. Elevated serum soluble Fas ligand is a promising marker of testicular toxicity induced by epirubicin in rats. Toxicol Lett. 2009;186:96–103. doi: 10.1016/j.toxlet.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Heikinheimo O, Mahony MC, Gordon K, Hsiu JG, Hodgen GD, Gibbons WE. Estrogen and progesterone receptor mRNA are expressed in distinct pattern in male primate reproductive organs. J Assist Reprod Genet. 1995;12:198–204. doi: 10.1007/BF02211799. [DOI] [PubMed] [Google Scholar]
- 9.Hikim AP, Lue Y, Yamamoto CM, Vera Y, Rodriguez S, Yen PH, Soeng K, Wang C, Swerdloff RS. Key apoptotic pathways for heat-induced programmed germ cell death in the testis. Endocrinology. 2003;144:3167–3175. doi: 10.1210/en.2003-0175. [DOI] [PubMed] [Google Scholar]
- 10.Kim IH, Son HY, Cho SW, Ha CS, Kang BH. Zearalenone induces male germ cell apoptosis in rats. Toxicol Lett. 2003;138:185–192. doi: 10.1016/s0378-4274(02)00405-8. [DOI] [PubMed] [Google Scholar]
- 11.Koji T, Hishikawa Y. Germ cell apoptosis and its molecular trigger in mouse testes. Arch Histol Cytol. 2003;66:1–16. doi: 10.1679/aohc.66.1. [DOI] [PubMed] [Google Scholar]
- 12.Krammer P, Behrmann I, Bier V, Daniel P, Dhein J, Falk M, Garcin G, Klas C, Knipping E, Lucking-Famira KM, Matzku S, Oehm A, Richards S, Trauth B, Bornkamm G, Falk W, Moller P, Debatin KM. Apoptosis in the APO-1 system. In: Tomei LD, Cope FO, editors. Apoptosis: The Molecular Basis of Cell Death. New York: Cold Spring Harbor Laboratory Press; 1991. pp. 7–99. [Google Scholar]
- 13.Kuiper-Goodman T, Scott PM, Watanabe H. Risk assessment of the mycotoxin zearalenone. Regul Toxicol Pharmacol. 1987;7:253–306. doi: 10.1016/0273-2300(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Xu L, Dunbar JC, Dhabuwala CB. Role of mitochondrial cytochrome c in cocaine-induced apoptosis in rat testes. Urology. 2003;61:646–650. doi: 10.1016/s0090-4295(02)02263-x. [DOI] [PubMed] [Google Scholar]
- 15.Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, Leung A, Wang C. Single exposure to heat induces stagespecific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology. 1999;140:1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- 16.Milano GD, Becú-Villalobos D, Tapia MO. Effects of long-term zearalenone administration on spermatogenesis and serum luteinizing hormone, follicle-stimulating hormone, and prolactin values in male rats. Am J Vet Res. 1995;56:954–958. [PubMed] [Google Scholar]
- 17.Moréno SG, Dutrillaux B, Coffigny H. Status of p53, p21, mdm2, pRb proteins, and DNA methylation in gonocytes of control and gamma-irradiated rats during testicular development. Biol Reprod. 2001;64:1422–1431. doi: 10.1095/biolreprod64.5.1422. [DOI] [PubMed] [Google Scholar]
- 18.National Toxicology Program. Carcinogenesis bioassay of zearalenone (CAS No. 17924-92-4) in F344/N rats and B6C3F1 mice (feed study) Natl Toxicol Program Tech Rep Ser. 1982;235:1–155. [PubMed] [Google Scholar]
- 19.Richburg JH, Nañez A. Fas- or FasL-deficient mice display an increased sensitivity to nitrobenzene-induced testicular germ cell apoptosis. Toxicol Lett. 2003;139:1–10. doi: 10.1016/s0378-4274(02)00419-8. [DOI] [PubMed] [Google Scholar]
- 20.Riley RT. Mechanistic interaction of mycotoxins: theoretical considerations. In: Sinha KK, Bhatnagar D, editors. Mycotoxins in Agriculture and Food Safety. New York: Marcel Dekker; 1998. pp. 227–253. [Google Scholar]
- 21.Ruhr LP, Osweiler GD, Foley CW. Effect of the estrogenic mycotoxin zearalenone on reproductive potential in the boar. Am J Vet Res. 1983;44:483–485. [PubMed] [Google Scholar]
- 22.Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River Press; 1990. pp. 59–118. [Google Scholar]
- 23.Sasso-Cerri E. Enhanced ERbeta immunoexpression and apoptosis in the germ cells of cimetidine-treated rats. Reprod Biol Endocrinol. 2009;7:127–134. doi: 10.1186/1477-7827-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibayama T, Fukata H, Sakurai K, Adachi T, Komiyama M, Iguchi T, Mori C. Neonatal exposure to genistein reduces expression of estrogen receptor alpha and androgen receptor in testes of adult mice. Endocr J. 2001;48:655–663. doi: 10.1507/endocrj.48.655. [DOI] [PubMed] [Google Scholar]
- 25.Sinha Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod. 1999;4:38–47. doi: 10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- 26.Ueno Y, Tashiro F. α-Zearalenol, a major hepatic metabolite in rats of zearalenone, an estrogenic mycotoxin of Fusarium species. J Biochem. 1981;89:563–571. doi: 10.1093/oxfordjournals.jbchem.a133232. [DOI] [PubMed] [Google Scholar]
- 27.Xu JP, Li X, Mori E, Guo MW, Matsuda I, Takaichi H, Amano T, Mori T. Expression of Fas-Fas ligand in murine testis. Am J Reprod Immunol. 1999;42:381–388. doi: 10.1111/j.1600-0897.1999.tb00116.x. [DOI] [PubMed] [Google Scholar]