Abstract
Cytokines produced during infection/inflammation activate adaptive CNS responses, including acute stress responses mediated by the hypothalamo-pituitary-adrenal (HPA) axis. The mechanisms by which cytokines engage HPA control circuitry remain unclear, though stimulated release of prostanoids from neighboring vascular cells has been implicated in this regard. How specific vascular cell types, endothelial cells (ECs) vs. perivascular cells (PVCs; a subset of brain-resident macrophages), participate in this response remains unsettled. We exploited the phagocytic activity of PVCs to deplete them in rats by central injection of a liposome-encapsulated pro-apoptotic drug. This manipulation abrogated CNS and hormonal indices of HPA activation under immune challenge conditions (interleukin-1; IL-1) that activated prostanoid synthesis only in PVCs, while enhancing these responses to stimuli (lipopolysaccaride; LPS) that engaged prostanoid production by ECs as well. Thus, PVCs provide both prostanoid-mediated drive to the HPA axis, and an anti-inflammatory action that constrains endothelial, and overall CNS, responses to inflammatory insults.
Introduction
Episodes of systemic infection or inflammation engage the innate immune system to release pro-inflammatory cytokines that act on the brain to initiate specific CNS responses. These include a constellation of acute phase reactions, including somnolence, fever, lethargy, anorexia and metabolic effects (Hart, 1988; Konsman et al., 2002), which facilitate adaptation to the challenge at hand. Such insults can also impact the brain’s intrinsic immune effector mechanisms, notably microglia, to precipitate or exacerbate a host of neurodegenerative disorders (Choi et al., 2009; Phillis et al., 2006). Clarifying the cellular-molecular mechanisms of immune-to-brain communication thus has implications not only for understanding basic central processes involved in coping with acute illness but also for identifying targets for intervention in neurological disease.
Here we focus on one key acute phase response system, the hypothalamo-pituitary-adrenal (HPA) axis, an integral part of the brain’s stress response machinery (Turnbull and Rivier, 1999; van der Meer et al., 1996). Glucocorticoid mediators of HPA function exert catabolic effects that mobilize energy reserves to facilitate coping with inflammatory insults, and powerfully suppress immune-inflammatory reactions. This latter effect plays a critical regulatory role in preventing excess cytokine production and immune cell proliferation (Webster et al., 2002). Dysfunction of the central arm of this feedback loop is implicated in the genesis of autoimmune disorders in susceptible animal models (Harbuz et al., 1997) and in humans (Wick et al., 1993).
The mechanisms by which immune stimuli impact the brain to engage the HPA axis remain unsettled. Multiple routes of access have been supported, whose involvement may vary with the strength and nature of the insult (Dantzer and Kelley, 2007; Quan, 2008). For stimuli involving intravenous administration of individual pro-inflammatory cytokines (interleukin-1; IL-1) or pathogen analogs (bacterial lipopolysaccharide; LPS), which model systemic infection, substantial evidence indicates that circulating cytokines can be monitored by non-neuronal cells of the cerebral vasculature, which appear capable of engaging proximate afferent projections to relevant effector neurons by releasing local signaling molecules, notably prostaglandin E2 (PGE2; (Elmquist et al., 1997; Schiltz and Sawchenko, 2003). In the case of HPA control circuitry, evidence supports a role for PGE2 acting on brainstem catecholaminergic neurons that project to corticotropin-releasing factor- (CRF-) expressing hypothalamic neurosecretory cells in initiating IL-1- or LPS-stimulated drive on the axis (Ericsson et al., 1994, 1997; Schiltz and Sawchenko, 2007; van der Meer et al., 1996).
Questions remain as to the manner and extent to which inducible prostaglandin-dependent mechanisms within the brain contribute to HPA responses, and the identity of the vascular cell type(s) involved in transducing immune signals and mounting prostanoid responses. Endothelial cells (ECs) of the cerebral vasculature are optimally positioned to record circulating immune signals, but their threshold to inducible cyclooxygenase (COX-2) expression is high (Schiltz and Sawchenko, 2002). Perivascular cells (PVCs), a subset of brain-resident macrophages, are more sensitive to COX-2 induction (Schiltz and Sawchenko, 2002), but their position in the perivascular space between the EC basement membrane and the glia limitans (Thomas, 1999; Williams et al., 2001), makes them unlikely to be accessed directly by blood-borne cytokines.
To address these issues, we used a method that exploits the phagocytic capabilities of PVCs to selectively deplete them by central administration of a liposome-encapsulated drug, clodronate (Polfliet et al., 2001b). The results support a prominent and selective involvement of brain macrophages, and their capacity to mount prostanoid responses, in immune challenge-induced HPA activation. They also identify a novel, anti-inflammatory, influence of this cell type in restraining endothelial involvement in CNS host-defense responses to inflammatory insults.
Results
Liposome-mediated targeting of brain macrophages
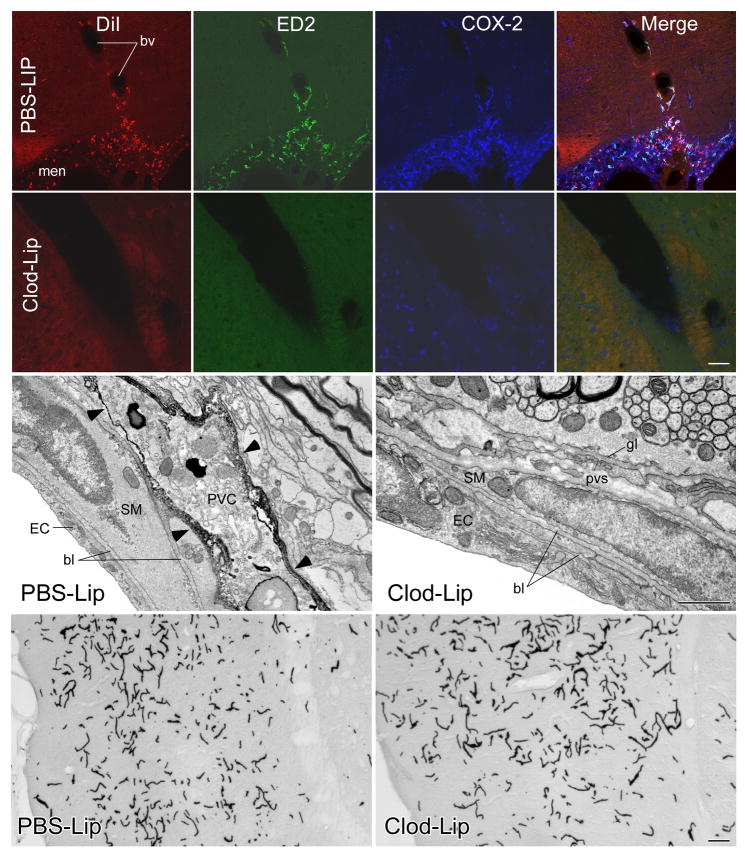
We sought first to validate a method (Polfliet et al., 2001b) that exploits the phagocytic activity of PVCs and meningeal macrophages (MMs) to selectively deplete them by icv injection of liposome-encapsulated clodronate. Clodronate is a bisphosphonate drug used to treat osteolytic disease, which can cause irreversible metabolic damage and apoptosis at high intracellular concentrations (Van Rooijen and Sanders, 1994). Liposomes were labeled with the fluorophore, DiI, to allow visualization of cells that have incorporated them (Polfliet et al., 2001b). Injection of control liposomes, encapsulating PBS (PBS-Lips), resulted in selective DiI labeling of meningeal and vascular cells that expressed the ED2 macrophage differentiation antigen, a definitive PVC/MM marker (Dijkstra et al., 1985); Fig. 1). Using clodronate liposomes (Clod-Lips), ED2 positive cells were frankly reduced in rats sacrificed at 2–3 d, and virtually absent in animals killed 5–7 d, after injection.
Figure 1.
Liposome targeting of brain macrophages. Top: Fluorescence images showing triple labeling for DiI (left panels, red), ED2 (middle left panels, green), and COX-2 (middle right panel, blue) in the meninges and a blood vessel of a rat given DiI-labeled PBS-liposomes into a lateral ventricle 7 d prior to an iv injection of IL-1 (2 μg/kg, iv). Merged images (right panels) show that virtually all Dil-labeled profiles colocalize with ED2- and COX-2-irs indicating that the liposomes were selectively taken up by ED2-positive PVCs and MMs, and that inducible COX-2 expression is discretely localized to this cell type. Second row: Images showing the same markers, but from a rat that received icv injection of clodronate-filled liposomes (Clod-Lips) prior to an IL-1 challenge. Clod-Lip pretreatment results in a loss of detectable DiI, ED2 and COX-2 labeling in the meninges and blood vessels. Scale bar: 100 μm. Third row: Immunoelectron micrographs of arterioles from rats injected icv with control or Clod-Lips 7 d earlier. Surface labeling for the ED2 antigen (arrowheads) is seen in cellular elements displaying macrophage-like features (e.g., numerous lysosomes, multivesicular bodies) in the perivascular spaces (pvs) of PBS-, but not Clod-Lip, treated animals. Other aspects of vascular structure, including the morphology of endothelial (EC) and smooth muscle (SM) cells and basal laminae (bl), are ostensibly unaffected by Clod-Lip treatment. Scale bar: 1 μm. Bottom row: Light micrographs through comparable regions of temporal cortex from control and Clod-Lip pretreated rats, showing vascular labeling for HRP 60 min after iv injection. No evidence of leakage of enzyme into brain parenchyma is evident. Scale bar: 100 μm.
The effectiveness of liposome-mediated targeting of brain macrophages was evaluated after each experiment by co-staining series of brain sections for ED2, challenge-induced COX-2 expression and/or the DiI fluorophore. Rats killed 5–7 d after icv injection of PBS-Lips and iv injection of vehicle displayed DiI labeling strictly limited to ED2-stained cells associated with the meninges and cerebral vasculature. The efficiency of labeling was high, with >95% of all ED2 positive cells co-labeled for DiI in samplings from multiple brain regions (see methods). COX-2 was not detected in DiI-and/or ED2-stained cells of control animals. In separate series of sections prepared for concurrent localization of DiI and Iba1, a marker that labels microglia, as well as PVCs/MMs, we failed to detect DiI labeling of parenchymal microglia in any experiment (not shown).
Rats that received icv injection of control liposomes and were challenged 7 d later with IL-1 (2 μg/kg, iv) displayed a similar disposition of DiI/ED2 labeling, which was overlain by induced COX-2 expression. Vascular and meningeal COX-2 immunoreactivity was strictly confined to ED2-labeled cells. By contrast, Clod-Lip-treated animals challenged with IL-1 showed in most cases a complete absence of vascular and meningeal labeling for DiI, ED2 and COX-2. In these cases, reliable COX-2 labeling was seen only in neurons (mainly in cortical regions) that express the enzyme constitutively.
Ancillary analyses were carried out to assess the broader impact of central liposome treatment. Electron microscopy (conventional and of ED2-immunolabeled material) of brain tissue from control and Clod Lip-treated rats revealed no major disruption of vascular structure in the latter, save for instances of vacant areas and/or apparent cellular debris in the perivascular spaces, presumably reflecting the loss of ED2+ PVCs (Fig. 1). Basal laminae and junctions (zonulae occludens) between adjoining endothelial cells appeared unaffected, and there was no evidence of infiltration by circulating lymphocytes. The integrity of the blood-brain barrier was assessed by injecting experimental and control animals intravenously with horseradish peroxidase. Light level analysis of histochemical preparations revealed no detectable penetration of enzyme into the brain parenchyma in either group, aside from that expected at circumventricular organs. Finally, as central injection of Clod-Lips is reported to variably and transiently attenuate peripheral macrophage populations (Polfliet et al., 2001b), we assessed the most sensitive of these, hepatic Kupffer cells, during the time window (5–7 d post-injection) in which immune challenges were administered (Supplemental Fig. 1). Comparisons of the density of ED2-labeled cells in comparable series through the liver of control and Clod-Lip-treated rats (n=3) revealed no significant difference (P>0.1).
These observations support the efficacy and selectivity of icv injection of Clod-Lips in depleting brain-resident vascular and meningeal macrophages.
Vascular responses to immune insults
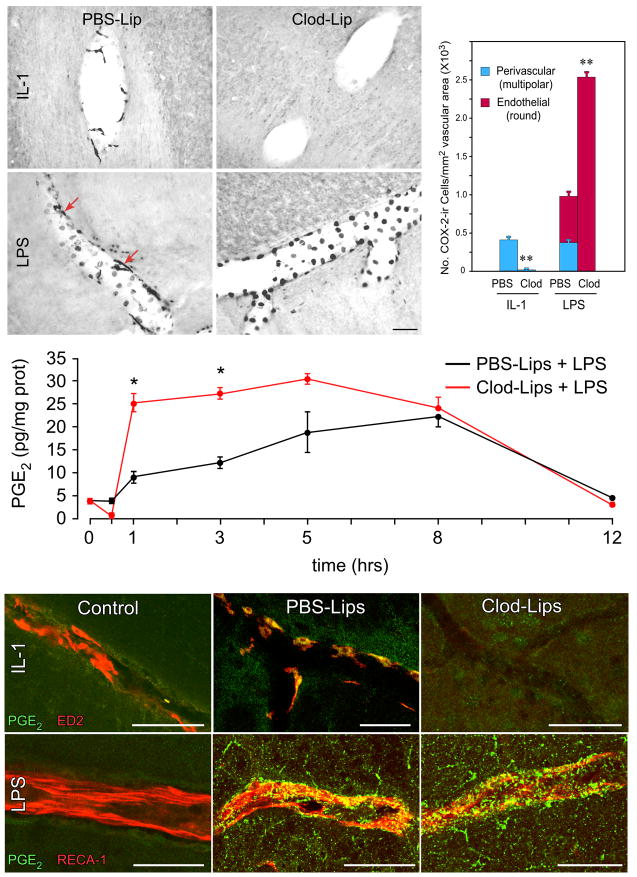
Control rats sacrificed 3 h after IL-1 injection (2 μg/kg, iv) displayed the expected induction of COX-2 in the cerebral vasculature and meninges; pretreatment with icv injection of Clod-Lips 5 d earlier completely eliminated detectable IL-1-induced COX-2 expression at these loci (Fig. 2). LPS injections (2 μg/kg, iv) in PBS-Lip rats provoked the expected COX-2 induction in both multipolar and round vascular profiles shown previously by dual staining to conform to PVCs (ED2+) and ECs (RECA-1+), respectively (Schiltz and Sawchenko, 2002). Predictably, LPS challenges of Clod-Lip pretreated rats elicited COX-2 responses only in ECs, but this effect was remarkably potentiated, in that COX-2-ir ECs were far greater in number (2.4-fold increase) and staining intensity in macrophage-depleted than control rats. Because ECs far outnumber PVCs, the overall density of COX-2-labeled vascular cells in Clod-Lip rats challenged with LPS more than doubled that of PBS-Lip-pretreated controls (Fig. 2).
Figure 2.
Liposome effects on indices of vascular prostanoid production. Top: Differential effects of liposome treatment on induced vascular COX-2 induction. IL-1 treatment in control (PBS-Lip) animals induces COX-2 in multipolar cells (shown previously to correspond to PVCs), while LPS activates the enzyme in both PVCs (arrows) and in round profiles that conform to nuclear/perinuclear regions of endothelia. While Clod-Lip treatment eliminates IL-1-induced COX-2 expression by ablating PVCs, it enhances LPS effects on the number and staining intensity of COX-2-ir in endothelia, indicating that PVCs restrain endothelial cell responsiveness. Histogram shows quantitative comparison of the density (number of cells/mm2 vascular area) of COX-2-ir PVCs, endothelia and total vascular cells as function of treatment status. N=6/group. *, p<0.001 versus PBS-liposome-treated controls. Middle: LPS effects on tissue PGE2 levels in control and Clod-Lip pretreated rats. LPS-induced elevations in mean (± SEM) PGE2 concentration in medulla are significantly greater over 1–3 h post-injection in brain macrophage-deficient rats. Bottom: Effects of brain macrophage depletion on immune challenge-induced PGE2-ir in vascular cell types. Merged confocal images from rats treated with IL-1 (top row) or LPS (bottom row) co-stained for PGE2-ir (green) markers (red) for perivascular (ED2; top) or endothelial cell (RECA-1; bottom) in untreated (left), and IL-1- or LPS-challenged rats pretreated with control (middle) or clodronate liposomes (right). Doubly-stained elements appear as yellow. Under control conditions, PGE2-ir is not detectable in either vascular cell type. In intact rats, IL-1 provokes robust cytoplasmic PGE2 staining localized discretely to PVCs, while cytokine-induced labeling is absent in PVC-depleted animals. LPS induces more widespread punctate PGE2 staining, a portion of which localizes to ECs; this labeling is enhanced in Clod-Lip-pretreated animals. Scale bars: 100 μm.
Analysis of more specific indices of PGE2 production as function of treatment status yielded generally compatible findings. Thus, vascular expression of microsomal prostaglandin E synthase-1 (mPGES-1), a terminal enzyme in PGE2 synthesis which is commonly coupled to COX-2 activity, was not detectable under basal conditions, but was mildly upregulated in response to IL-1 and more strongly in response to LPS in control (PBS-Lip) rats (Supplemental Fig. 2). Concurrent dual localization of mPGES-1 mRNA and ED2-ir indicated that enzyme expression included PVCs in both challenge paradigms, though these were sparse. Clod-Lip pretreated animals displayed massively potentiated upregulation of mPGES-1 mRNA in presumed endothelial cells following an LPS challenge, consistent with the COX-2 findings in this cell type described above, but this was also seen in IL-1-injected rats, whose endothelia failed to mount a detectable COX-2 response.
To determine whether altered expression of prostaglandin biosynthetic enzymes in Clod-Lip pretreated rats is reflected in altered release of PGE2, we attempted time course analyses in which prostanoid levels were measured by enzyme immunoassay in extracts of hypothalamus or medulla. This was not feasible in IL-1 challenged animals, as basal and stimulated PGE2 were below the detection limits of the assay. With LPS, however, Clod-Lip pretreated rats displayed a more rapid, pronounced and prolonged LPS-induced rise in brain tissue levels of PGE2 than controls (Fig. 2). To overcome the lack of sensitivity in the IL-1 model, we established a protocol for immunolocalization of PGE2, itself (see Methods), and found a clear and discrete upregulation of PGE2-ir in identified PVCs of intact rats injected with IL-1; similarly challenged Clod-Lip pretreated animals failed to display detectable prostanoid signals in brain (Fig. 2, Supplemental Fig. 3). LPS challenge in control rats also gave rise to detectable PGE2 immunostaining, which was punctate in appearance and codistributed in part with an endothelial marker. This staining was enhanced in macrophage-depleted rats, and, in addition, extended into the parenchyma, with some taking the form of microglial-like cellular profiles, a localization confirmed by co-staining with a microglial marker (Iba-1; not shown).
Overall, the enhanced inducible PGE2 responses to LPS in the brain macrophage-depleted model indicates that PVCs exert a potent restraining influence on EC activity in transducing circulating immune signals. The starkly differential effects of liposome treatment on multiple indices of PGE2 production after IL-1 versus LPS challenges define models for evaluating the role of vascular prostanoid synthesis in acute phase responses.
Effects on central stress-related circuitry
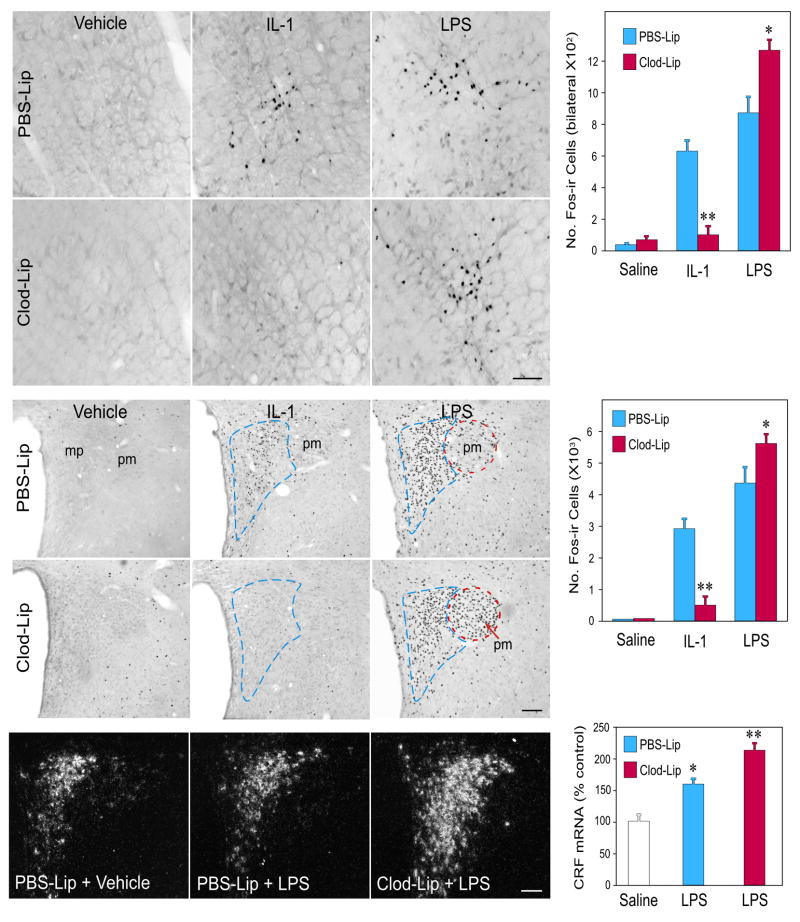
Differences in IL-1- and LPS-induced vascular COX-2 induction in Clod-Lip animals were mirrored in challenge effects on HPA control circuitry, assessed using Fos immunostaining as a generic marker of cellular activation (Fig. 3). Projections to the hypothalamus arising from catecholamine-containing (adrenergic and noradrenergic) neurons in the caudal brainstem (nucleus of the solitary tract and ventrolateral medulla) have been specifically implicated in mediating IL-1 and LPS effects on HPA output (Ericsson et al., 1994; Schiltz and Sawchenko, 2007; van der Meer et al., 1996). Here, we confirmed that both cell groups display markedly increased Fos expression in response to standard doses of IL-1 or LPS (2 μg/kg, iv). Co-staining for Fos and the catecholamine biosynthetic enzymes, dopamine-β-hydroxylase and phenylethanolamine-N-methyltransferase, identified the overwhelming majority of responsive neurons as displaying the adrenergic or noradrenergic phenotype (Supplemental Fig. 4). Whereas Clod-Lip pretreatment exerted no discernible effect on Fos expression in saline-injected controls, it resulted in markedly reduced responses to IL-1, to levels that did not differ reliably from those of controls. By contrast, brain macrophage depletion yielded significant increases in the number of NTS and VLM neurons displaying activational responses to LPS, over and above that seen after similar injection in PBS-Lip pretreated animals (P<0.01).
Figure 3.
Liposome effects on immune challenge-induced activation of HPA control circuitry. Brightfield images of IL-1- and LPS-induced Fos expression in the ventrolateral medulla (top) and PVH (middle) as a function of brain macrophage status. Histograms show mean ± SEM number of cells in the A1 and C1 regions of the ventrolateral medulla and PVH in each condition (n=5–7 per group). Liposome treatment did not significantly affect basal levels of Fos expression in either region, and data from PBS- and Clod-Lip rats that received iv saline injections are pooled for presentation. Clod-Lip treatment reduced IL-1 stimulated Fos expression in medullary neurons whose projections are required for HPA activation, and their hypothalamic targets in the parvocellular part of PVH (mp; outlined in blue) that governs HPA output. By contrast, brain macrophage depletion enhanced activational responses induced by LPS at both levels of the HPA control circuit. This includes recruitment of additional cell types in the magnocellular division of the nucleus (pm; outlined in red). Bottom: Darkfield photomicrographs showing CRF mRNA under basal (saline-injected) and 3 h after LPS challenge in control- and Clod-Lip-injected rats. Mean ± SEM relative levels of this transcript in these treatment groups (n= 5–8) are shown. Clod-Lip pretreated rats display significantly enhanced upregulation of CRF mRNA, relative to similarly challenged rats that received control liposome injections. *, p<0.05; **, p<0.01 versus saline-injected controls. Scale bars: 100 μm.
At the level of hypothalamic effectors that control HPA output, IL-1-induced Fos expression in control rats was focused in the CRF-rich hypophysiotropic zone of the PVH, and this response was reduced (by 80%) in Clod-Lip pretreated animals to levels that did not differ from those of vehicle-injected control animals (P>0.1). LPS provoked a similarly focused, but more robust, activation of PVH neurons in PBS-Lip rats. Clod-Lip pretreatment resulted in a reliable increase in the number of responsive PVH neurons, attributable to recruitment of cells in the magnocellular division of the nucleus, which have also been implicated as contributing to central drive on the HPA axis (Holmes et al., 1986). This analysis was carried out in material prepared under staining conditions that optimize sensitivity of immunolocalization, and the robustness of LPS-induced Fos in the parvocellular neurosecretory zone of the PVH might impose a ceiling effect. Staining of additional series of sections from control and Clod-Lip animals challenged with LPS using higher dilutions of Fos antiserum revealed a reliable (31%) increase in the number of labeled cells in the parvocellular PVH, supporting the contention that macrophage depletion results in enhanced drive to both major functional compartments of the nucleus. This finding was further supported by comparisons of relative levels of CRF mRNA in the PVH, which revealed a reliably greater increase in the Clod-Lip (2.2 fold) than PBS-Lip group (1.6 fold) in response to LPS (p<0.05).
Together, these findings indicate that brain macrophages are required for IL-1 engagement of HPA control circuitry, which at both medullary and hypothalamic levels responds to brain macrophage depletion in a manner indicative of a positive relationship with vascular COX-2/PGE2 production. This extends to the exaggerated HPA-regulatory responses seen in the LPS model.
Stress hormone secretion
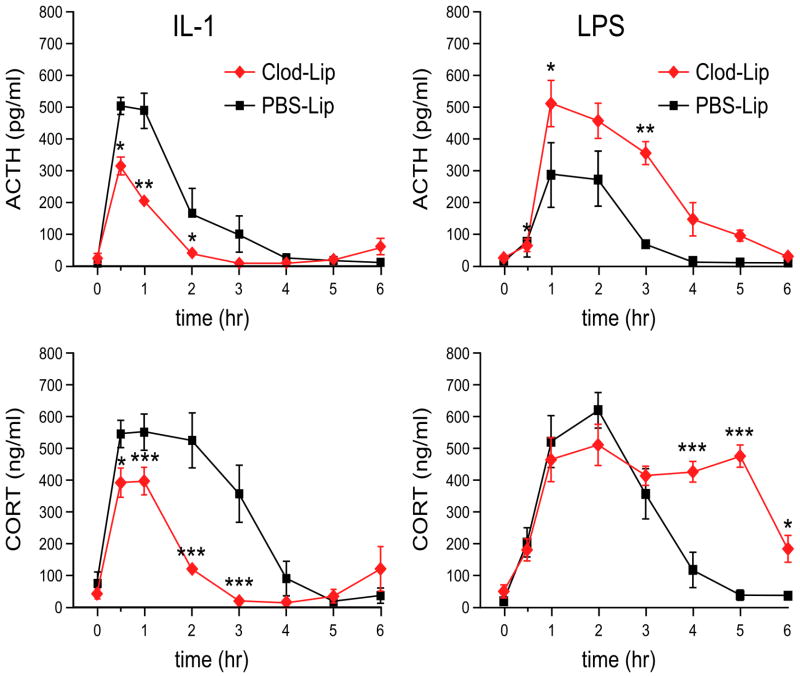
To determine whether altered responsiveness of HPA control circuitry as a function of brain macrophage status is mirrored by changes in hormonal output, separate groups of rats (n=5) pretreated with control or Clod-Lips were implanted with jugular catheters, challenged 2 d later with IL-1 or LPS as above, and repeated blood samples were collected for radioimmunoassay of plasma adrenocorticotropic hormone (ACTH) and corticosterone. Basal titers of neither hormone varied reliably as a function of brain macrophage status (Ps>0.1; Fig. 4). An IL-1 challenge elicited rapid and reliable increases in plasma levels of both hormones that peaked at similar time points. However, both the ACTH and corticosterone secretory responses of Clod-Lip-treated rats were of significantly lesser magnitude and duration than those of control animals. Total integrated responses, as assessed by calculating areas under the curve (AUC), were reduced to 49% (ACTH) and 37% (corticosterone) of control values (Ps<0.001).
Figure 4.
Liposome effects on challenge-induced stress hormone secretion. Mean ± SEM plasma ACTH (top) and corticosterone (CORT; bottom) levels in control rats (black) and animals pretreated with Clod Lips (red), and subsequently challenged with iv injection of 3 μg/kg IL-1 (left) or 2 μg/kg LPS (right). In IL-1-challenged rats, macrophage ablation did not affect the timing of peak ACTH and CORT responses, but attenuated their magnitude, and markedly reduced the time over which significant elevations were observed. Conversely, and in line with the COX-2 and Fos data, the principal effect of Clod Lip pretreatment in LPS-challenged rats was to significantly enhance the longevity of hormonal responses. n = 5–6 per group. *, differs significantly from control liposome group, p<0.05; **, p<0.01; ***, p<0.001.
LPS also elicited significant increases in ACTH and corticosterone secretion in both groups that peaked at similar time points, and in the case of corticosterone achieved similar maxima (Fig. 4). But the magnitude of peak ACTH titers and the duration of both hormonal responses were substantially greater in Clod-Lip-treated rats, as were AUC measures (2.4- and 1.5-fold increases for ACTH and corticosterone, respectively; Ps<0.001). Thus, the differential effects of brain macrophage depletion on IL-1- and LPS-driven engagement of HPA control circuitry are predictive of alterations in stress hormone secretion.
Other acute phase responses
Groups of rats (n=9) pretreated centrally with clodronate or control liposomes were implanted with telemetry transducers to allow continuous remote monitoring of body temperature and locomotor activity. Two days later, they were challenged with IL-1 or LPS 1 h prior to the onset of the nocturnal phase of the lighting cycle, when animals are most active.
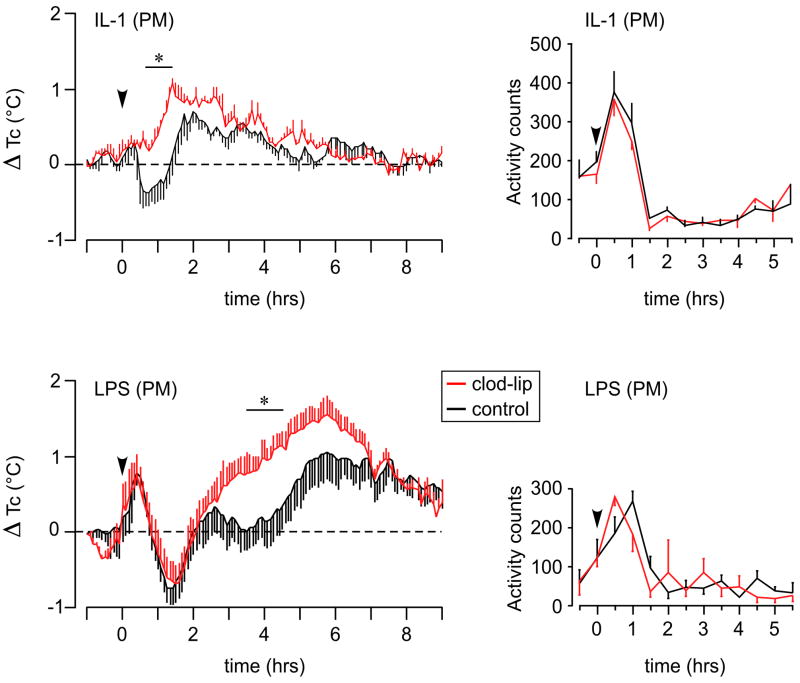
In rats pretreated with control liposomes, IL-1 elicited a biphasic core temperature response, with an initial mild hypothermia preceding a more substantial fever that peaked at ~2 h after injection (Fig. 5). Clod-Lip treated animals failed to exhibit a significant decline in core temperature, but the peak magnitude of their febrile responses did not differ reliably from those of controls. LPS provoked in both groups an early rise and decline in core temperature, whose timing and magnitude were similar. Thereafter, controls exhibited a biphasic rise in temperature with peaks at roughly 2.75 and 6 h after injection, whereas brain macrophage-depleted rats displayed a monophasic fever that peaked at ~6 h and whose magnitude was significantly greater over the 3.5–4.5 h time interval (Ps <0.05).
Figure 5.
Liposome effects on IL-1- and LPS-induced fever and lethargy. Left: Mean ± SEM change in core body temperature (Tc; data points in 3 min bins) of rats pretreated with control (black) or Clod Lips (red), and challenged (arrowhead) with iv injection of IL-1 (top) or LPS (bottom) 1 h before lights out. Brain macrophage ablation eliminated an early hypothermic response to IL-1, but did not significantly affect the magnitude or duration of subsequent fever. By contrast, LPS-induced fever was enhanced at 3.5–4.5 h post-injection. Right: Locomotor activity data (averaged into 30 min bins) from the same animals. Clod-Lip treatment did not affect the hypoactivity elicited by either treatment. n = 9 per group. *, differs significantly from control liposome group, p<0.05.
By contrast, activity responses were unaffected by Clod-Lip pretreatment. Both IL-1 and LPS treatments (Fig. 5) gave rise to rapid and significant (Ps<0.01) decrements in activity beginning at 0.5–1.5 h after injection, whose magnitude did not differ reliably as a function of brain macrophage status at any time point through 6 h post-injection (Ps>0.10; Fig. 5).
Repetition of this study in separate groups challenged with IL-1 or LPS during the subjective morning hours yielded compatible results (Supplemental Fig. 5).
Discussion
Differential effects of brain macrophage ablation on indices of cerebrovascular PGE2 production induced by IL-1 (reduced) versus LPS (exaggerated) were paralleled by altered responses of the HPA axis and its CNS control circuitry. Other acute phase responses were affected less profoundly (fever) or not at all (lethargy). The results support a dependence of HPA responses to proinflammatory insults on the integrity of perivascular cells and their capacity to mount prostanoid responses, and define a novel anti-inflammatory interaction between perivascular and endothelial cells in transducing circulating cytokine signals and sculpting specific CNS responses to them.
Model and implications
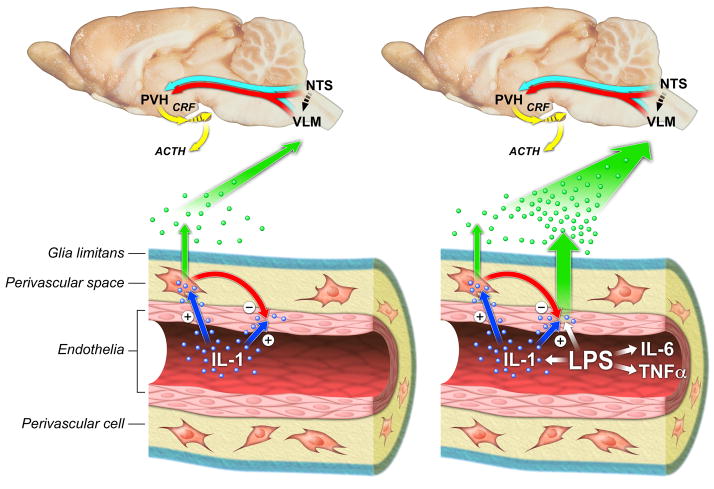
A model to summarize these findings is provided in Figure 6. In response to IL-1, the circulating cytokine activates PVCs most likely indirectly, after first being bound by endothelial type 1 IL-1 receptors (Ericsson et al., 1995). ECs are responsive to this stimulus, and signaling through them is required for upstream CNS effects (Ching et al., 2007; Gosselin and Rivest, 2008), but they do not display detectable indices of PGE2 production, due at least partly to a restraining influence exerted on them by PVCs. PVC activation leads to production and release of PGE2, which acts in a paracrine manner to engage HPA control circuitry and, consequently, its effector arm in the endocrine hypothalamus. Accordingly, elimination of PVCs abrogates vascular PGE2 production and the HPA response. Moderate to high doses of LPS engage PVCs in a similar manner, and are able to partially overcome the PVC-imposed brake, leading to endothelial PGE2 synthesis, and a greater total vascular prostanoid response. PVC ablation removes the brake, leading to further enhancement of EC and overall PGE2 production, whose effects are manifest particularly during the later stages of HPA and febrile responses.
Figure 6.
Cellular mechanisms for engaging HPA control circuitry by IL-1 (left) or LPS (right) challenges. Both reprise the core circuitry required for activation of the HPA axis, highlighting projections from medullary adrenergic and noradrenergic (blue and red arrows) cell groups to CRF-expressing neurosecretory neurons in the PVH. Bottom: Vascular mechanisms for transducing circulating cytokine signals, involving initial monitoring by endothelial cells with consequent activation of PGE2 synthesis/release (green dots) by perivascular cells under IL-1 treatment and by both perivascular and endothelial cells under the more complex stimulus presented by LPS. Factor(s) produced by PVCs restrain (red arrow) endothelial responsiveness under basal and challenge conditions. Paracrine mediators of bidirectional interactions between the two cell types remain to be identified.
The immune challenge parameters employed here were chosen based on their ability to elicit vascular COX-2 responses exclusively from PVCs (IL-1) or from both these and endothelial cells (LPS; Schiltz and Sawchenko, 2002). Bolus administration of IL-1 is an admittedly artificial situation, but presents a discrete stimulus that mimics many CNS effects of systemic infection (Dinarello, 1991). LPS is a component of the cell wall of gram-negative bacteria, and is widely used to model infection or sepsis (Ulevitch and Tobias, 1995). It stimulates the release of several proinflammatory cytokines, prominently including IL-1 (Turnbull and Rivier, 1999), and can also act directly by binding a toll-like receptor 4/MD2/CD14 complex (Laflamme and Rivest, 2001). Importantly, like IL-1, low doses of LPS stimulate COX-2 only in PVCs (Schiltz and Sawchenko, 2002), and we would predict that results obtained in the IL-1 model would generalize to mild LPS challenges.
These findings clarify the cellular mechanisms by which challenges that model systemic infection access the brain to engage specific CNS response systems, notably HPA axis activation and fever, that facilitate coping with insult. Glucocorticoid mediators of HPA function serve to mobilize energy reserves and constrain systemic immune responses, while fever may create a suboptimal thermal environment for pathogen proliferation and can directly enhance certain host defense mechanisms (Mackowiak, 1998).
Understanding the transit of immune signals across the blood-brain-barrier also has implications for the many neurodegenerative diseases in which central inflammatory mechanisms are believed to play a contributing role. Systemic LPS and/or IL-1 treatment is commonly reported to exacerbate neuropathology and cognitive/behavioral symptoms in animal models of Alzheimer’s, Parkinson’s and Prion Diseases, and ALS (Byler et al., 2009; Cunningham et al., 2005; Kitazawa et al., 2005; Letiembre et al., 2009). A single (high) dose of LPS, alone, can cause persistent CNS inflammation and neuronal loss (Qin et al., 2007). Moreover, animals challenged neonatally with IL-1 or LPS display altered central regulation of the HPA axis that persists into adulthood (Shanks et al., 1995), and may predispose individuals to an even broader array of stress-related pathologies. In this light, the identification of a potent anti-inflammatory mechanism at the blood-brain interface should provide leverage in identifying targets for intervention in a range of pathologies. The liposome targeting approach employed here has been shown to modulate disease progression in animal models of multiple sclerosis, meningitis and Alzheimer’s Disease (Hawkes and McLaurin, 2009; Polfliet et al., 2001a, 2002) and the feasibility of using the stem cells that give rise to them as vehicles for gene therapy has been established (Hahn et al., 1998; Priller et al., 2001).
What are perivascular cells?
Along with meningeal macrophages, PVCs are derived from bone marrow precursors that populate the brain in early postnatal life and turn over slowly throughout adulthood (Bechmann et al., 2001a; Vallieres and Sawchenko, 2003). They are distinct from pericytes and parenchymal microglia, though they may share a common lineage with them (Gehrmann et al., 1995; Thomas, 1999). As evidenced in the present study, PVCs are constitutively phagocytic, with their definitive ED2 marker having been identified as a macrophage scavenger receptor, CD163 (Fabriek et al., 2007). PVCs can serve as antigen-presenting cells (Hickey and Kimura, 1988), participate in the immune surveillance of the nervous system (Bechmann et al., 2001b; Hickey, 2001), and have been implicated in a host of pathological processes, including the entry of HIV into the nervous system (Stoll and Jander, 1999; Thomas, 1999).
Prostaglandin involvement in CNS host-defense
PGE2 levels in brain are increased following systemic IL-1 or LPS injection (Dinarello, 1991; Sehic et al., 1996), and a wealth of evidence indicates that central or peripheral blockade of prostanoid synthesis using COX inhibitors (NSAIDs) can disrupt the HPA activation normally elicited by these challenges (Ericsson et al., 1997; Katsuura et al., 1988; Lacroix and Rivest, 1998; Watanabe et al., 1990). The cellular source(s) of central prostaglandin production have remained a major point of contention, with previous reports being divided as to whether PVCs (Elmquist et al., 1997; Schiltz and Sawchenko, 2002) or endothelial cells (Cao et al., 1996; Matsumura et al., 1998; Quan et al., 1998) are the dominant seats of LPS- and/or IL-1-driven vascular COX-2 induction. We offered a potential reconciliation by identifying PVCs as the sole site of COX-2 expression following IL-1 or low doses of LPS, with endothelia recruited at higher endotoxin doses (Schiltz and Sawchenko, 2002).
Multiple indices of IL-1-driven HPA activity were suppressed in brain macrophage-depleted rats, supporting a prominent role for PVCs in the activation of central stress circuitry and hormone secretion under this condition. As IL-1 is the most potent of the proinflammatory cytokines induced by LPS in stimulating HPA output (Turnbull and Rivier, 1999), we conclude that PVCs provide a generalized, low-threshold impetus for the engagement of this system by infection/inflammation. Hormonal responses to IL-1 were not completely eliminated by central macrophage depletion, leaving open possible contributions of adjunct central or peripheral mechanisms. Direct IL-1 actions on the pituitary and adrenal glands to stimulate ACTH and corticosterone secretion, respectively, have been described (Bernton et al., 1987; Engstrom et al., 2008).
In addition to indicating a requirement for PVCs in IL-1-induced HPA activation, our findings are also consistent with COX-dependent PGE2 production in mediating this influence. PGE2 production from the COX-dependent intermediate, PGH2, may be catalyzed by several terminal PGE2 synthases, with mPGES-1 being commonly coupled to COX-2 activity (Ivanov and Romanovsky, 2004). While we found that vascular mPGES-1 expression co-varied directly with COX-2, PGE2 and HPA-related endpoints across most treatment conditions, it was expressed only weakly in PVCs of IL-1-stimulated rats. This raises the question of how PGE2 is generated in this cell type with the alacrity required of acute phase mechanisms. There are recent data to implicate the constitutively-expressed cyclooxygenase isoform, COX-1, in the initial phase of LPS-induced HPA activation (Elander et al., 2009; Garcia-Bueno et al., 2009; Zhang et al., 2003). New findings that COX-1 is expressed under basal conditions by PVCs and microglia, and is LPS-inducible in endothelia (Garcia-Bueno et al., 2009), are consistent with a role in the present context. It remains to be determined whether other PGE2 synthases, notably COX-1-associated cytosolic PGES, are positioned to support COX-1 involvement.
Involvement of PGE2, itself, in CNS responses to inflammatory stimuli is commonly inferred on the basis of COX-2 expression. Here, we provide biochemical/histochemical data supporting discrete PGE2 induction in PVCs after IL-1 treatment, more broadly in response to LPS, and for regulation in step with HPA endpoints in macrophage-depleted rats. This evidence is correlative, and it must be noted that PGE2 is one of several prostanoids whose levels increase in brain following insults of the kind we employed (Choi et al., 2008; de Vries et al., 1995). Supporting specific involvement of PGE2 are the findings that IL-1 activates medullary catecholaminergic neurons that express EP3 (and inducible EP4) PGE2 receptors (Ek et al., 2000), and that microinjection of PGE2 into these cell groups discretely activates them and their hypothalamic targets that govern HPA output, in a manner closely mimicking the response to systemic immune challenge (Ericsson et al., 1997). In addition, genetic deletion of PGE2 receptor subtypes (EP1, EP3) attenuates HPA responses to LPS (Matsuoka et al., 2003).
Response-specificity of PVC involvement
The disruptive effect of brain macrophage depletion on IL-1-induced HPA activation did not generalize to other acute phase responses. The body temperature profile was not affected, save for an attenuation of an early hypothermic response. By contrast, later phases of LPS-induced fever (stages 2–3 in the terminology of Romanovsky et al. (2005) were enhanced. The timing of this effect correlates well with observed elevations in endothelial COX-2, mPGES-1 expression and PGE2 levels in Clod-Lip treated rats, again reflecting PVC-imposed restraint of endothelial PGE2 production. Our failure to detect any effect of brain macrophage depletion on IL-1- or LPS-induced reductions in locomotor activity is consistent with reports that COX inhibitors affect this response subtly, if at all (Otterness et al., 1991; Wieczorek and Dunn, 2006). Overall, the results indicate differential involvement of the mechanisms we describe in acute phase responses.
Perivascular-endothelial cell interactions
The shift in the EC COX-2/PGE2 production that we describe following ablation of an adjacent cell type (PVCs) has an intriguing parallel in recent work on cardiac myocytes and fibroblasts (Wang et al., 2009). In the present case, the throttling influence of PVCs is also evident using more generic markers of EC activation, including the vascular early response gene, Verge, and components of the nuclear factor-κB (NF-κB) signaling pathway (Serrats and Sawchenko, unpublished). In view of the potential for exploiting this mechanism to intervene in inflammatory CNS disease, its molecular underpinnings are of particular interest. One candidate for fulfilling such a role is nitric oxide; its expression can be induced by LPS or IL-1 in a number of tissue macrophage populations and cerebrovascular elements (Wong et al., 1996a and b), and it can inhibit cytokine-stimulated endothelial COX-2 expression (Blais and Rivest, 2001), as well as HPA activation (Turnbull and Rivier, 1999). Another potential mediator is 15-deoxy Δ12,14 prostaglandin J2 (15d-PGJ2), an alternate, COX-dependent prostanoid product. 15d-PGJ2 has been shown capable of suppressing LPS-induced COX-2 synthesis by serving as an endogenous ligand for the peroxisome proliferator-activated receptor-γ (PPARγ) and/or by direct interference with NF-κB signaling pathway, both of which are present and active in the vascular endothelium (Bianchi et al., 2005; Inoue et al., 2000; Simonin et al., 2002). Inhibitory effects of 15d-PGJ2 on LPS-induced fever have been described (Mouihate et al., 2004), though it remains unclear whether this or other PPARγ ligands are produced in meaningful concentrations in the cellular context of interest here.
Concluding remarks
The results indicate dual, time-dependent roles of brain resident macrophages in CNS responses to inflammatory insults. These cells are required for full manifestation of HPA responses to IL-1 challenge, and this requirement exhibits acute phase response-specificity. This same cell type serves normally to restrain endothelial prostanoid production; in its absence, the latter stages of HPA and febrile responses to LPS are enhanced. Across conditions, HPA and febrile responses co-varied closely with overall vascular COX-2/PGE2 expression, supporting a dynamic, prostanoid-based transduction mechanism at the brain-vascular interface. Further elaboration of this interplay is expected to clarify central mechanisms of adaptation to acute sickness, and to provide leverage in the many CNS disease states in which inflammatory mechanisms are involved.
Experimental Procedures
Animals
Adult male Sprague-Dawley albino rats (260–340 g) were housed under standard vivarium conditions with food and water freely available, and were adapted to handling for at least 5 d prior to manipulation. All protocols were approved by the Institutional Animal Care and Use Committee of the Salk Institute.
Liposome preparation and delivery
Liposomes were prepared as previously described (Van Rooijen and Sanders, 1994). These are polylamellar phosphatidylcholine-cholesterol membranes that encapsulate clodronate (used at a concentration of 250 mg/ml), are mannosylated, to facilitate receptor-mediated uptake, and labeled with carbocyanine dye, DiI (D282, Molecular Probes), to enable detection of cells that have incorporated them. For icv injection, rats were anesthetized with ketamine-xylazine-acepromazine (25:5:1 mg/kg, s.c.) and mounted in a stereotaxic frame. Control (encapsulating PBS) or clodronate-liposomes were equilibrated to room temperature, gently shaken to resuspend them, and injected in a volume of 50 μl over 10 min into a lateral ventricle using a 26 ga needle mounted onto a stereotaxic arm and attached via PE tubing to a 1000 μl gastight syringe (Bee Stinger, BAS). Rats were allowed 5–7d to recover prior to testing, the point at which depletion of brain macrophages is maximal, and prior to repopulation by bone marrow-derived progenitors (Polfliet et al., 2001b; Van Rooijen and Sanders, 1994).
Intravenous administration of IL-1β and LPS
Indwelling jugular catheters (PE 50) containing sterile, heparin-saline (50 U/ml) were implanted under isoflurane anesthesia (Ericsson et al., 1994). After 2 d recovery, awake and freely moving rats were injected with 2 μg/kg of recombinant rat IL-1β (generously provided by Dr. Ron Hart, Rutgers University), or its vehicle (1 ml/kg, 0.01% BSA, 0.01% ascorbic acid, 10 mM Tris-HCl, 36 mM sodium phosphate buffer, pH 7.4) and returned to their home cages. In similar experiments, groups of rats were injected with lipopolysaccharide from E. coli at 2.0 μg/kg (Sigma, serotype 055:B5) or sterile saline (1 ml/kg).
Perfusion and histology
At appropriate time points (2–4 h post-injection), rats were anesthetized and perfused via the ascending aorta with 4% paraformaldehyde in borate buffer, pH 8.0, at 4 C and the brains were removed, postfixed for 3 h, and cryoprotected overnight. Regularly spaced series of coronal sections (30 μm thick) were collected in cryoprotectant solution and stored at −20°C until processing.
Immunohistochemistry
Immunolocalization was achieved using conventional avidin-biotin immunoperoxidase (Vectastain Elite kit; Vector Laboratories) and dual indirect immunofluorescence methods (Sawchenko et al., 1990), the latter employing fluorescein-, Cy5- and/or Alexa 488-conjugated secondary antisera (Molecular Probes). Combined immunoperoxidase staining with dual fluorescence or with in situ hybridization was carried out using protocols described previously (Chan et al., 1993; Ericsson et al., 1994).
Descriptions of all but one of the antisera employed are provided as Supplemental Data. To localize PGE2-ir, a rabbit polyclonal antiserum raised against PGE2 (Cat. No. PG 31, Oxford Biomedical Research) was used. This serum recognizes PGE2 and associated pathway-specific arachidonate derivatives, including PGE1, PGA2 and PGB2, but does not cross-react with other prostanoids. After screening a range of fixation protocols in sections from rat tissues that express PGE2 constitutively (kidney, gut) and the brains of IL-1- or LPS-treated animals, the following yielded the most sensitive and discrete localization in the cerebral vasculature. Rats were rapidly anesthetized and perfused briefly via the ascending aorta with saline (100 ml over ~ 2 min). Brains were then extracted, immersed in ice-cold saline for 1 min, transected in the coronal plane into forebrain and brainstem blocks, and immersion fixed overnight in 4% paraformaldehyde in borate buffer, pH 9.5, with 15% sucrose added. Adsorption tests were carried out by incubating antiserum overnight in the presence of graded concentrations of PGE2 (half order of magnitude increments between 2.8 μM and 2.8 mM) prior to application to brain sections from IL-1- or LPS-challenged rats. Concentrations ≥ 280 μM abolished all detectable labeling.
Hybridization histochemistry
Techniques for probe synthesis, hybridization, and autoradiographic localization of mRNA signal were adapted from (Simmons et al., 1989), and are detailed as Supplemental Data.
Electron microscopy
These procedures are described as Supplemental Data.
Intravenous HRP injections
This procedure is described as Supplemental Data.
Stress hormone assays
Separate groups of animals were implanted with indwelling jugular catheters 5 d after icv injection of clodronate or control liposomes, and 2 d before stress exposure. Blood samples (300 μl) were taken prior to iv injection of IL-1 or LPS, at 30 min and then hourly through 6 h post-injection. They were collected into chilled EDTA-containing tubes and centrifuged; plasma was stored at −20°C until assay. ACTH was measured using a two-site immunoradiometric assay obtained in kit form (DiaSorin), with intra- and interassay coefficients of variation of 3 and 9%, respectively, and a sensitivity of 5 pg/ml. Plasma corticosterone was measured without extraction, using an antiserum raised in rabbits against a corticosterone-BSA conjugate, and 125I-corticosterone-BSA as tracer (MP Biomedicals). The sensitivity of the assay was 0.8 μg/dl; intra- and inter-assay coefficients of variation were 5 and 10%, respectively.
PGE2 assays
Tissue PGE2 levels were measured by enzyme immunoassay (EIA) using reagents in kit form (Prostaglandin E2 EIA Kit-Monoclonal; Cayman Chemical Company). Details are given as Supplemental Data.
Telemetry
Separate groups of rats received icv injections of control or Clod-Lips and 5 d later were implanted with jugular catheters for iv injection of IL-1, LPS or their respective vehicles, as described above. Under the same anesthetic dose telemetry transmitters (Mini-Mitter) were implanted intra-abdominally. Two d later, core body temperature and activity levels were recorded continuously for 24 h before and after iv injection. Transmitter output was monitored by a receiver board beneath the animals’ cages, which recorded core temperature (to ± 0.1°C) and episodes (counts) of gross horizontal movement.
Supplementary Material
Acknowledgments
The authors thank Carlos Arias, Kris Trulock and Casey Peto for excellent assistance with surgery/histology, imaging/graphics and electron microscopy, respectively. This research was supported by NS-21182, and was conducted in part by the Clayton Medical Research Foundation. PES is a Senior Investigator of the Clayton Medical Research Foundation. Fellowship support was provided by the NIH (NS10695 to JCS; DK064086 to TMR), IBRO (JS) and the Spanish Ministry of Education and Science (JS, BGB) and CIBERsam (BGB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechmann I, Kwidzinski E, Kovac AD, Simburger E, Horvath T, Gimsa U, Dirnagl U, Priller J, Nitsch R. Turnover of rat brain perivascular cells. Exp Neurol. 2001a;168:242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001b;14:1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- Bernton EW, Beach JE, Holaday JW, Smallridge RC, Fein HG. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science. 1987;238:519–521. doi: 10.1126/science.2821620. [DOI] [PubMed] [Google Scholar]
- Bianchi A, Moulin D, Sebillaud S, Koufany M, Galteau MM, Netter P, Terlain B, Jouzeau JY. Contrasting effects of peroxisome-proliferator-activated receptor (PPAR)gamma agonists on membrane-associated prostaglandin E2 synthase-1 in IL-1beta-stimulated rat chondrocytes: evidence for PPARgamma-independent inhibition by 15-deoxy-Delta12,14prostaglandin J2. Arthritis Res Ther. 2005;7:R1325–1337. doi: 10.1186/ar1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais V, Rivest S. Inhibitory action of nitric oxide on circulating tumor necrosis factor-induced NF-kappaB activity and COX-2 transcription in the endothelium of the brain capillaries. J Neuropathol Exp Neurol. 2001;60:893–905. doi: 10.1093/jnen/60.9.893. [DOI] [PubMed] [Google Scholar]
- Byler SL, Boehm GW, Karp JD, Kohman RA, Tarr AJ, Schallert T, Barth TM. Systemic lipopolysaccharide plus MPTP as a model of dopamine loss and gait instability in C57Bl/6J mice. Behav Brain Res. 2009;198:434–439. doi: 10.1016/j.bbr.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Cao C, Matsumura K, Yamagata K, Watanabe Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1[beta]: a possible site of prostaglandin synthesis responsible for fever. Brain Res. 1996;733:263–272. doi: 10.1016/0006-8993(96)00575-6. [DOI] [PubMed] [Google Scholar]
- Chan RKW, Brown ER, Ericsson A, Kovács KJ, Sawchenko PE. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci. 1993;13:5125–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Zhang H, Belevych N, He L, Lai W, Pu XA, Jaeger LB, Chen Q, Quan N. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci. 2007;27:10476–10486. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, Hoogendoorn KH, van Dijk J, Zijlstra FJ, van Dam AM, Breimer DD, van Berkel TJ, de Boer AG, Kuiper J. Eicosanoid production by rat cerebral endothelial cells: stimulation by lipopolysaccharide, interleukin-1 and interleukin-6. J Neuroimmunol. 1995;59:1–8. doi: 10.1016/0165-5728(95)00009-q. [DOI] [PubMed] [Google Scholar]
- Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985;54:589–599. [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- Ek M, Arias C, Sawchenko PE, Ericsson-Dahlstrand A. Distribution of the EP3 prostaglandin E2 receptor subtype in the rat brain: Relationship to sites of interleukin-1-induced cellular responsiveness. J Comp Neurol. 2000;428:5–20. doi: 10.1002/1096-9861(20001204)428:1<5::aid-cne2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Elander L, Engstrom L, Ruud J, Mackerlova L, Jakobsson PJ, Engblom D, Nilsberth C, Blomqvist A. Inducible prostaglandin E2 synthesis interacts in a temporally supplementary sequence with constitutive prostaglandin-synthesizing enzymes in creating the hypothalamic-pituitary-adrenal axis response to immune challenge. J Neurosci. 2009;29:1404–1413. doi: 10.1523/JNEUROSCI.5247-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. TINS. 1997;20:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- Engstrom L, Rosen K, Angel A, Fyrberg A, Mackerlova L, Konsman JP, Engblom D, Blomqvist A. Systemic immune challenge activates an intrinsically regulated local inflammatory circuit in the adrenal gland. Endocrinology. 2008;149:1436–1450. doi: 10.1210/en.2007-1456. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Kovács KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Liu C, Hart RP, Sawchenko PE. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J Comp Neurol. 1995;361:681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- Fabriek BO, Polfliet MM, Vloet RP, van der Schors RC, Ligtenberg AJ, Weaver LK, Geest C, Matsuno K, Moestrup SK, Dijkstra CD, van den Berg TK. The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood. 2007;109:5223–5229. doi: 10.1182/blood-2006-08-036467. [DOI] [PubMed] [Google Scholar]
- Garcia-Bueno B, Serrats J, Sawchenko PE. Cerebrovascular cyclooxygenase-1 expression, regulationand role in HPA axis activation by inflammatory stimuli. J Neurosci. 2009 doi: 10.1523/JNEUROSCI.2373-09.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol Psychiatry. 2008;13:480–497. doi: 10.1038/sj.mp.4002122. [DOI] [PubMed] [Google Scholar]
- Hahn CN, del Pilar Martin M, Zhou XY, Mann LW, d’Azzo A. Correction of murine galactosialidosis by bone marrow-derived macrophages overexpressing human protective protein/cathepsin A under control of the colony-stimulating factor-1 receptor promoter. Proc Natl Acad Sci U S A. 1998;95:14880–14885. doi: 10.1073/pnas.95.25.14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbuz MS, Conde GL, Marti O, Lightman SL, Jessop DS. The hypothalamic-pituitary-adrenal axis in autoimmunity. Ann N Y Acad Sci. 1997;823:214–224. doi: 10.1111/j.1749-6632.1997.tb48393.x. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Antoni FA, Aguilera G, Catt KJ. Magnocellular axons in passage through the median eminence release vasopressin. Nature. 1986;319:326–329. doi: 10.1038/319326a0. [DOI] [PubMed] [Google Scholar]
- Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPARgamma. J Biol Chem. 2000;275:28028–28032. doi: 10.1074/jbc.M001387200. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci. 2004;9:1977–1993. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- Katsuura G, Gottschall PE, Dahl RR, Arimura A. Adrenocorticotropin release induced by intracerebroventricular injection of recombinant human interleukin-1 in rats: possible involvement of prostaglandin. Endocrinology. 1988;122:1773–1779. doi: 10.1210/endo-122-5-1773. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Rivest S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. J Neurochem. 1998;70:452–466. doi: 10.1046/j.1471-4159.1998.70020452.x. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 2001;15:155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Mackowiak PA. Concepts of fever. Arch Intern Med. 1998;158:1870–1881. doi: 10.1001/archinte.158.17.1870. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Cao C, Ozaki M, Morii H, Nakadate K, Watanabe Y. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: Light and electron microscopic immunocytochemical studies. J Neurosci. 1998;18:6279–6289. doi: 10.1523/JNEUROSCI.18-16-06279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Furuyashiki T, Bito H, Ushikubi F, Tanaka Y, Kobayashi T, Muro S, Satoh N, Kayahara T, Higashi M, et al. Impaired adrenocorticotropic hormone response to bacterial endotoxin in mice deficient in prostaglandin E receptor EP1 and EP3 subtypes. Proc Natl Acad Sci U S A. 2003;100:4132–4137. doi: 10.1073/pnas.0633341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouihate A, Boisse L, Pittman QJ. A novel antipyretic action of 15-deoxy-Delta12,14-prostaglandin J2 in the rat brain. J Neurosci. 2004;24:1312–1318. doi: 10.1523/JNEUROSCI.3145-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterness IG, Golden HW, Seymour PA, Eskra JD, Daumy GO. Role of prostaglandins in the behavioral changes induced by murine interleukin 1 alpha in the rat. Cytokine. 1991;3:333–338. doi: 10.1016/1043-4666(91)90502-5. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev. 2006;52:201–243. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Polfliet MM, van de Veerdonk F, Dopp EA, van Kesteren-Hendrikx EM, van Rooijen N, Dijkstra CD, van den Berg TK. The role of perivascular and meningeal macrophages in experimental allergic encephalomyelitis. J Neuroimmunol. 2002;122:1–8. doi: 10.1016/s0165-5728(01)00445-3. [DOI] [PubMed] [Google Scholar]
- Polfliet MM, Zwijnenburg PJ, van Furth AM, van der Poll T, Dopp EA, Renardel de Lavalette C, van Kesteren-Hendrikx EM, van Rooijen N, Dijkstra CD, van den Berg TK. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J Immunol. 2001a;167:4644–4650. doi: 10.4049/jimmunol.167.8.4644. [DOI] [PubMed] [Google Scholar]
- Polfliet MMJ, Goede PH, van Kesteren-Hendrikx EML, van Rooijen N, Dijkstra CD, van den Berg TK. A method for the selective depletion of perivascular and meningeal macrophages in the central nervous system. J Neuroimmunol. 2001b;116:188–195. doi: 10.1016/s0165-5728(01)00282-x. [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N. Immune-To-Brain Signaling: How Important are the Blood-Brain Barrier-independent Pathways? Mol Neurobiol. 2008;37:142–152. doi: 10.1007/s12035-008-8026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Whiteside M, Herkenham M. Cyclooxygenase 2 mRNA expression in rat brain after peripheral injection of lipopolysaccharide. Brain Res. 1998;802:189–197. doi: 10.1016/s0006-8993(98)00402-8. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2005;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Cunningham ET, Jr, Mortrud MT, Pfeiffer SW, Gerfen CR. Phaseolus vulgaris-leucoagglutinin (PHA-L) anterograde axonal transport technique. Meth Neurosci. 1990;3:247–260. [Google Scholar]
- Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and natrue of immune insults. J Neurosci. 2002;22:5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: The role of perivascular cells. Front Biosci. 2003;8:1321–1329. doi: 10.2741/1211. [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Specificity and generality of the involvement of catecholaminergic afferents in hypothalamic responses to immune insults. J Comp Neurol. 2007;502:455–467. doi: 10.1002/cne.21329. [DOI] [PubMed] [Google Scholar]
- Sehic E, Szekely M, Ungar AL, Oladehin A, Blatteis CM. Hypothalamic prostaglandin E2 during lipopolysaccharide-induced fever in guinea pigs. Brain Res Bull. 1996;39:391–399. doi: 10.1016/0361-9230(96)00037-8. [DOI] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol. 1989;12:169–181. [Google Scholar]
- Simonin MA, Bordji K, Boyault S, Bianchi A, Gouze E, Becuwe P, Dauca M, Netter P, Terlain B. PPAR-gamma ligands modulate effects of LPS in stimulated rat synovial fibroblasts. Am J Physiol Cell Physiol. 2002;282:C125–133. doi: 10.1152/ajpcell.2002.282.1.C125. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Rev. 1999;31:42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: Actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer MJ, Sweep CG, Rijnkels CE, Pesman GJ, Tilders FJ, Kloppenborg PW, Hermus AR. Acute stimulation of the hypothalamic-pituitary-adrenal axis by IL-1 beta, TNF alpha and IL-6: a dose response study. J Endocrinol Invest. 1996;19:175–182. doi: 10.1007/BF03349862. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Wang D, Patel VV, Ricciotti E, Zhou R, Levin MD, Gao E, Yu Z, Ferrari VA, Lu MM, Xu J, Zhang H, Hui Y, Cheng Y, Petrenko N, Yu Y, FitzGerald GA. Cardiomyocyte cyclooxygenase-2 influences cardiac rhythm and function. Proc Natl Acad Sci USA. 2009;106:7548–7552. doi: 10.1073/pnas.0805806106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Morimoto A, Sakata Y, Murakami N. ACTH response induced by interleukin-1 is mediated by CRF secretion stimulated by hypothalamic PGE. Experientia. 1990;46:481–484. doi: 10.1007/BF01954238. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Wick G, Hu Y, Schwarz S, Kroemer G. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocr Rev. 1993;14:539–563. doi: 10.1210/edrv-14-5-539. [DOI] [PubMed] [Google Scholar]
- Wieczorek M, Dunn AJ. Relationships among the behavioral, noradrenergic, and pituitary-adrenal responses to interleukin-1 and the effects of indomethacin. Brain Behav Immun. 2006;20:477–487. doi: 10.1016/j.bbi.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- Wong ML, Bongiorno PB, al-Shekhlee A, Esposito A, Khatri P, Licinio J. IL-1 beta, IL-1 receptor type I and iNOS gene expression in rat brain vasculature and perivascular areas. Neuroreport. 1996a;7:2445–2448. doi: 10.1097/00001756-199611040-00008. [DOI] [PubMed] [Google Scholar]
- Wong ML, Rettori V, al-Shekhlee A, Bongiorno PB, Canteros G, McCann SM, Gold PW, Licinio J. Inducible nitric oxide synthase gene expression in the brain during systemic inflammation. Nat Med. 1996b;2:581–584. doi: 10.1038/nm0596-581. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Lu J, Elmquist JK, Saper CB. Specific roles of cyclooxygenase-1 and cyclooxygenase-2 in lipopolysaccharide-induced fever and Fos expression in rat brain. J Comp Neurol. 2003;463:3–12. doi: 10.1002/cne.10743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.