Abstract
Objective
The Hartley guinea pig develops articular cartilage degeneration similar to that seen in idiopathic human osteoarthritis. We investigated whether the application of pulsed low-intensity ultrasound (PLIUS) to the Hartley guinea pig joint would prevent or attenuate the progression of this degenerative process.
Methods
Treatment of male Hartley guinea pigs was initiated at the onset of degeneration (8 weeks of age) to assess the ability of PLIUS to prevent osteoarthritis, or at a later age (12 months) to assess the degree to which PLIUS acted to attenuate the progression of established disease. PLIUS (30 mW/cm2) was applied to stifle joints for 20 minutes per day over periods ranging from three to ten months, with contralateral limbs serving as controls. Joint cartilage histology was graded according to a modified Mankin scale to evaluate treatment effect. Immunohistochemical staining for IL-1 receptor antagonist (IL-1ra), MMP-3, MMP-13, and TGF-β1 was performed on the cartilage to evaluate patterns of expression of these proteins.
Results
PLIUS did not fully prevent cartilage degeneration in the prevention groups, but diminished the severity of the disease, with the treated joints showing markedly decreased surface irregularities and a much smaller degree of loss of matrix staining as compared to controls. PLIUS also attenuated disease progression in the groups with established disease, although to a somewhat lesser extent as compared to the prevention groups. Immunohistochemical staining demonstrated a markedly decreased degree of TGF-β1 production in the PLIUS-treated joints. This indicates less active endogenous repair, consistent with the marked reduction in cartilage degradation.
Conclusions
PLIUS exhibits the ability to attenuate the progression of cartilage degeneration in an animal model of idiopathic human OA. The effect was greater in the treatment of early, rather than established, degeneration.
Introduction
Osteoarthritis (OA) is a slowly progressive, highly prevalent, degenerative disease of diarthrodial joints which leads to loss of normative articular cartilage structure and function. While progress has been made, the search for a clinically useful disease-modifying drug for OA remains ongoing [1–3]. A different approach is suggested by the well-established fact that appropriate mechanical input acts as an anabolic stimulus to chondrocyte metabolism and matrix production [4–9]. While readily implemented in in vitro cartilage studies, mechanical input can be difficult to apply to an animal or human joint. A convenient mode of implementing mechanical stimulation of cartilage is through low-intensity ultrasound. A number of studies have found an ultrasound-induced increase in aggrecan gene expression and chondroitin sulfate synthesis; the systems studied include chondrocytes harvested from embryonic chick sterna [10]; chondrocytes from the rabbit and cultured in a type I collagen gel [11]; rat chondrocytes [12]; the healing fracture callus in the rat [13]; cells harvested from bovine [14] or rabbit [15] intervertebral disc; chondrocytes obtained from human OA patients [16]; and cultured explants from embryonic chick sterna [17] and from human joints [18]. Type II collagen augmentation has also been found in several of these ultrasound-stimulated systems [10, 14, 16–18]. This is of particular interest due to the low collagen-to-proteoglycan ratio typical of engineered cartilage constructs [19].
Ultrasound has also been applied to animal models of degenerative cartilage lesions. In two studies [20, 21] knee cartilage degeneration was induced in the rat by injection of papain. It was found that cartilage repair was enhanced by ultrasound exposure in cases of mild pathology, while progression of established disease was attenuated. In another study, an acute chemical arthritis was induced in the radiocarpal joint of calves by intraarticular injections of turpentine oil [22]. Again, the effect of ultrasound applied over the joint was to improve the histologic state of the cartilage. Salutary effects of ultrasound were similarly observed in donkeys [23]. It was found that application of ultrasound to surgically-induced full-thickness osteochondral lesions in the rabbit knee resulted in a statistically significant improvement in joint pathology and in the histology of repair cartilage [24]. Ultrasound in conjunction with high-molecular weight hyaluronate was found to be effective in reducing OA pathology in a rabbit model [25].
Ultrasound signals incorporating a variety of pulse and power parameters were used in these in vitro and in vivo studies, but in general, the power applied was considerably lower than that used in physical therapy applications [26–28].
The animal studies of ultrasound therapy for OA performed to date have significant limitations in terms of relevance to human idiopathic OA due to the artificial nature of the induced arthritic lesions. In contrast, the Hartley guinea pig is known to develop a progressive osteoarthritic pathology over a period of approximately two years that is similar in many respects to that which develops in humans over decades [29–32]. Thus, it is a particularly appropriate model with which to study the potential effects of therapeutics on idiopathic OA [33–36].
The aim of the current study was therefore to examine the efficacy of pulsed low intensity ultrasound (PLIUS) in the prevention and treatment of idiopathic age-associated OA in the guinea pig. PLIUS was applied to guinea pigs at early and at more advanced stages of the development of OA, with efficacy assessed through use of a histologic scoring system. In a subset of samples, immunohistochemical studies were also performed to provide semi-quantitative analysis of the expression of proteins of particular importance in the pathogenesis of OA.
Materials and Methods
Experimental Groups
A total of 32 outbred male Hartley guinea pigs, forming four groups of eight animals each, were used in these studies (Table 1). For groups E2/T4 and E2/T10, animals were entered into treatment at 2 months of age, with ultrasound applied for 4 and 10 months, respectively. This is prior to the development of macroscopically evident osteoarthritis based on literature studies, so that this represented a disease prevention protocol. For groups E12/T3 and E12/T6, animals were entered into treatment at 12 months of age, with ultrasound applied for 3 and 6 months, respectively. Application of PLIUS to these groups represented treatment of established disease. PLIUS was applied externally for the 20 minute duration of daily treatments to the medial aspect of the left knee of all animals while they were gently restrained in a custom-built harness. The contralateral limb was used as a control in order to account for the substantial inter-animal variability in the development of OA in this model. Accordingly, care was taken to handle both limbs equivalently.
Table 1.
Description of Experimental Groups
| Early or late intervention | Age at enrollment for treatment | Duration of treatment | Age at histologic evaluation | |
|---|---|---|---|---|
| Group E2/T4 n=8 | Early | 2 months | 4 months | 6 months |
| Group E2/T10 n=8 | Early | 2 months | 10 months | 12 months |
| Group E12/T3 n=7 | Late | 12 months | 3 months | 15 months |
| Group E12/T6 n=8 | Late | 12 months | 6 months | 18 months |
Group designations indicate age of animal when enrolled for treatment and duration of treatment, in months. One animal in group E2/T4 died after 11 weeks of treatment; data included. One animal in Group E12/T3 died early in the course of treatment and was not evaluated.
Ultrasound Therapy and Test Device Description
PLIUS Treatment
PLIUS was generated using the Sonic Accelerated Fracture Healing System (SAFHS; Smith and Nephew, Inc., Memphis, TN), with an intensity of 30 mW/cm2 (spatial average-temporal average; SATA) and a sinusoidal waveform of frequency 1.5 MHz. The pulse burst frequency was 1 kHz and the burst duration was 200 μs. After sedation with 5 mg/kg xylazine and 35 mg/kg ketamine, legs were shaved and the animals were immobilized on home-built supports permitting full access to their limbs. Ultrasound transducers were attached to the supports and fixed to the medial aspect of the left knee. Standard coupling gel was applied between the transducer head and the limb to minimize reflection and attenuation of the ultrasound energy. The right knees were handled in an equivalent manner but were shielded from the transducer by a plastic barrier. The duration of PLIUS exposure was 20 minutes five days per week, in conformity with the standard protocol for the PLIUS device when used for bone fracture healing [37].
Sample preparation
Sedated animals were sacrificed by the administration of intravenous KCl, 0.5 mg/kg body weight. Right and left knees were harvested intact and were placed in neutral buffered formalin prior to histologic analysis. Decalcification was initiated by repeated rinsing with PBS for 60 minutes, followed by immersion in formic acid. Uniform exposure was ensured by agitation of the samples. After complete decalcification, the knees were processed with EtOH and xylenes, followed by paraffin embedding. Sections were prepared with a thickness of 6 μm and were stained with 0.1% thionin, a metchromatic stain that is sensitive primarily to the glycosaminoglycan component of the matrix, for histologic analysis. We have found thionin to exhibit particularly stable staining characteristics with respect to sample variations, including hydration [38, 39]. All compared sections (treated vs control) were stained in the same batch to control insofar as possible for variability in the process. The fixation and sectioning process was performed with the joints articulated and intact.
Histologic examination
Microscopic joint pathology was assessed at several sites in the femorotibial joint according to a histological grading system (Table 2) modified from Mankin [40]. Fibrillation, matrix distribution and staining, chondrocyte loss, and chondrocyte cloning were assessed by two independent observers blinded to treatment group, with reported scores representing consensus. Images were obtained with a 4X fixed objective.
Table 2.
Modified Mankin scoring scale
| Fibrillation |
|
| Matrix distribution |
|
| Chondrocyte loss |
|
| Chondrocyte cloning |
|
Grading was performed separately for the medial femoral condyle, lateral femoral condyle, medial tibial plateau, and lateral tibial plateau. Minimum total score is 4 and maximum total score is 16.
Immunohistochemical analysis
Knees were dissected and tissue was processed, embedded, and sectioned according to standard procedures. Antigens were unmasked by treating sections with 0.1units/mL chondroitinase ABC (Sigma, St. Louis, MO) for 30 minutes at 37°C. The following antibodies were used: 1) interleukin 1 receptor antagonist (IL-1ra; R&D Systems, Inc., Minneapolis, MN, # AF-480-NA), at a 1:50 dilution for a final concentration of 2ug/mL; 2) MMP-3 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, # SC-6839), at a 1:100 dilution for a final concentration of 2ug/mL; 3) MMP-13 (Santa Cruz Biotechnology, # SC-12363), at a 1:100 dilution for a final concentration of 2ug/mL; and, 4) TGF-β1 (Santa Cruz Biotechnology, # SC-146), at a 1:100 dilution for a final concentration of 2ug/mL. Femoral condyle tissue was exposed to primary antibody overnight at 37°C. Secondary antibody staining and visualization was performed using the ABC system (Santa Cruz Biotechnology). The percentage of cells showing positive staining for antibodies was evaluated using Bioquant Nova v5.00.8 software (R&M Biometrics, Nashville, TN).
Statistical Comparisons
Numerical data are given as mean ± SD. Comparison of differences between modified Mankin scores for control and PLIUS-treated limbs was performed using the Wilcoxon signed rank test. Comparisons of percent of cells showing positive staining for IL-1ra, MMP-13, and MMP-3 between treated and contralateral limbs were performed using the two-tailed Student t-test for paired data. Statistical significance was taken as p < 0.05 in all cases.
Results
As noted, treatment of groups E2/T4 and E2/T10 represented early intervention (prevention), while treatment of groups E12/T3 and E12/T6 represented treatment of more established disease.
Histology
Progression of OA in Untreated Limbs
As expected, the modified Mankin score (MMS) was observed to increase over time in the untreated limbs (Tables 3 and 4), consistent with the known progression of OA in the guinea pig [41]. The greatest progression occurred in the medial tibial plateau, where the MMS increased from 8.12 ±1.08 at 6 months to 10.78 ± 1.22 at 18 months, an increase of 33% (p< 0.001). In the lateral tibial plateau, the increase was 7.18 ±1.00 to 8.15 ± 0.80, a 14% change (p=0.05). The MMS for the medial femoral condyle was 7.01 ±1.01 at 6 months, which increased to a maximum of 8.39 ± 0.96 at 15 months, a 19.7 % increase (p=0.014). The corresponding values for the lateral femoral condyle, 6.35 ±1.03 at 6 months and 7.22 ± 0.75 at 15 months, a 13.7 % change, also showed a trend towards progression (p=0.074).
Table 3.
Modified Mankin scores for tibial plateau
| Medial tibial plateau | Lateral tibial Plateau | Average tibial plateau | ||||
|---|---|---|---|---|---|---|
| Right Knee: Control | Left Knee: PLIUS | Right Knee: Control | Left Knee: PLIUS | Right Knee: Control | Left Knee: PLIUS | |
| Group E2/T4— | 8.12 ± 1.08 | 7.45 ± 0.81 * | 7.18 ± 1.00 | 7.10 ± 0.99 | 7.65 ± 0.84 | 7.28 ± 0.77& |
| Group E2/T10 | 10.12 ± 1.49 | 9.49± 0.68 | 7.70 ± 0.83 | 7.15 ± 0.55 * | 8.91 ± 0.54 | 8.32 ± 0.38 * |
| Group E12/T3 | 10.03 ± 1.58 | 9.41 ± 1.29 | 8.08 ± 0.53 | 7.69 ± 0.50 | 9.05± 0.94 | 8.55± 0.61 |
| Group E12/T6 | 10.78 ± 1.22 | 10.79± 1.11 | 8.15 ± 0.80 | 8.14 ± 1.30 | 9.46 ± 0.78 | 9.47 ± 1.03 |
| Groups E2/T4 + E2/T10 | 9.12 ± 1.67 | 8.47± 1.28 * | 7.44 ± 0.95 | 7.13 ± 0.78& | 8.28± 0.96 | 7.80± 0.80 ** |
| Groups E12/T3 + E12/T6 | 10.43 ± 1.40 | 10.15 ± 1.35 | 8.12 ± 0.66 | 7.93 ± 1.00 | 9.27 ± 0.85 | 9.04 ± 0.96 |
—E2/T4 indicates animals for which treatment was initiated at 2 months of age and applied for 4 months, and similarly for the other group designations. The results for the combined groups E2/T4 + E2/T10 indicate the efficacy of PLIUS for disease prevention overall, while results for the combined groups E12/T3 + E12/T6 indicate overall efficacy for treatment of established disease. Values show are mean modified Mankin score ± SD. Statistical comparisons are between control and treated knees.
p < 0.01;
p < 0.05;
p=0.10
Table 4.
Modified Mankin scores for femoral condyle
| Medial femoral condyle | Lateral femoral condyle | Average femoral condyle | ||||
|---|---|---|---|---|---|---|
| Right Knee: Control | Left Knee: PLIUS | Right Knee: Control | Left Knee: PLIUS | Right Knee: Control | Left Knee: PLIUS | |
| Group E2/T4— | 7.01 ± 1.01 | 6.77 ± 0.94 | 6.35 ± 1.03 | 6.17 ± 0.99 | 6.68 ± 0.92 | 6.47 ± 0.82 |
| Group E2/T10 | 7.67± 0.93 | 7.57 ± 0.47 | 7.10 ± 0.59 | 7.61 ± 0.84 | 7.39 ± 0.66 | 7.59 ± 0.60 |
| Group E12/T3 | 8.39 ± 0.96 | 7.81 ± 0.43 | 7.22± 0.75 | 6.99 ± 0.76 | 7.81 ± 0.66 | 7.40 ± 0.42 |
| Group E12/T6 | 8.09 ± 1.81 | 7.97 ±1.10 | 7.1 ± 0.78 | 5.40 ± 1.05 ** | 7.59 ± 1.17 | 6.83 ± 0.98& |
| Groups E2/T4 + E2/T10 | 7.34 ± 1.00 | 7.17 ± 0.83 | 6.73 ± 0.90 | 6.89 ± 1.15 | 7.03 ± 0.86 | 7.03 ± 0.90 |
| Groups E12/T3 + E12/T6 | 8.24 ±1.39 | 7.89 ± 0.83 | 7.16 ± 0.74 | 6.14 ± 1.22 ** | 7.70 ± 0.92 | 7.07 ± 0.77 * |
—E2/T4 indicates animals for which treatment was initiated at 2 months of age and applied for 4 months, and similarly for the other group designations. The results for the combined groups E2/T4 + E2/T10 indicate the efficacy of PLIUS for disease prevention overall, while results for the combined groups E12/T3 + E12/T6 indicate overall efficacy for treatment of established disease. Values show are mean modified Mankin score ± SD. Statistical comparisons are between control and treated knees.
p < 0.01;
p < 0.05;
p=0.10
Effect of Treatment
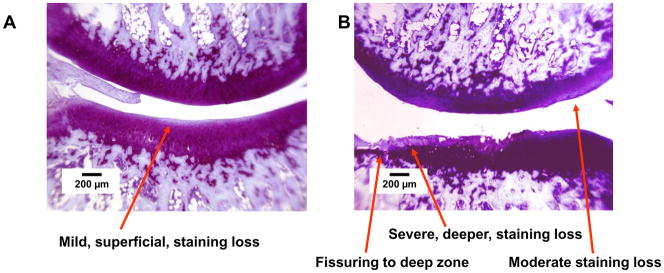
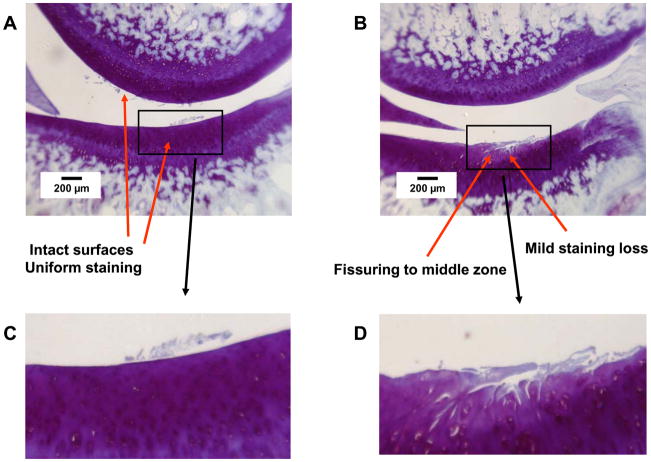
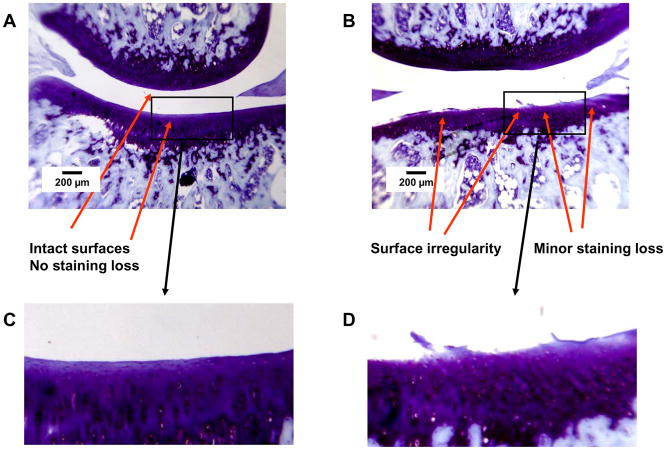
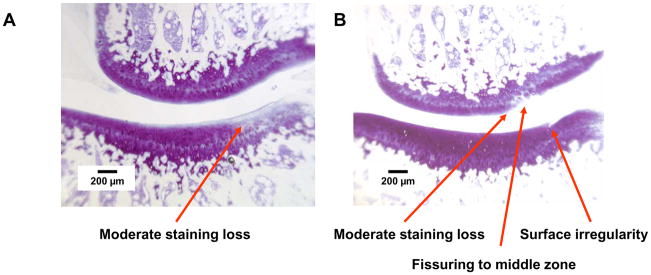
Representative histologic findings are shown in Figures 1–4. The figures are oriented with the femoral condyle above the tibial plateau, and each shows the control and PLIUS-treated knees for one animal. The effect of PLIUS treatment on group E2/T4 is shown in Figs. 1 and 2. Although the pathology was relatively modest even in the medial compartment of this group, the control knees demonstrated surface fibrillation, fissuring, and loss of matrix staining, particularly in the medial tibial plateau (Fig. 1). The degree of pathologic change was decreased in the PLIUS-treated knees. In the lateral compartment as well (Fig. 2), surface irregularities and staining deficits were seen in the control limb which were not seen in the PLIUS-treated knee. Fig. 3 shows an example of PLIUS treatment for group E2/T10. In the medial compartment (Fig. 3), the tibial plateau of the treated limb shows moderate staining loss similar to that seen in the femoral condyle of the untreated limb, but the untreated knee demonstrates a greater degree of surface irregularities as well as more pathologic changes in both the tibial plateau and femoral condyle. In the lateral compartments of group E2/T10 as well, the effect of treatment with PLIUS to attenuate the development of OA was also readily observed, with the control knees demonstrating greater morphologic irregularities and staining loss than the PLIUS-treated knees. For group E12/T6, a greater degree of surface irregularity, fissuring, and staining loss in the medial compartment of the tibial plateau of the untreated limbs was again seen. Fig. 4 illustrates the effects of treatment in the lateral compartment of that group for an animal with relatively mild OA. Again, there are more pronounced morphologic irregularities and staining loss in the control limb. Treatment effects were also seen in the limbs of animals with much more severe disease; staining loss and fissuring are seen in both the control and PLIUS-treated limbs in these animals with more advanced disease but the deficits are more pronounced in the control limb. Similar results were obtained for group E12/T3.
Figure 1.
Group E2/T4, medial compartment. Thionin stain. A. Left side, PLIUS treated. Medial tibial plateau: Intact surface with mild loss of matrix staining in the superficial region. Medial femoral condyle: Intact surface with uniform matrix staining. B. Right side, control. Medial tibial plateau: Extensive surface fibrillation with fissuring to the deep zone and superficial loss of matrix staining. Medial femoral condyle: Intact surface with moderate loss of matrix staining.
Figure 4.
Group E12/T6, Lateral compartment. Thionin stain. A. Left side, PLIUS treated. Lateral tibial plateau: Intact surface and staining. Lateral femoral condyle: Intact surface and staining. B. Right side, control. Lateral tibial plateau: Fissuring to middle zone with focal mild matrix staining loss. Lateral femoral condyle: Intact surface and uniform staining. C and D. Expansion of indicated regions of Panels A and B.
Figure 2.
Group E2/T4, lateral compartment. Thionin stain. A. Left side, PLIUS treated. Tibial plateau: Intact surface, no loss of staining. Femoral condyle: Intact surface, no loss of staining. B. Right side, control. Tibial plateau: extensive surface irregularities with mild loss of matrix staining. Femoral condyle: Intact surface, no loss of staining. C and D. Expansion of indicated regions of Panels A and B.
Figure 3.
Group E2/T10, medial compartment. Thionin stain. A. Left side, PLIUS treated. Tibial plateau: Intact surface with moderate staining loss. Femoral condyle: Intact surface with minimal matrix staining loss. B. Right side, control. Tibial plateau: Small surface irregularity. Femoral condyle: Fissuring to the middle zone and moderate loss of matrix staining.
Tables 3 and 4 show averaged values for the MMS for the tibial plateau and femoral condyle, respectively, for the control and PLIUS-treated animals.
As shown in Table 3, the effectiveness of PLIUS therapy was particularly evident in the medial tibial plateau of group E2/T4 and the lateral tibial plateau of group E2/T10. The greatest efficacy was seen when the data for the prevention groups, E2/T4 and E2/T10, were combined. For the overall prevention group, indicated in Table 3 by “Groups E2/T4 + E2/T10”, there was a statistically significant therapeutic effect of PLIUS treatment for the medial tibial plateau, a clear trend in the lateral tibial plateau, and a strong statistically significant improvement in MMS for the averaged tibial plateau scores.
The results shown in Table 4 demonstrate that the ability of PLIUS to attenuate the progression of established disease was particularly evident in the lateral femoral condyles of group E12/T6. For the overall treatment of established disease, as seen by the combined results for “Groups E12/T3 + E12/T6” in Table 4, there was a statistically significant treatment effect seen in the lateral compartment and for the averaged medial condyle scores. Of note is that in no case did the treated limb demonstrate a trend towards a greater MMS.
Immunohistochemistry
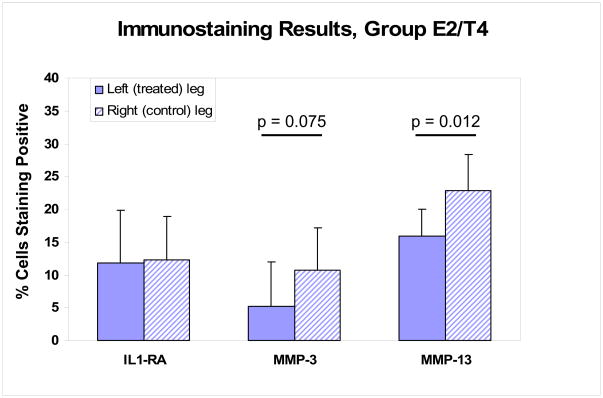
Samples from 8 animals (group E2/T4) were analyzed for the percent chondrocytes expressing MMP-3 (stromelysin-1), MMP-13 (collagenase-3), IL-1RA, or TGF-β1 in the femoral condyles of knees exposed to the PLIUS treatment as compared to the contralateral control knee (Figures 5 and 6). For IL-1Ra, 4 animals showed increased expression on the treated side and while the other 4 animals showed decreased expression; there was no difference on average between the treated and untreated limbs. In the case of MMP-3, there was no consistent pattern observed, with 3 animals showing increased expression in the treated cartilage and 5 animals showing decreased expression. Overall, however there was a trend to a smaller average MMP-3 value in the treated limbs (p = 0.075). In contrast to these mixed results, the percentage of cells expressing MMP-13 was lower in the PLIUS treated side as compared to the control side in all 8 animals studied, with a statistically significant difference being observed between the two sides (p<0.02).
Figure 5.
Percent of cells showing positive staining for IL1-RA, MMP-3, and MMP-13 in group E2/T4. On average, PLIUS treatment resulted in a trend for a smaller percentage of cells to express MMP-3, and a statistically significant reduction in the percentage of cells expressing MMP-13.
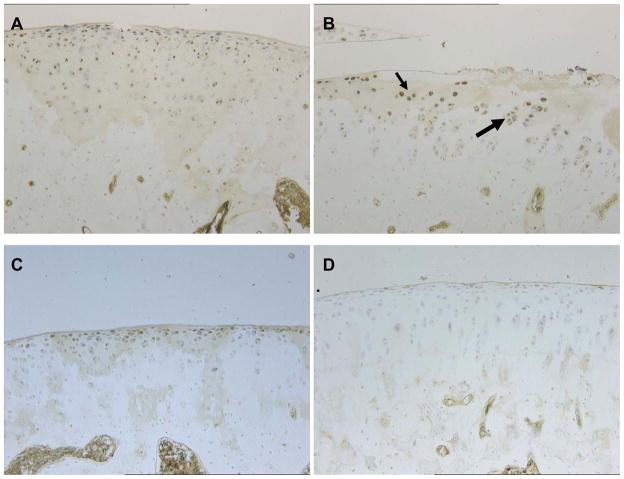
Figure 6.
Representative results of immunostaining for TGF-β1 in the femoral condyles of group E2/T4. A. Expression of TGF-β1 is seen throughout the articular surface of non-treated joint. 4X fixed objective. B. Expansion of region of Panel A. Association of TGF-β1 expression with chondrocytes in clusters. 10X fixed objective. C. Representative region of a different untreated limb, again indicating widespread TGF-β1 expression. 4X fixed objective. D. Dramatic reduction of TGF-β1 expression seen in articular cartilage exposed to PLIUS treatment; shown is the treated joint contralateral to the control joint shown in Figure 6A. 10X fixed objective. In all animals, PLIUS-treated articular cartilage displayed minimal expression of TGF-β1.
A further striking effect of PLIUS treatment was observed on the expression level of TGF-β1. In all 8 animals of group E2/T4, TGF-β1 was widely expressed by chondrocytes throughout the full extent of the articular cartilage of the femoral condyles of control limbs (Figure 6). Figure 6A shows a typical example of the widespread expression of TGF-β1 throughout the articular surface of a non-treated joint. The expression of TGF-β1 was often observed associated with chondrocytes found in colonies which are thought to be part of a repair response (Figure 6B; expansion of region of Figure 6A). Figure 6C shows the widespread expression of TGF-β1 in a non-treated joint from another representative animal. In contrast, the expression of TGF-β1 was dramatically reduced in the articular cartilage exposed to the PLIUS treatment; Figure 6D shows the treated joint contralateral to the control joint shown in Figure 6A. In general, the PLIUS treated articular cartilage displayed minimal expression of TGF-β1. In both the treated and non-treated limbs, chondrocytes in the superficial zone showed the highest level of expression.
Discussion
PLIUS has been applied to a number of in vitro systems and animal models of OA in an effort to characterize its ability to increase the self-repair capacity of cartilage [10–18]. Molecular and cellular studies have indicated that application of PLIUS results in up-regulation of genes involved in anabolic and repair processes, and production of the corresponding proteins has been shown to follow. Similarly, the efficacy of PLIUS has been demonstrated in a number of animal models of localized arthritis. However, to date, these experiments have all involved application of PLIUS to artificially-induced arthritis, whether a focal surgically-induced lesion, or whole-joint pathology, produced by injection of an irritant into the joint capsule or through disruption of the normal anatomy of the joint. In contrast, the current study evaluates PLIUS treatment in the guinea pig model of spontaneous OA, which closely resembles idiopathic OA in the human.
As expected, even the 6 month old untreated animals demonstrated OA pathology. The progression of the disease was particularly rapid over the first year, with relative stability thereafter. These results are consistent with previous reports of the natural history of OA in guinea pigs. In general, OA changes are first observed in the medial tibial plateau at approximately 3 months of age, and later in the medial femoral condyle at approximately 12 – 18 months [32]. Tokuda [42] reported femoral condyle changes as early as 5 months. Lateral tibial plateau lesions are reported to occur at approximately 30 – 40 weeks [30, 42]. In the present study, the extent of these degenerative cartilage changes was attenuated by ultrasound exposure, especially in the overall prevention groups and overall treatment groups, as described in detail above.
The most striking finding from immunostaining was a strong reduction in TGF-β1 with PLIUS treatment, as seen throughout the immunohistologic section. Previous studies have investigated the effects of PLIUS on TGF-β production. Mukai [43] showed that TGF-β1 production was promoted over a time course of up to 12 hours after application of PLIUS to aggregates of rat chondrocytes harvested from the distal femur of 2-day old rats, and that both PLIUS and direct application of TFG-β1 itself to the culture system resulted in greater expression of type II collagen and aggrecan mRNA. Application of anti-TGF-β1 neutralizing antibody rendered the application of PLIUS ineffective. Thus, in this setting, TGF-β1 indicates an acute repair response. However, in nucleus pulposus cells harvested from mature dogs, Hiyama [44] found that PLIUS did not result in an increase in TGF-β1 production when assayed on the same day as the second of two PLIUS applications. Park et al. [45] applied PLIUS over a period of five days to 3-dimensional constructs of chondrocytes harvested from the knees of two week-old pigs, finding that TGF-β1 expression was minimally responsive to application of PLIUS. In our experiments, application of PLIUS extended over a period of months. The decreased level of TGF-β1 protein expression seen after 4 months of PLIUS treatment indicates a less active ongoing repair response; this is consistent with the marked reduction in cartilage degradation seen with treatment, resulting in a much lower requirement for active endogenous repair in the treated limb as compared to the untreated limb. Further, the results of immunostaining indicated an overall decrease in MMP-13 with treatment, consistent with PLIUS acting to attenuate type II collagen digestion. This contrasts with the results of Park et al. [45], in which the mRNA level of MMP-13 was unchanged with PLIUS stimulation in, however, a very different experimental protocol.
There has been increasing recognition that effective therapeutics for OA may require more than chondroprotection and repair [46, 47]. In particular, other joint structures besides cartilage, including bone, synovium, and periarticular muscle, may be involved in the initiation of OA and may also sustain damage from the process itself [48, 49]. According to this concept, PLIUS may have particular potential for OA treatment due to its documented effects on other tissues in addition to cartilage [50, 51].
The mechanism by which ultrasound and other forms of mechanical input provide an anabolic stimulus to cartilage has been investigated in a number of studies. Activation of integrins and a downstream Rho/ROCK/Src/ERK signaling pathway were found to result from PLIUS application to human skin fibroblasts [52]. This suggests a mechanism by which PLIUS may accelerate wound healing and, by extension, increase metabolism in other connective tissues. A subsequent study of PLIUS in an immortalized chondrocyte line identified the activation of a comparable signaling pathway accompanied by upregulation of mRNA for type II collagen and aggrecan [53]. The central role of integrins in initiating chondrocyte mechanotransduction pathways has been extensively reviewed [54, 55].
Pulsed electromagnetic field (PEMF) therapy has also been applied to cartilage disease [33, 56, 57]. Indeed, clear disease modification effects of PEMF in animal models of OA, including the guinea pig model studied here, have been documented. However, in spite of some evident success in clinical trials with endpoints of decreased pain and increased function [58–60], no disease modifying activity of this intervention in clinical studies has been documented to date.
Although there are currently no well-accepted disease-modifying interventions for OA, it is generally agreed that treatment of the disease in its earlier stages is likely to be more effective than treatment of more advanced pathology. This notion is supported by the current results, in which treatment of the medial compartment of the tibial plateau, which demonstrated the most pathology, was more effective in the groups E2/T4 and E2/T10 groups than in groups E12/T3 and E12/T6. It is likely that this may be due to gradual loss of the ability of chondrocytes to produce matrix molecules with age, although this hypothesis was not tested in the present study. Current approaches to early detection are centered on non-invasive MRI studies, although defining the stage of pathology at which anatomic abnormalities can be visualized using this approach remains an open question. Other, non-anatomic, methods for assessing matrix quality by MRI hold a great deal of promise for detection of early OA [61] Non-imaging methodologies based on synovial fluid and blood markers are also under development [62, 63].
There were a number of limitations to this study. It incorporated a relatively small sample size per treatment group. In spite of this, the duration of the study permitted the development of significant pathology in the untreated limbs and the emergence of statistically significant treatment effects in the PLIUS limbs. In addition, virtually all of the non-statistically significant results trended towards efficacy of PLIUS, so that it is likely that a larger sample size would have resulted in even stronger results. We further note that selection of an appropriate control group is problematic for unilateral joint intervention or treatment experiments. Use of a separate group of animals provides a true physiologic control, with outcome measures unaffected by the indirect effects, such as gait alterations, due to the condition of the index joint. However, this greatly limits the power of the analysis in the setting of substantial inter-animal variability; this effect was minimized in our study through choice of the contralateral limb as control. This choice was also consistent with a conservative evaluation of treatment efficacy; improvement in the treated limbs would be expected to, if anything, limit degeneration in the contralateral leg. A further limitation is the possibility that a detailed dose-response investigation of the application of PLIUS in this OA model may indicate that a different dose or application regimen is more effective than the one applied. We applied PLIUS at the dose approved for fracture treatment using the SAFHS. This dose has been documented to result in anabolic effects on chondrocytes and cartilage explants, and in animal models of OA [10, 17, 24]. Limited dose-response data related to this effect is available, with a doubling of the treatment time from 20 to 40 minutes per day significantly improving the quality of repair tissue in surgically-created full-thickness osteochondral defects in the rabbit [24]. It has also been reported that a reduction in the intensity from 30 mW/cm2 (SATA) to 2 mW/cm2 resulted in further improvement in collagen gene expression and protein production in chondrocytes isolated from embryonic chick sterna and cultured in alginate beads [10]. In spite of the lack of dose-response data in the current study, application of the FDA-approved dose was shown to result in the salutary effects reported. Finally, the present study does not address the important points of potential effects on bone or osteophyte formation, and whether the effects of PLIUS would persist after cessation of the therapy, or whether ongoing treatment is required.
We conclude that PLIUS exhibits clear disease-modifying activity in an animal model of idiopathic human OA. These results support the investigation of this modality in a clinical trial.
Acknowledgments
We thank Kenneth Fishbein for his interest and comments. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging, by Smith and Nephew Ltd., London, UK, and by NIH R01 EB000744 (NP).
Footnotes
Conflict of Interest Statement
Drs. Huckle and Todman are employees of Smith and Nephew, Ltd., which provided partial sponsorship for this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moskowitz RW, Hooper M. State-of-the-art disease-modifying osteoarthritis drugs. Curr Rheumatol Rep. 2005;7:15–21. doi: 10.1007/s11926-005-0004-0. [DOI] [PubMed] [Google Scholar]
- 2.Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov. 2005;4:331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DJ, Hellio Le Graverand-Gastineau M-P. How Close are We to Having Structure-Modifying Drugs Available? Medical Clinics of North America. 2009;93:223–234. doi: 10.1016/j.mcna.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Hunter CJ, Mouw JK, Levenston ME. Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthritis Cartilage. 2004;12:117–130. doi: 10.1016/j.joca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Kandel RA. Long-term intermittent compressive stimulation improves the composition and mechanical properties of tissue-engineered cartilage. Tissue Eng. 2004;10:1323–1331. doi: 10.1089/ten.2004.10.1633. [DOI] [PubMed] [Google Scholar]
- 6.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Kandel RA. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res. 2003;21:590–596. doi: 10.1016/S0736-0266(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 7.Waldman SD, Couto DC, Grynpas MD, Pilliar RM, Kandel RA. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthritis Cartilage. 2006;14:323–330. doi: 10.1016/j.joca.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Stoddart MJ, Ettinger L, Hauselmann HJ. Enhanced matrix synthesis in de novo, scaffold free cartilage-like tissue subjected to compression and shear. Biotechnol Bioeng. 2006;95:1043–1051. doi: 10.1002/bit.21052. [DOI] [PubMed] [Google Scholar]
- 9.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZJ, Huckle J, Francomano CA, Spencer RG. The effects of pulsed low-intensity ultrasound on chondrocyte viability, proliferation, gene expression and matrix production. Ultrasound Med Biol. 2003;29:1645–1651. doi: 10.1016/j.ultrasmedbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Nishikori T, Ochi M, Uchio Y, Maniwa S, Kataoka H, Kawasaki K, et al. Effects of low-intensity pulsed ultrasound on proliferation and chondroitin sulfate synthesis of cultured chondrocytes embedded in Atelocollagen gel. J Biomed Mater Res. 2002;59:201–206. doi: 10.1002/jbm.1226. [DOI] [PubMed] [Google Scholar]
- 12.Parvizi J, Wu CC, Lewallen DG, Greenleaf JF, Bolander ME. Low-intensity ultrasound stimulates proteoglycan synthesis in rat chondrocytes by increasing aggrecan gene expression. J Orthop Res. 1999;17:488–494. doi: 10.1002/jor.1100170405. [DOI] [PubMed] [Google Scholar]
- 13.Yang KH, Parvizi J, Wang SJ, Lewallen DG, Kinnick RR, Greenleaf JF, et al. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J Orthop Res. 1996;14:802–809. doi: 10.1002/jor.1100140518. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto K, An HS, Sah RL, Akeda K, Okuma M, Otten L, et al. Exposure to pulsed low intensity ultrasound stimulates extracellular matrix metabolism of bovine intervertebral disc cells cultured in alginate beads. Spine. 2005;30:2398–2405. doi: 10.1097/01.brs.0000184558.44874.c0. [DOI] [PubMed] [Google Scholar]
- 15.Iwashina T, Mochida J, Miyazaki T, Watanabe T, Iwabuchi S, Ando K, et al. Low-intensity pulsed ultrasound stimulates cell proliferation and proteoglycan production in rabbit intervertebral disc cells cultured in alginate. Biomaterials. 2006;27:354–361. doi: 10.1016/j.biomaterials.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Choi BH, Woo JI, Min BH, Park SR. Low-intensity ultrasound stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture. J Biomed Mater Res A. 2006;79:858–864. doi: 10.1002/jbm.a.30816. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZJ, Huckle J, Francomano CA, Spencer RG. The influence of pulsed low-intensity ultrasound on matrix production of chondrocytes at different stages of differentiation: an explant study. Ultrasound Med Biol. 2002;28:1547–1553. doi: 10.1016/s0301-5629(02)00659-2. [DOI] [PubMed] [Google Scholar]
- 18.Min BH, Woo JI, Cho HS, Choi BH, Park SJ, Choi MJ, et al. Effects of low-intensity ultrasound (LIUS) stimulation on human cartilage explants. Scand J Rheumatol. 2006;35:305–311. doi: 10.1080/03009740600588418. [DOI] [PubMed] [Google Scholar]
- 19.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Bursac PM, Vunjak-Novakovic G, et al. IGF-I and mechanical environment interact to modulate engineered cartilage development. Biochem Biophys Res Commun. 2001;286:909–915. doi: 10.1006/bbrc.2001.5486. [DOI] [PubMed] [Google Scholar]
- 20.Huang MH, Ding HJ, Chai CY, Huang YF, Yang RC. Effects of sonication on articular cartilage in experimental osteoarthritis. J Rheumatol. 1997;24:1978–1984. [PubMed] [Google Scholar]
- 21.Huang MH, Yang RC, Ding HJ, Chai CY. Ultrasound effect on level of stress proteins and arthritic histology in experimental arthritis. Arch Phys Med Rehabil. 1999;80:551–556. doi: 10.1016/s0003-9993(99)90198-3. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia R, Sobti VK, Roy KS. Gross and histopathological observations on the effects of therapeutic ultrasound in experimental acute chemical arthritis in calves. Zentralbl Veterinarmed [A] (Journal of Vet Med A) 1992;39:168–173. doi: 10.1111/j.1439-0442.1992.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 23.Singh KI, Sobti VK, Roy KS. Gross and histomorphological effects of therapeutic ultrasound (1 Watt/cm2) in experimental acute traumatic arthritis in donkeys. Journal of Equine Veterinary Science. 1997;17:150–155. [Google Scholar]
- 24.Cook SD, Salkeld SL, Popich-Patron LS, Ryaby JP, Jones DG, Barrack RL. Improved cartilage repair after treatment with low-intensity pulsed ultrasound. Clin Orthop. 2001:S231–243. doi: 10.1097/00003086-200110001-00022. [DOI] [PubMed] [Google Scholar]
- 25.Park SR, Park SH, Jang KW, Cho HS, Cui JH, An HJ, et al. The effect of sonication on simulated osteoarthritis. Part II: alleviation of osteoarthritis pathogenesis by 1 MHz ultrasound with simultaneous hyaluronate injection. Ultrasound Med Biol. 2005;31:1559–1566. doi: 10.1016/j.ultrasmedbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Baker KG, Robertson VJ, Duck FA. A Review of Therapeutic Ultrasound: Biophysical Effects. PHYS THER. 2001;81:1351–1358. [PubMed] [Google Scholar]
- 27.Bjordal JM, Johnson MI, Lopes-Martins RA, Bogen B, Chow R, Ljunggren AE. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord. 2007;8:51. doi: 10.1186/1471-2474-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Özgönenel L, Aytekin E, Durmusog, lu G. A Double-Blind Trial of Clinical Effects of Therapeutic Ultrasound in Knee Osteoarthritis. Ultrasound in Medicine & Biology. 2009;35:44–49. doi: 10.1016/j.ultrasmedbio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Bendele AM, Hulman JF. Effects of body weight restriction on the development and progression of spontaneous osteoarthritis in guinea pigs. Arthritis Rheum. 1991;34:1180–1184. doi: 10.1002/art.1780340916. [DOI] [PubMed] [Google Scholar]
- 30.Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol (Oxford) 1990;71:279–293. [PMC free article] [PubMed] [Google Scholar]
- 31.Bendele AM, White SL, Hulman JF. Osteoarthrosis in guinea pigs: histopathologic and scanning electron microscopic features. Lab Anim Sci. 1989;39:115–121. [PubMed] [Google Scholar]
- 32.Bendele AM, Hulman JF. Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum. 1988;31:561–565. doi: 10.1002/art.1780310416. [DOI] [PubMed] [Google Scholar]
- 33.Ciombor DM, Aaron RK, Wang S, Simon B. Modification of osteoarthritis by pulsed electromagnetic field--a morphological study. Osteoarthritis Cartilage. 2003;11:455–462. doi: 10.1016/s1063-4584(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 34.Kraus VB, Huebner JL, Stabler T, Flahiff CM, Setton LA, Fink C, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50:1822–1831. doi: 10.1002/art.20291. [DOI] [PubMed] [Google Scholar]
- 35.Sabatini M, Lesur C, Thomas M, Chomel A, Anract P, de Nanteuil G, et al. Effect of inhibition of matrix metalloproteinases on cartilage loss in vitro and in a guinea pig model of osteoarthritis. Arthritis Rheum. 2005;52:171–180. doi: 10.1002/art.20900. [DOI] [PubMed] [Google Scholar]
- 36.Bowyer J, Heapy CG, Flannelly JK, Waterton JC, Maciewicz RA. Evaluation of a magnetic resonance biomarker of osteoarthritis disease progression: doxycycline slows tibial cartilage loss in the Dunkin Hartley guinea pig. International Journal of Experimental Pathology. 2009;90:174–181. doi: 10.1111/j.1365-2613.2008.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parvizi J, Vegari D. Pulsed low-intensity ultrasound for fracture healing. Foot Ankle Clin. 2005;10:595–608. vii. doi: 10.1016/j.fcl.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Bulstra SK, Drukker J, Kuijer R, Buurman WA, van der Linden AJ. Thionin staining of paraffin and plastic embedded sections of cartilage. Biotech Histochem. 1993;68:20–28. doi: 10.3109/10520299309105572. [DOI] [PubMed] [Google Scholar]
- 39.Yagi R, McBurney D, Laverty D, Weiner S, Walter E, Horton J. Intrajoint comparisons of gene expression patterns in human osteoarthritis suggest a change in chondrocyte phenotype. Journal of Orthopaedic Research. 2005;23:1128–1138. doi: 10.1016/j.orthres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 41.Han B, Cole AA, Shen Y, Brodie T, Williams JM. Early alterations in the collagen meshwork and lesions in the ankles are associated with spontaneous osteoarthritis in guinea-pigs. Osteoarthritis Cartilage. 2002;10:778–784. doi: 10.1053/joca.2002.0822. [DOI] [PubMed] [Google Scholar]
- 42.Tokuda M. Histological study of spontaneous osteoarthritis in the knee joint of guinea pigs. J Orth Sci (Japan) 1997;2:248–258. [Google Scholar]
- 43.Mukai S, Ito H, Nakagawa Y, Akiyama H, Miyamoto M, Nakamura T. Transforming growth factor-beta1 mediates the effects of low-intensity pulsed ultrasound in chondrocytes. Ultrasound Med Biol. 2005;31:1713–1721. doi: 10.1016/j.ultrasmedbio.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, et al. Synergistic effect of low-intensity pulsed ultrasound on growth factor stimulation of nucleus pulposus cells. J Orthop Res. 2007 doi: 10.1002/jor.20460. [DOI] [PubMed] [Google Scholar]
- 45.Park K, Hoffmeister B, Han DK, Hasty K. Therapeutic ultrasound effects on interleukin-1beta stimulated cartilage construct in vitro. Ultrasound Med Biol. 2007;33:286–295. doi: 10.1016/j.ultrasmedbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Felson DT, Kim YJ. The futility of current approaches to chondroprotection. Arthritis Rheum. 2007;56:1378–1383. doi: 10.1002/art.22526. [DOI] [PubMed] [Google Scholar]
- 47.Brandt KD, Radin EL, Dieppe PA, van de Putte L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 2006;65:1261–1264. doi: 10.1136/ard.2006.058347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quasnichka HL, Anderson-MacKenzie JM, Bailey AJ. Subchondral bone and ligament changes precede cartilage degradation in guinea pig osteoarthritis. Biorheology. 2006;43:389–397. [PubMed] [Google Scholar]
- 49.Muraoka T, Hagino H, Okano T, Enokida M, Teshima R. Role of subchondral bone in osteoarthritis development: a comparative study of two strains of guinea pigs with and without spontaneously occurring osteoarthritis. Arthritis Rheum. 2007;56:3366–3374. doi: 10.1002/art.22921. [DOI] [PubMed] [Google Scholar]
- 50.Yeung CK, Guo X, Ng YF. Pulsed ultrasound treatment accelerates the repair of Achilles tendon rupture in rats. J Orthop Res. 2006;24:193–201. doi: 10.1002/jor.20020. [DOI] [PubMed] [Google Scholar]
- 51.Sparrow KJ, Finucane SD, Owen JR, Wayne JS. The effects of low-intensity ultrasound on medial collateral ligament healing in the rabbit model. Am J Sports Med. 2005;33:1048–1056. doi: 10.1177/0363546504267356. [DOI] [PubMed] [Google Scholar]
- 52.Zhou S, Schmelz A, Seufferlein T, Li Y, Zhao J, Bachem MG. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J Biol Chem. 2004;279:54463–54469. doi: 10.1074/jbc.M404786200. [DOI] [PubMed] [Google Scholar]
- 53.Choi BH, Choi MH, Kwak MG, Min BH, Woo ZH, Park SR. Mechanotransduction pathways of low-intensity ultrasound in C-28/I2 human chondrocyte cell line. Proc Inst Mech Eng H. 2007;221:527–535. doi: 10.1243/09544119JEIM201. [DOI] [PubMed] [Google Scholar]
- 54.Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32:435–446. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- 55.Loeser RF. Integrins and cell signaling in chondrocytes. Biorheology. 2002;39:119–124. [PubMed] [Google Scholar]
- 56.Fini M, Giavaresi G, Carpi A, Nicolini A, Setti S, Giardino R. Effects of pulsed electromagnetic fields on articular hyaline cartilage: review of experimental and clinical studies. Biomed Pharmacother. 2005;59:388–394. doi: 10.1016/j.biopha.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Fini M, Torricelli P, Giavaresi G, Aldini NN, Cavani F, Setti S, et al. Effect of pulsed electromagnetic field stimulation on knee cartilage, subchondral and epyphiseal trabecular bone of aged Dunkin Hartley guinea pigs. Biomed Pharmacother. 2007 doi: 10.1016/j.biopha.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Pipitone N, Scott DL. Magnetic pulse treatment for knee osteoarthritis: a randomised, double-blind, placebo-controlled study. Curr Med Res Opin. 2001;17:190–196. doi: 10.1185/0300799039117061. [DOI] [PubMed] [Google Scholar]
- 59.Trock DH, Bollet AJ, Dyer RH, Jr, Fielding LP, Miner WK, Markoll R. A double-blind trial of the clinical effects of pulsed electromagnetic fields in osteoarthritis. J Rheumatol. 1993;20:456–460. [PubMed] [Google Scholar]
- 60.Trock DH, Bollet AJ, Markoll R. The effect of pulsed electromagnetic fields in the treatment of osteoarthritis of the knee and cervical spine. Report of randomized, double blind, placebo controlled trials. J Rheumatol. 1994;21:1903–1911. [PubMed] [Google Scholar]
- 61.Burstein D, Bashir A, Gray ML. MRI techniques in early stages of cartilage disease. Invest Radiol. 2000;35:622–638. doi: 10.1097/00004424-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Marshall KW, Zhang H, Yager TD, Nossova N, Dempsey A, Zheng R, et al. Blood-based biomarkers for detecting mild osteoarthritis in the human knee. Osteoarthritis Cartilage. 2005;13:861–871. doi: 10.1016/j.joca.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Gobezie R, Kho A, Krastins B, Sarracino DA, Thornhill TS, Chase M, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther. 2007;9:R36. doi: 10.1186/ar2172. [DOI] [PMC free article] [PubMed] [Google Scholar]