Abstract
BACKGROUND
The V617F mutation, which causes the substitution of phenylalanine for valine at position 617 of the Janus kinase (JAK) 2 gene (JAK2), is often present in patients with polycythemia vera, essential thrombocythemia, and idiopathic myelofibrosis. However, the molecular basis of these myeloproliferative disorders in patients without the V617F mutation is unclear.
METHODS
We searched for new mutations in members of the JAK and signal transducer and activator of transcription (STAT) gene families in patients with V617F-negative polycythemia vera or idiopathic erythrocytosis. The mutations were characterized biochemically and in a murine model of bone marrow transplantation.
RESULTS
We identified four somatic gain-of-function mutations affecting JAK2 exon 12 in 10 V617F-negative patients. Those with a JAK2 exon 12 mutation presented with an isolated erythrocytosis and distinctive bone marrow morphology, and several also had reduced serum erythropoietin levels. Erythroid colonies could be grown from their blood samples in the absence of exogenous erythropoietin. All such erythroid colonies were heterozygous for the mutation, whereas colonies homozygous for the mutation occur in most patients with V617F-positive polycythemia vera. BaF3 cells expressing the murine erythropoietin receptor and also carrying exon 12 mutations could proliferate without added interleukin-3. They also exhibited increased phosphorylation of JAK2 and extracellular regulated kinase 1 and 2, as compared with cells transduced by wild-type JAK2 or V617F JAK2. Three of the exon 12 mutations included a substitution of leucine for lysine at position 539 of JAK2. This mutation resulted in a myeloproliferative phenotype, including erythrocytosis, in a murine model of retroviral bone marrow transplantation.
CONCLUSIONS
JAK2 exon 12 mutations define a distinctive myeloproliferative syndrome that affects patients who currently receive a diagnosis of polycythemia vera or idiopathic erythrocytosis.
The myeloproliferative disorders comprise a spectrum of chronic hematologic diseases that are likely to arise from a mutant multipotent hematopoietic stem cell.1,2 The V617F somatic mutation in the Janus kinase (JAK) 2 gene (JAK2), which causes the substitution of phenylalanine for valine at position 617, has recently been found in the majority of patients with polycythemia vera and in many with essential thrombocythemia or idiopathic myelofibrosis.3-7 This gene encodes a cytoplasmic tyrosine kinase. The mutation, which occurs in the JAK homology 2 (JH2) negative regulatory domain, increases JAK2 kinase activity and causes cytokine-independent growth of cell lines and cultured bone marrow cells. Mutant JAK2 transfected into murine bone marrow cells produces erythrocytosis and subsequent myelofibrosis in recipient animals,3,8,9 suggesting a causal role for the mutation.
Allele-specific polymerase chain reaction (PCR) can be used to detect the V617F mutation in approximately 95% of patients with polycythemia vera and in 50 to 60% of patients with essential thrombocythemia or idiopathic myelofibrosis.4,10,11 The mutation is also present in hematopoietic progenitors committed to granulocytic or erythroid differentiation4,12 and in purified hematopoietic stem cells from patients with polycythemia vera.13 Many patients with polycythemia vera or idiopathic myelofibrosis are homozygous for the V617F mutation, as a result of mitotic recombination affecting chromosome 9p,3-6 but homozygosity is rare in patients with essential thrombocythemia.12 The mutation occurs infrequently in patients with myelodysplasia or acute myeloid leukemia but does not occur in those with lymphoid tumors, epithelial cancers, or sarcomas.14-18
The JAK2 mutation allows for a distinction between two subtypes of idiopathic myelofibrosis and essential thrombocythemia.19-21 The phenotype of V617F-positive, but not V617F-negative, essential thrombocythemia resembles that of polycythemia vera.20 However, patients with V617F-negative essential thrombocythemia do have cytogenetic abnormalities, dysplastic megakaryocytes, and a risk of transformation to myelofibrosis or acute myeloid leukemia, all of which are features of a myeloproliferative disorder.20 Activating mutations in the thrombopoietin receptor have been reported in 10% of patients with V617F-negative idiopathic myelofibrosis22 and in a few patients with essential thrombocythemia.23 However, the molecular basis of V617F-negative polycythemia vera is unknown.
METHODS
PATIENTS
We recruited patients from Addenbrooke's Hospital in Cambridge, St. Thomas' Hospital in London, and Belfast City Hospital in Belfast (all in the United Kingdom) and from those enrolled in the Myeloproliferative Disorders Study of Harvard University in Boston.5 Diagnoses assigned by local physicians were reviewed centrally and revised according to established criteria for polycythemia vera,24 essential thrombocythemia,25 and idiopathic myelofibrosis.26 The Addenbrooke's National Health Service Trust Research Ethics Committee approved this study. Written informed consent was obtained from each patient.
MUTATION SCREENING
The isolation of granulocytes and T lymphocytes and hematopoietic colony assays were performed as previously described.4 Individual burst-forming units and erythropoietin-independent erythroid colonies were harvested into water and boiled. Primers for the coding exons of JAK1, JAK2, JAK3, the tyrosine kinase 2 gene (TYK2), and of two signal transducer and activator of transcription genes (STAT5A and STAT5B) are listed at www.sanger.ac.uk/genetics/CGP; all additional primers used are listed in Table 1 in the Supplementary Appendix (available with the full text of this article at www.nejm.org). We performed allele-specific PCR using DNA from granulocytes or from total peripheral blood, an annealing temperature of 62°C, JAK2 exon 12 control primers, and primers specific for the alleles containing the K539L mutation (leading to the replacement of lysine at position 539 with a leucine), the N542-E543del mutation (causing the deletion of asparagine at position 542 and glutamic acid at position 543), the F537-K539delinsL mutation (leading to the replacement of phenylalanine at position 537 through lysine at position 539 by a single leucine), or the H538QK539L mutation (causing a substitution of glutamine for histidine at position 538 and leucine for lysine at position 539). We amplified DNA from in vitro colonies using exon 12 primers and sequenced or genotyped the PCR products using digestion with AseI.
Table 1. Clinical Features of Patients with JAK2 Exon 12 Mutations at Diagnosis.*.
| Patient No. |
Sex | Age | JAK2 Mutation | Hemoglobin Level |
White-Cell Count† |
Platelet Count |
Serum Erythropoietin Level‡ |
Cytogenetic Karyotype |
Splenomegaly | Erythroid Colonies Indpendent of Erythropoietin |
Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| yr | g/liter | ×10−3/mm3 | IU/liter | ||||||||
| 1 | M | 50 | F537-K539delinsL | 234 | 7.9 | 450 | ND | Deletion in 20q | Present on palpation | ND | PV |
| 2 | F | 59 | F537-K539delinsL | 179 | 14.4 | 294 | 9.9 | Deletion in 20q | Absent | ND | PV |
| 3 | F | 50 | F537-K539delinsL | 201 | 5.1 | 308 | <2.5 | Normal | Absent | Yes | IE |
| 4 | M | 27 | H538QK539L | 211 | 11.1 | 286 | <5.0 | Normal | Present on ultrasonography | Yes | PV |
| 5 | F | 62 | K539L | 181 | 7.5 | 301 | <2.5 | Normal | Absent | Yes | IE |
| 6 | M | 54 | K539L | 207 | 8.6 | Normal | 8.4 | ND | Absent | ND | IE |
| 7 | F | 17 | N542-E543del | 220 | 5.6 | 310 | <1.0 | Normal | Present on palpation | Yes | PV |
| 8 | F | 29 | N542-E543del | 198 | 5.8 | 285 | 2.0 | Normal | Absent | Yes | IE |
| 9 | F | 57 | N542-E543del | 199 | 4.4 | 204 | 5.0 | ND | Present on ultrasonography | Yes | PV |
| 10 | M | 33 | N542-E543del | 225 | 12.1 | 425 | ND | Normal | Present on palpation | ND | PV |
The normal range of serum erythropoietin before therapy is 5 to 25 IU per liter. The diagnosis was determined on the basis of criteria of the Polycythemia Vera Study Group.24 The presence or absence of erythroid colonies independent of erythropoietin were assessed in 2005 or 2006. The deletion in chromosome 20q was confirmed by microsatellite polymerase chain reaction of DNA from peripheral-blood granulocytes, bone marrow cells, or both (data not shown). PV denotes polycythemia vera, IE idiopathic erythrocytosis, and ND not determined.
Neutrophil counts were 8960 per cubic millimeter for Patient 2, 9000 per cubic millimeter for Patient 4, and 7300 per cubic millimeter for Patient 10.
The serum erythropoietin level was measured after venesection therapy in Patient 2 and before therapy in all other patients.
BONE MARROW BIOPSY
Bone marrow biopsy specimens from the iliac crest were fixed in neutral buffered formalin. Some were processed in paraffin and others in methylmethacralate after decalcification in 5.5% EDTA. Sections (1 to 3 μm thick) were cut and visualized using hematoxylin and eosin or Wright-Giemsa stain. All stained sections were viewed under a light microscope (Olympus-BX51) equipped with a 10×-H26.5 ocular lens. Low-power (20×) and high-power (40×) images were obtained with a digital camera (Pixera Pro150ES) and Studio 3.0.1 software (Adobe Systems).
SITE-DIRECTED MUTAGENESIS AND PRODUCTION OF RETROVIRUS
We introduced the mutations V617F, H538QK539L, K539L, N542-E543del, and F537-K539delinsL into murine Jak2 complementary DNA in a bicistronic retroviral vector encoding green fluorescent protein (MSCViresGFP),8 using QuikChange site-directed mutagenesis (Stratagene). The complete nucleotide sequence of each retroviral vector was confirmed before use. For the production of each retrovirus, equal amounts of Jak2 retroviral vector and packaging plasmids (Ecopak) were combined, incubated with FuGene (Roche) for 15 minutes, and then added to the human embryonic kidney-cell line, 293T. The supernatants were harvested 48 hours later and were used to transduce BaF3 cells expressing the murine erythropoietin receptor (BaF3/EpoR cells)27 or murine bone marrow cells.
BAF3-CELL PROLIFERATION ASSAYS AND WESTERN BLOTTING
BaF3/EpoR cells were maintained in RPMI-1640 medium containing 10% fetal-calf serum and 10% medium conditioned with WEHI-3B cells, as a source of interleukin-3, and infected with retroviral supernatants containing MSCViresGFP vectors encoding mutant or wild-type Jak2. The green fluorescent protein–positive population from each transduction was purified by flow-cytometric sorting 2 days later and was then expanded in RPMI-1640 medium with 10% fetal-calf serum and 10% WEHI-3B–conditioned medium for 3 to 8 days. To assay for growth-factor hypersensitivity, transduced BaF3/EpoR cells were cultured in the absence of interleukin-3, and the number of viable cells was measured at days 2 and 4 with the use of trypan-blue exclusion. Data from four independent experiments were combined in analyses.
For immunoprecipitation and Western blot studies, BaF3/EpoR cells expressing wild-type or mutant Jak2 were starved for 4 to 5 hours in RPMI-1640 medium containing 1% bovine serum albumin and were then pelleted and frozen for subsequent analysis. Cells stimulated with 10 U per milliliter of erythropoietin for 10 minutes served as a positive control. For the analysis of Jak2 and Stat5, 3×107 cells were lysed in 10 mM TRIS–hydrochloric acid (pH 7.4) with 150 mM sodium chloride and 0.5% NP-40 buffer containing phosphatase and protease inhibitors. The protein supernatant was precipitated with anti-Jak2 antibody (Upstate Cell Signaling Solutions) or anti-Stat5 antibody (Santa Cruz Biotechnology). Precipitates were blotted with antibodies against phosphorylated Stat5 (phosphotyrosine at position 694) (Cell Signaling Technology), phosphotyrosine (4G10) (Upstate Cell Signaling Solutions), Jak2, or Stat5 (Santa Cruz Biotechnology). Alternatively, total cell lysates were resuspended in lithium dodecyl sulfate sample buffer (Invitrogen) and then blotted with antibodies against phosphorylated extracellular regulated kinase 1 and 2 (Erk1 and Erk2) (phosphothreonine at position 202 and phosphotyrosine at position 204 in Erk) or against total Erk (Cell Signaling Technology).
BONE MARROW TRANSPLANTATION ASSAY IN MICE
Bone marrow transplantation was performed as previously described.28 Briefly, retroviral supernatants were titrated by determining the percentage of BaF3 cells that were positive for green fluorescent protein 48 hours after the introduction of the retroviral vector. Supernatants containing equal titers of wild-type Jak2 or V617F or K539L Jak2 were used to transfect bone marrow cells. BALB/c donor mice were treated with 150 mg of 5-fluorouracil per kilogram of body weight, and cells harvested from femurs and tibias 7 days later were cultured for 24 hours in transplantation medium (RPMI-1640 medium, 10% fetal-calf serum, 6 ng of murine interleukin-3 per milliliter, 10 ng of human interleukin-6 per milliliter, and 10 ng of murine stem-cell factor per milliliter). Bone marrow cells were centrifuged at 2500 rpm for 90 minutes in the presence of 1 ml of retroviral supernatant and 10 μg of polybrene per 4×106 cells. Exposure to retroviral supernatant and centrifugation were repeated 1 day later. Aliquots of 1×106 bone marrow cells were resuspended in 0.7 ml of Hank's balanced salt solution and then injected into lethally irradiated BALB/c mice. Peripheral-blood counts and cell morphology were evaluated for each recipient 38 days after transplantation.
STATISTICAL ANALYSIS
We used an unpaired Student's t-test to compare demographic and laboratory features at the time of diagnosis between patients with a V617F JAK2 mutation and those with a JAK2 exon 12 mutation and to compare peripheral-blood counts among mouse recipients of bone marrow cells expressing wild-type, V617F, or K539L Jak2. Fisher's exact test was used to compare frequencies of mutation-positive erythroid colonies and of colonies homozygous for the mutation between patients with the V617F mutation and patients with an exon 12 mutation.
RESULTS
SOMATIC MUTATIONS AFFECTING JAK2 EXON 12
Of the 73 patients with polycythemia vera in our original cohort, 2 did not have the V617F mutation4 and were studied further. In these two patients, mutations were not found in the coding exons of JAK1, JAK3, TYK2, STAT5A, or STAT5B. However, both patients had alterations in JAK2 exon 12 that affected residues lying approximately 80 amino acids before V617. One patient had a 6-bp in-frame deletion affecting positions 1611 to 1616, resulting in an F537-K539delinsL mutation. The second patient had a CAA→ATT mutation at positions 1614 through 1616, resulting in an H538QK539L mutation (Fig. 1A). These mutations were acquired, since they could be detected in peripheral-blood granulocytes but not in T lymphocytes.
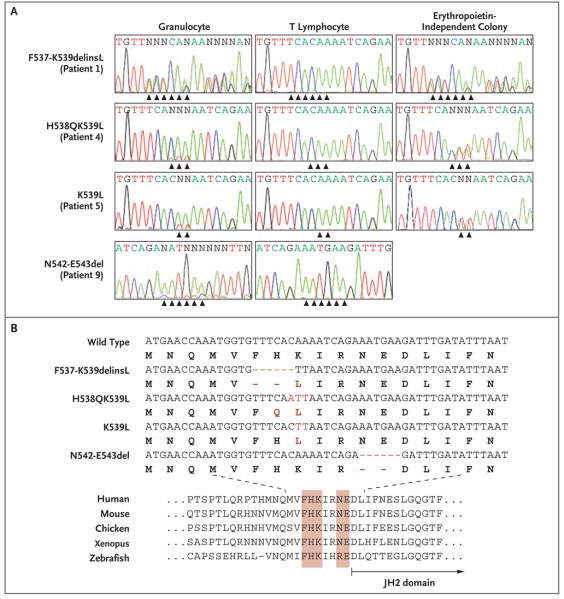
Figure 1. Somatic Mutations of JAK2 Exon 12 in Patients with Polycythemia Vera or Idiopathic Erythrocytosis.
Panel A shows DNA-sequence traces from peripheral-blood granulocytes and T lymphocytes and from erythropoietin-independent erythroid colonies. Nucleotides are indicated by capital letters, with N representing sites at which wild-type and mutant nucleotides are apparent at the same position. The traces reveal four acquired mutations within JAK2 exon 12 (indicated by arrowheads), often with low-level involvement in granulocytes. Panel B (top) shows the alignment of wild-type and mutant exon 12 JAK2 alleles (shown in red) (nucleotides are indicated by capital letters and amino acids by bold capital letters; dashes indicate the positions of deleted nucleotides). The amino acid alignment across multiple species (Panel B, bottom) shows conservation of the mutated amino acids, indicated in red.
JAK2 exon 12 mutations were identified in eight of an additional nine patients who received a diagnosis of V617F-negative polycythemia vera from their local physicians. The mutations were frequently present at low levels in granulocyte DNA but were readily identifiable in clonally derived erythropoietin-independent erythroid colonies (Fig. 1A). In total, four exon 12 alleles were identified, all of which had changes affecting conserved residues between K537 and E543 (Fig. 1); three of the alleles (in Patients 1 through 6) contained a K539L substitution (Fig. 1B). JAK2 exon 12 mutations were not detected by sequencing granulocyte DNA from 55 patients with V617F-positive polycythemia vera, 25 patients with V617F-negative essential thrombocythemia, and 12 patients with V617F-negative cases of idiopathic myelofibrosis14 (and data not shown). Since mutation-bearing granulocytes may represent only a minority of peripheral blood granulocytes,4,10,29 DNA from an additional 90 patients with V617-negative essential thrombocythemia was screened using sensitive allele-specific PCR assays for each exon 12 mutation, but no mutations were detected (data not shown). These results indicate that JAK2 exon 12 mutations occur only in patients with a myeloproliferative syndrome who present with erythrocytosis.
CLINICAL PHENOTYPE ASSOCIATED WITH JAK2 EXON 12 MUTATIONS
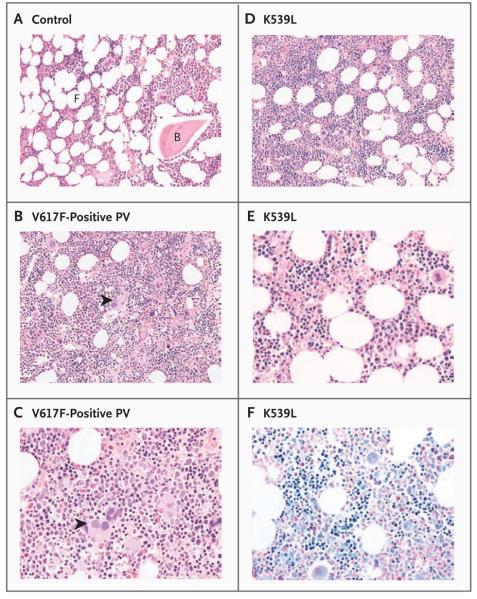
Table 1 shows the clinical and laboratory features of the patients with exon 12 mutations. All had platelet counts of 450×103 or less per cubic millimeter and neutrophil counts that were within the normal range or were insufficiently raised to fulfill the criteria for a diagnosis of polycythemia vera.24 A low serum erythropoietin level was found in four of eight tested patients, and in six of six tested patients, erythropoietin-independent erythroid colonies could be grown from peripheral-blood cells, a key feature of the myeloproliferative disorders.30 Central review of clinical and laboratory features revealed that six patients fulfilled the criteria of the Polycythemia Vera Study Group for polycythemia vera,24 and four patients fulfilled criteria for idiopathic erythrocytosis. Patients with exon 12 mutations were significantly younger at diagnosis than 86 patients from Addenbrooke's Hospital who had V617F-positive polycythemia vera (median age, 52 years vs. 58 years; P = 0.003) and had significantly higher hemoglobin levels (mean, 202 g per liter vs. 180 g per liter; P = 0.002), lower white-cell counts (mean, 8.4×103 per cubic millimeter vs. 14.1×103 per cubic millimeter; P = 0.008), and lower platelet counts (mean, 311×103 per cubic millimeter vs. 605×103 per cubic millimeter; P<0.001) (Table 2 in the Supplementary Appendix). Bone marrow trephine biopsy was performed in five patients at diagnosis; the biopsy specimens were examined in a blinded manner. All showed a characteristic pattern of erythroid hyperplasia without morphologic abnormalities of the megakaryocyte or granulocyte lineages (Fig. 2, and Fig. 1A in the Supplementary Appendix).
Figure 2. Erythroid Hyperplasia with Normal Granulopoiesis and Megakaryopoiesis in Patients with JAK2 Exon 12 Mutations.
Bone marrow trephine sections, stained with hematoxylin and eosin, from a control patient (Panel A) and from one patient with V617F-positive polycythemia vera (PV) (Panel B; shown at twice the magnification in Panel C) show the marked hypercellularity with trilineage hyperplasia that is characteristic of patients with polycythemia vera. There are increased numbers of megakaryocytes, some of which are morphologically abnormal and present in clusters (arrowheads). In contrast, trephine sections from Patient 5, with a K539L JAK2 mutation, were only mildly hypercellular and showed isolated erythroid hyperplasia (Panel D; shown at twice the magnification in Panel E; hematoxylin and eosin). Megakaryocytes appear to be morphologically normal and are not clustered. In Panel F (shown at the same magnification as that in Panel E), Wright–Giemsa staining highlights cells of the erythroid lineage. B denotes bone, and F fat.
Hematopoietic progenitors that are homozygous for the V617F mutation are detectable in most patients with polycythemia vera.12 To seek such homozygosity in patients with exon 12 mutations, individual hematopoietic progenitors from Patients 3, 4, 5, and 7 were genotyped with the use of AseI digestion (Fig. 2B in the Supplementary Appendix), sequence analysis, or both. Homozygosity was not observed in any of the 151 erythroid colonies carrying an exon 12 mutation, whether they were grown in the presence or absence of erythropoietin (Fig. 2C in the Supplementary Appendix). In one patient, granulocyte-macrophage colonies were also heterozygous for the exon 12 mutation, demonstrating that this genetic change occurred at the level of the common myeloid progenitor or the hematopoietic stem cell.
PROLIFERATION AND SIGNALING IN CELLS BEARING EXON 12 MUTATIONS
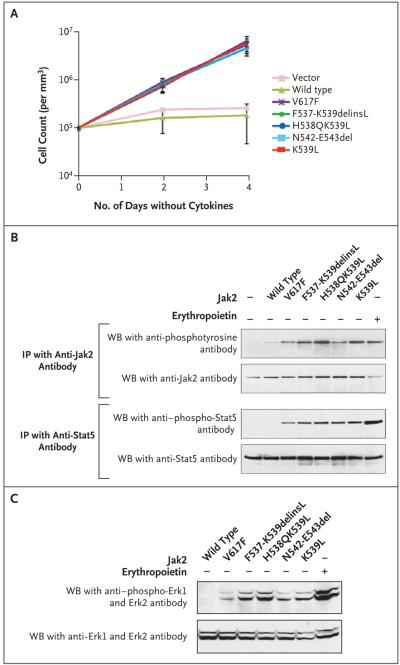
The expression of each Jak2 exon 12 mutant in interleukin-3-dependent BaF3/EpoR cells caused the cells to proliferate in the absence of added exogenous cytokine, with kinetics indistinguishable from those observed for cells with the V617F mutation (Fig. 3A). This proliferation required expression of the erythropoietin receptor; it was not observed in parental BaF3 cells (data not shown). In the absence of stimulation with erythropoietin, all mutants were consistently associated with increased levels of tyrosine-phosphorylated Jak2 and Stat5, as compared with wild-type Jak2 (Fig. 3B). Moreover, the three alleles containing a K539L substitution all generated consistently higher levels of phosphorylated Jak2 than those with the V617F mutation (Fig. 3B). The exon 12 mutants also constitutively activated the Ras–ERK signaling pathway, generating levels of phosphorylated Erk1 and Erk2 that were markedly higher than those obtained with wild-type Jak2 and higher than those obtained with V617F Jak2 (Fig. 3C). In summary, when transduced into BaF3/EpoR cells, all four Jak2 exon 12 mutations caused growth-factor hypersensitivity and activated biochemical pathways associated with erythropoietin signaling.
Figure 3. Proliferation and Increased Signaling in the Absence of Exogenous Cytokine from Jak2 Exon 12 Mutations.
BaF3/EpoR cells (105 per cubic millimeter) — transduced with an empty retroviral vector or stably expressing wild-type murine Jak2 or Jak2 with V617F, F537-K539delinsL, H538QK539L, K539L, or N542-E543del mutations — were cultured in the absence of interleukin-3 for 4 days (Panel A). On days 2 and 4, we assessed cell numbers and viability in quadruplicate using trypan-blue exclusion. Results reflect four independent experiments; mean (±SD) counts for each cell line at both time points are shown. BaF3/EpoR cells transduced with an empty MSCViresGFP retroviral vector (Panel B), or BaF3/ EpoR cells containing wild-type Jak2 or Jak2 with V617F, F537-K539delinsL, H538QK539L, N542-E543del, or K539L mutations were depleted of cytokines for 4 hours. Cells were lysed and underwent immunoprecipitation (IP) with antibody specific for Jak2 or Stat5; Western blot (WB) was then performed with antibodies against phosphotyrosine (4G10), total Jak2, phosphotyrosine-694 Stat5, or total Stat5 (Panel B). BaF3/EpoR cells expressing the Jak2 alleles were analyzed by Western blot with antibodies specific for phosphorylated or total extracellular regulated kinase 1 (Erk1) and 2 (Erk2) (Panel C). BaF3/EpoR cells stimulated with 10 U per milliliter of erythropoietin for 10 minutes were used as positive controls in Panels B and C. Plus signs indicate presence and minus signs absence of exogenous Jak2 or erythropoietin.
RETROVIRAL TRANSFER OF JAK2 MUTATIONS INTO MICE
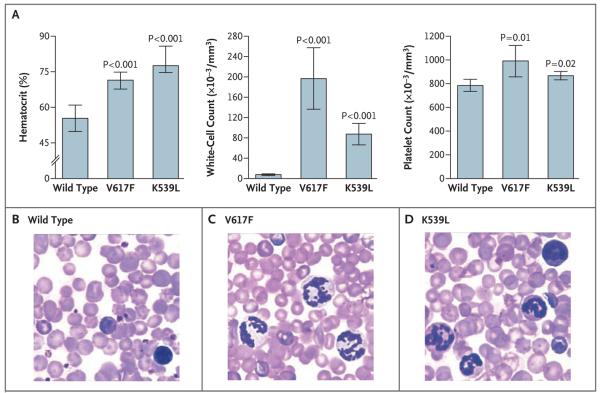
To assess the effects of exon 12 mutations in vivo, murine bone marrow cells were transduced with retroviral vectors encoding wild-type, V617F, or K539L Jak2 and then were transplanted into lethally irradiated BALB/c mice, which are especially susceptible to the development of myeloid disorders after transfer of the V617F mutant.8 Five weeks after transplantation, animals that received V617F-transduced bone marrow cells had erythrocytosis and leukocytosis (Fig. 4A), results that are consistent with previous observations,8 as well as a modest thrombocytosis. Recipients of K539L-transduced cells also had an elevated hematocrit, reticulocytosis, and leukocytosis and a modest thrombocytosis (Fig. 4). Consistent with the human phenotypes associated with exon 12 and V617F mutations, the mean white-cell and platelet counts were lower in recipients of K539Ltransduced cells than in recipients of V617F-transduced cells (P = 0.005 and P = 0.07, respectively). Fluorescence-activated cell-sorting analysis of bone marrow cells from these mice showed that, as compared with wild-type Jak2, K539L-transduced cells resulted in expansion of the erythroid and granulocytic lineages but not those of T lymphocytes, B lymphocytes, or megakaryocytes (data not shown).
Figure 4. A Myeloproliferative Phenotype, Resulting from Retroviral Expression of K539L Jak2, in a Murine Model of Bone Marrow Transplantation.
Panel A shows the mean (±SD) hematocrit, white-cell count, and platelet count in the peripheral blood of BALB/c mouse recipients of bone marrow expressing wild-type, V617F, or K539L Jak2. Mice (five in each group) were evaluated 38 days after transplantation. P values are shown for the comparison with recipients of wild-type Jak2. Panels 2nd 3rd B, C, and D (hematoxylin and eosin) show representative peripheral-blood smears from mice 38 days after transplanation.
DISCUSSION
We have identified a distinctive myeloproliferative syndrome, associated with gain-of-function JAK2 exon 12 mutations, that includes patients who are currently given a diagnosis of polycythemia vera or idiopathic erythrocytosis. Patients with JAK2 exon 12 mutations present with erythrocytosis, low serum erythropoietin levels, and a distinctive histologic appearance of the bone marrow. As in other myeloproliferative diseases, erythropoietin-independent erythroid progenitors can be cultured from peripheral-blood cells, and cytogenetic abnormalities, splenomegaly, or transformation to myelofibrosis has been observed in some patients. Unlike erythroid colonies in patients with V617F-positive polycythemia vera, those in patients with exon 12 mutations are not homozygous for the JAK2 mutation.
The diagnosis of individual patients with a myeloproliferative disorder can be difficult.31 Different centers use different diagnostic criteria, and several diagnostic tests are not widely used. A patient may therefore be given a diagnosis of polycythemia vera by one clinician and a diagnosis of idiopathic erythrocytosis by another. Our results emphasize the importance of molecular classification of these diseases. Exon 12 mutations may have previously been missed when peripheral-blood leukocyte DNA was analyzed, since granulocyte involvement in patients with these mutations is often low. For the molecular diagnosis of this syndrome, it is therefore important to sequence DNA from bone marrow cells or, preferably, from individual clonogenic hematopoietic colonies.
It is not clear how mutations that affect residues 537 through 543 result in unregulated JAK2 activity. To date, only the structure of the JAK2 kinase domain has been elucidated,32 and for this reason the details of interdomain interactions in JAK2 are unknown. However, homology-based molecular modeling suggests that residues 537 through 543 lie within a region linking the predicted SRC homology 2 (SH2) and JH2 domains of JAK2.33 These residues are near the predicted loop carrying V617 in a theoretical model of the full-length JAK2 protein (Fig. 3 in the Supplementary Appendix). Verification of this model awaits detailed structural and biochemical analysis.
Our results also shed light on the various clinical phenotypes associated with exon 12 and V617F mutations. Compared with the V617F mutation, exon 12 mutations result in stronger ligand-independent signaling through JAK2; exon 12 mutations generate higher levels of JAK2 and ERK1 and ERK2 phosphorylation than does the V617F mutation. Moreover, the absence of exon 12 mutations in patients with essential thrombocythemia accords with the proposal that low levels of JAK2 signaling favor thrombocytosis, whereas more-active signaling favors erythrocytosis.9
Acknowledgments
Supported by grants from the U.K. Leukaemia Research Fund and the Wellcome Trust (to Dr. Green), the Leukemia and Lymphoma Society, the Doris Duke Charitable Foundation, and the Howard Hughes Medical Institute (to Dr. Gilliland), Amgen (to Dr. Lodish), the National Cancer Institute (KO1 CA115679, to Dr. Tong), the National Institutes of Health (P01 HL32262, to Dr. Lodish, and DK50654 and CA66996, to Dr. Gilliland), and the American Society of Hematology and the Doris Duke Charitable Foundation (to Dr. Levine).
We thank Romano Kroemer for the coordinates of the JAK2 model; Melanie Percy, Betty Cheung, Anthony Bench, and the staff of the Addenbrooke's Haematological Disorders Sample Bank for the processing of clinical samples; Sara Zarnegar for technical assistance; Brian Huntly for comments on the manuscript; Yana Pikman for assistance with transplant experiments; and Martha Wadleigh for providing clinical details.
Footnotes
No potential conflict of interest relevant to this article was reported.
In Figure 1 (page 462), the DNA-sequence trace for Erythropoietin-Independent Colony for K539L (Patient 5) was incorrect. The figure has been corrected on the Journal's Web site at www.nejm.org.
References
- 1.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–5. [PubMed] [Google Scholar]
- 2.Adamson JW, Fialkow PJ, Murphy S, Prchal JF, Steinmann L. Polycythemia vera: stem-cell and probable clonal origin of the disease. N Engl J Med. 1976;295:913–6. doi: 10.1056/NEJM197610212951702. [DOI] [PubMed] [Google Scholar]
- 3.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 4.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. Erratum, Lancet 2005;366:122. [DOI] [PubMed] [Google Scholar]
- 5.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 7.Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–92. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–81. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacout C, Pisani DF, Tulliez M, Moreau Gachelin F, Vainchenker W, Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–60. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 10.Levine RL, Belisle C, Wadleigh M, et al. X-inactivation-based clonality analysis and quantitative JAK2V617F assessment reveal a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F-negative ET and MMM patients with clonal hematopoiesis. Blood. 2006;107:4139–41. doi: 10.1182/blood-2005-09-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn T, Kremer M, Dechow T, et al. Detection of the activating JAK2 V617F mutation in paraffin-embedded trephine bone marrow biopsies of patients with chronic myeloproliferative diseases. J Mol Diagn. 2006;8:299–304. doi: 10.2353/jmoldx.2006.050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott LM, Scott MA, Campbell PJ, Green AR. Progenitors homozygous for the V617F mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood. 2006;108:2435–7. doi: 10.1182/blood-2006-04-018259. [DOI] [PubMed] [Google Scholar]
- 13.Jamieson CH, Gotlib J, Durocher JA, et al. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc Natl Acad Sci U S A. 2006;103:6224–9. doi: 10.1073/pnas.0601462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott LM, Campbell PJ, Baxter EJ, et al. The V617F JAK2 mutation is uncommon in cancers and in myeloid malignancies other than the classic myeloproliferative disorders. Blood. 2005;106:2920–1. doi: 10.1182/blood-2005-05-2087. [DOI] [PubMed] [Google Scholar]
- 15.Levine RL, Loriaux M, Huntly BJ, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005;106:3377–9. doi: 10.1182/blood-2005-05-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steensma DP, Dewald GW, Lasho TL, et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood. 2005;106:1207–9. doi: 10.1182/blood-2005-03-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones AV, Kreil S, Zoi K, et al. Wide-spread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–8. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 18.Jelinek J, Oki Y, Gharibyan V, et al. JAK2 mutation 1849G→T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood. 2005;106:3370–3. doi: 10.1182/blood-2005-05-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia. 2005;19:1847–9. doi: 10.1038/sj.leu.2403902. [DOI] [PubMed] [Google Scholar]
- 20.Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366:1945–53. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- 21.Campbell PJ, Griesshammer M, Dohner K, et al. V617F mutation in JAK2 is associated with poorer survival in idiopathic myelofibrosis. Blood. 2005;107:2098–100. doi: 10.1182/blood-2005-08-3395. [DOI] [PubMed] [Google Scholar]
- 22.Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:1140–51. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardanani A, Levine R, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–6. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 24.Pearson TC. Evaluation of diagnostic criteria in polycythemia vera. Semin Hematol. 2001;38(Suppl 2):21–4. doi: 10.1016/s0037-1963(01)90136-2. [DOI] [PubMed] [Google Scholar]
- 25.Murphy S, Peterson P, Iland H, Laszlo J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34:29–39. [PubMed] [Google Scholar]
- 26.Barosi G, Ambrosetti A, Finelli C, et al. The Italian Consensus Conference on diagnostic criteria for myelofibrosis with myeloid metaplasia. Br J Haematol. 1999;104:730–7. doi: 10.1046/j.1365-2141.1999.01262.x. [DOI] [PubMed] [Google Scholar]
- 27.D'Andrea AD, Yoshimura A, Yousssoufian H, Zon L, Koo J, Lodish HF. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth regulatory domains. Mol Cell Biol. 1991;11:1980–7. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwaller J, Frantsve J, Aster J, et al. Transformation of hematopoietic cell lines to growth-factor independence and induction of a fatal myelo- and lymphoproliferative disease in mice by retrovirally transduced TEL/JAK2 fusion genes. EMBO J. 1998;17:5321–33. doi: 10.1093/emboj/17.18.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kralovics R, Teo SS, Li S, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377–80. doi: 10.1182/blood-2005-11-009605. [DOI] [PubMed] [Google Scholar]
- 30.Prchal JF, Axelrad AA. Bone-marrow responses in polycythemia vera. N Engl J Med. 1974;290:1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- 31.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–66. doi: 10.1056/NEJMra063728. [DOI] [PubMed] [Google Scholar]
- 32.Lucet IS, Fantino E, Styles M, et al. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood. 2006;107:176–83. doi: 10.1182/blood-2005-06-2413. [DOI] [PubMed] [Google Scholar]
- 33.Giordanetto F, Kroemer RT. Prediction of the structure of human Janus kinase 2 (JAK2) comprising JAK homology domains 1 through 7. Protein Eng. 2002;15:727–37. doi: 10.1093/protein/15.9.727. [DOI] [PubMed] [Google Scholar]