Abstract
Background
Few data are available concerning the effect of human papillomavirus (HPV) infection on HIV acquisition.
Methods
HIV-seronegative, sexually active 18-24 year-old Kenyan men within a randomized trial of male circumcision provided penile exfoliated cells from two anatomical sites (glans/coronal sulcus, and shaft) at baseline. The GP5+/6+ PCR assay ascertained a wide range of HPV DNA types at the baseline visit. Risk of HIV infection [95% confidence interval (CI)] was estimated using Kaplan-Meier methods, and hazard ratios (HR)[95% CI] from proportional hazards models.
Results
Among 2,168 uncircumcised men with baseline HPV data, 1,089 (50%) were HPV DNA positive. Cumulative incidence of HIV infection by 42-months was 5.8% [95% CI 3.6, 7.9] in men with HPV-positive glans specimens versus 3.7% [1.8, 5.6] in men with HPV-negative glans specimens (p=0.01). Controlling for subsequent circumcision status, baseline HSV-2 serostatus, and sexual and sociodemographic risk factors, the HR of HIV infection in men with HPV-positive glans specimens was 1.8 [1.1, 2.9] compared to men with HPV-negative glans specimens.
Conclusion
Results suggest an independent, increased risk of HIV seroconversion among HPV positive men. If confirmed in other studies, HPV prevention could be another tool for HIV prevention.
Keywords: Human immunodeficiency virus (HIV), human papillomavirus (HPV), circumcision, Kenya, men, acquisition, risk factors
Introduction
While an effective human immunodeficiency virus (HIV) vaccine remains the greatest hope in curbing the AIDS pandemic, development of a successful vaccine has thus far been elusive. Thus, the identification of preventable sexually transmitted infections (STIs), which confer an increased risk of HIV acquisition, is currently an important component of HIV prevention research. Although several STIs have been shown to be associated with HIV, there are few data concerning the possible effect of human papillomavirus (HPV) infection on HIV acquisition. Clear evidence of an association between HPV and risk of HIV infection would suggest a new possible intervention for HIV prevention; unlike efforts to develop an effective HIV prophylactic vaccine, HPV vaccines have shown tremendous promise for the prevention of persistent HPV infections and HPV-associated clinical outcomes in both women and men(1;2).
No ecological or observational studies have been published comparing the risk of incident HIV infection between HPV-positive and HPV-negative heterosexual men. One observational study among 1,409 HIV-seronegative men having sex with men in San Francisco found infection with at least two types of anal HPV infection to be significantly associated with a higher risk of HIV seroconversion, although multivariate results did not reach statistical significance for men with single-type anal HPV infections and were not adjusted for HSV-2 serostatus or presence of other sexually transmitted infections(3).
We have previously reported that circumcision significantly reduced the risk of HIV acquisition in a randomized controlled trial (RCT) of male circumcision in Kisumu, Kenya(4). Here we report results among the same cohort on the relative risk of HIV incidence in HPV-DNA positive men compared to HPV-negative men at their baseline visit, controlling for circumcision status and other potential confounders, in order to better understand the impact of HPV infection on HIV incidence.
Methods
An RCT was conducted between February 2002 and December 2006 in men aged 18-24 years in Kisumu as previously described(4). The primary aim of the RCT was to determine the effectiveness of male circumcision in reducing HIV incidence. Inclusion criteria included being uncircumcised, HIV seronegative, sexually active, and having a hemoglobin ≥9.0 g/100 mL.
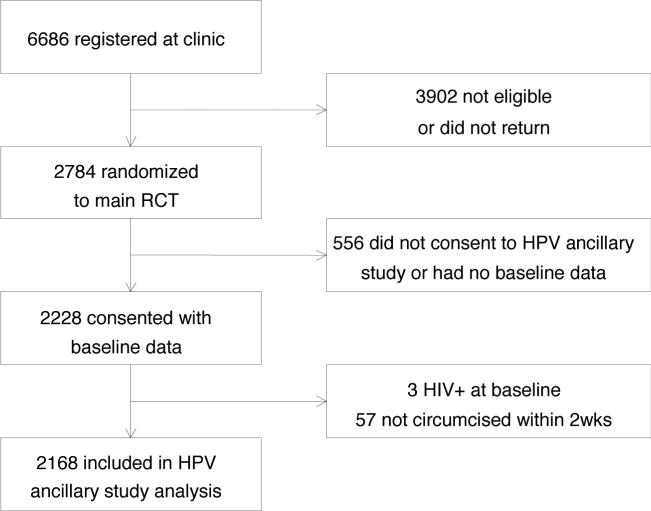
This present analysis includes men enrolled in the RCT who consented to penile exfoliated cell sample collection and its shipment overseas for HPV DNA testing (Figure 1). A total of 6,686 men were screened and 2,784 were randomized to the RCT(4). Of these, 2,228 consented to this ancillary study of HPV infection and had risk factor data collected at the baseline visit. The current analysis includes 2,168 of these men, with three men excluded since they were HIV positive at baseline, and 57 men excluded since they were randomized to be circumcised but did not receive surgery within the first two weeks of the trial. The original trial consented participants to randomization to circumcision or control (delayed circumcision) arms, and included 24 months of follow-up. Follow-up was extended on the last 1,740 men enrolled and 1,550 men entered extended follow-up. The current analysis reports on follow-up to 42 months. The study protocol was approved by the Institutional Review Boards of all collaborating institutions and the University of North Carolina.
Figure 1.
Study flow chart
Questionnaire, Clinical Examination, and Specimen Collection
After undergoing informed consent, participants were administered a standardized questionnaire on sociodemographic characteristics and sexual behavior by a trained male interviewer. Penile exfoliated cells were collected for HPV DNA detection from two anatomical sites: (i) glans/coronal sulcus and (ii) shaft, using techniques described previously(5).
HPV DNA Laboratory Testing
HPV DNA testing results presented here are from participating uncircumcised men at the baseline study visit. DNA was isolated from penile exfoliated cell samples using the NucleoSpin 96 Tissue kit (Macherey-Nagel, Germany) and a Microlab Star robotic system (Hamilton, Germany), according to manufacturers’ instructions. Presence of human DNA was evaluated by β-globin specific PCR, followed by agarose gel electrophoresis. HPV DNA positivity was assessed by GP5+/6+ PCR, followed by hybridisation of PCR products using an enzyme immunoassay readout with two HPV oligoprobe cocktail probes that, together, detect 44 HPV types: HPV 6, 11, 16, 18, 26, 30, 31, 32, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 61, 64, 66, 67, 68, 69, 70, 71 (equivalent to CP8061), 72, 73, 81(equivalent to CP8304), 82(IS39 and MM4 subtypes), 83 (equivalent to MM7), 84(equivalent to MM8), cand85, 86, cand89 (equivalent to CP6108) and JC9710. Subsequent HPV genotyping was performed by reverse line blot hybridisation of PCR products, as described previously (6). Primers and probe sequences, as well as cycling and staining conditions, have been detailed previously(6;7).
HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 were classified as high-risk (carcinogenic) HPV types. HPV infections with multiple HPV types were considered high-risk if one or more high-risk HPV types were detected. Low-risk types included all other HPV types. HPV types detected by enzyme immunoassay but not by reverse line blot genotyping were designated as HPV X, indicating a type, sub-type or variant not detectable with probes used for reverse line blot hybridization. Urine samples from the baseline visit were tested for N. gonorrhoeae and C. trachomatis infections by PCR-based methods (Roche Diagnostics), and serum was tested for herpes simplex virus type-2 antibodies using a type-specific ELISA (Kalon).
Circumcision procedure
Participants who met the study criteria were randomly assigned to either the intervention (circumcision) group (8) or the control (delayed circumcision) group after being questioned to ensure their understanding of all study procedures and requirements for participation.
HIV serological testing
HIV serostatus and timing of seroconversion were determined as follows. If a participant was double positive or discordant on two rapid tests with the synthetic peptide test Determine HIV 1/2 (Abbott Diagnostic Division, Hoofddorp, Netherlands) and the recombinant antigen test Unigold Recombigen HIV Test (Trinity Biotech, Wicklow, Ireland) taken from the same fingerprick sample, then serum was drawn and sent to the International STD/HIV Collaborative Group laboratory at the University of Nairobi for double ELISA (Detect HIV 1/2, Adaltis Inc, Montreal, Canada, and Recombigen HIV 1/2, Trinity Biotech, Wicklow, Ireland). Results were available within one week. Participants were deemed to be confirmed positive if the HIV ELISA tests were both positive. Two negative ELISA tests were considered negative; discordant ELISA tests were considered indeterminate, and the participant was asked to return for additional testing 1–6 months later, depending on the visit. For purposes of determining serostatus for analysis of study data, blood specimens from all participants who tested positive on at least one rapid test and one ELISA test were sent to the Health Canada National HIV Reference Laboratory (Ottawa, Canada) for confirmatory testing by line immunoassay (INNO-LIA HIV 1/2, Immunogenetics NV, Ghent, Belgium). Specimens indeterminate by line immunoassay were tested by PCR at Health Canada or the Fred Hutchinson Cancer Research Center (Seattle, WA, USA), with the PCR result deemed to be definitive. Any participant confirmed as positive at a follow-up visit had his baseline specimen tested at the Health Canada laboratory to ascertain HIV serostatus at enrollment. Participants who had a confirmed positive test at the month 3 follow-up visit had their month 1 specimen tested by PCR. The HIV seroconversion visit was judged to be the first visit at which the participant had at least one positive HIV rapid test and was confirmed as being HIV positive at the same or a subsequent visit according to the above procedure.
Statistical Methods
Associations between HPV infection and baseline characteristics were assessed using Pearson's χ2 test or Fisher's exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. McNemar's test was used to assess differences in the prevalence of HPV between the two anatomical sampling sites. The Kaplan-Meier method was used to estimate the cumulative incidence of HIV seroconversion over time. Differences in HIV seroconversion distributions were summarized using the log rank test. Hazard ratios (HRs) for the effect of HPV infection at baseline on HIV seroconversion and corresponding 95% confidence intervals (CIs) were estimated via Cox proportional hazards models ,using an exact method to adjust for ties. Multivariate proportional hazards models included circumcision status as well as risk factors with significant marginal associations (p<0.10) with HIV or variables believed a priori to be potential confounders. Variables not significant at the 0.10 level in multivariate models were eliminated sequentially in order of p-value magnitude. The Akaike Information Criteria (AIC) was used to compare non-nested models(9). All p-values reported are two-sided.
Unless noted otherwise, men randomized to the control arm who became circumcised prior to HIV seroconversion were right-censored at their last visit prior to circumcision. Analyses were also performed with circumcision as a time varying covariate. Sensitivity analyses were conducted using data only from the original 24 month follow-up period since only a subset of the original participants were observed at 42 months, and uncircumcised men could self-select to be circumcised or remain uncircumcised after 24 months.
Results
Among 2168 men included in the analyses, 1089 (50%) had HPV DNA detected in either the glans or shaft specimens at baseline. The prevalence of HPV was significantly higher in the glans specimens than in the shaft specimen (46% versus 18%, p<0.001). Of the 1,089 men with HPV, 991 (91%) had HPV DNA detected in the glans specimen. Baseline characteristics significantly associated with HPV infection in the glans or shaft included: less than secondary educational level (p=<0.001); salaried or self employment (p=0.001); less than daily bathing (p=0.01); presence of genital warts (p=0.04); HSV-2 seropositivity (p=0.02); gonorrhea (p=0.02); chlamydia infection (p=0.05); no use of condom with last partner (p=0.001); and greater reported number of lifetime female partners (p=0.04) (Table 1).
Table 1.
Baseline characteristics by human papillomavirus status
| All men N=2168 | HPV+ (glans or shaft) N=1089 | HPV− (glans and shaft) N=1079 | Odds Ratio (95% CI)* | p** | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age (years) | 20 (19-22; 17-28†) | 20 (19-22; 18-24) | 20 (19-22; 17-28) | 1.00 (0.96, 1.05) | 0.89 |
| Ethnic group | 0.45 | ||||
| Luo | 2138 (99%) | 1076 (99%) | 1062 (98%) | 1.32 (0.64, 2.74) | |
| Other | 30 ( 1%) | 13 ( 1%) | 17 ( 2%) | Ref | |
| Education level | <.001 | ||||
| Any secondary or above | 1414 (65%) | 669 (61%) | 745 (69%) | 0.71 (0.60, 0.85) | |
| Less than secondary | 754 (35%) | 420 (39%) | 334 (31%) | Ref | |
| Employment status | 0.001 | ||||
| Employed with salary | 192 ( 9%) | 107 (10%) | 85 ( 8%) | 1.40 (1.04, 1.90) | |
| Self-employed | 583 (27%) | 323 (30%) | 260 (24%) | 1.38 (1.14, 1.68) | |
| Unemployed | 1393 (64%) | 659 (61%) | 734 (68%) | Ref | |
| Martial status | 0.78 | ||||
| Not married (no live-in partner) | 2021 ( 94%) | 1013 ( 94%) | 1008 ( 94%) | 0.90 (0.62, 1.32) | |
| Not married (with live-in partner) | 13 (0.6%) | 5 (0.5%) | 8 (0.7%) | 0.75 (0.42, 1.35) | |
| Married (not living with wife) | 20 (0.9%) | 11 ( 1%) | 9 (0.8%) | 1.03 (0.75, 1.42) | |
| Married (living with wife) | 105 ( 5%) | 55 ( 5%) | 50 ( 5%) | Ref | |
| Bathing frequency | 0.01 | ||||
| Less than daily | 45 ( 2%) | 31 ( 3%) | 14 ( 1%) | 2.22 (1.18, 4.20) | |
| Daily | 2101 (98%) | 1048 (97%) | 1053 (99%) | Ref | |
| Physical and laboratory findings | |||||
| Genital warts | 0.04‡ | ||||
| Present | 12 ( 1%) | 10 ( 1%) | 2 (< 1%) | 4.99 (1.06, 46.9)‡ | |
| Absent | 2156 (99%) | 1079 (99%) | 1077 (>99%) | Ref | |
| Herpes simplex virus 2 serostatus | 0.02 | ||||
| Positive | 577 (27%) | 314 (29%) | 263 (24%) | 1.26 (1.04, 1.52) | |
| Negative | 1585 (73%) | 772 (71%) | 813 (76%) | Ref | |
| N. gonorrhea | 0.02 | ||||
| Positive | 44 ( 2%) | 30 ( 3%) | 14 ( 1%) | 2.15 (1.13, 4.08) | |
| Negative | 2098 (98%) | 1047 (97%) | 1051 (99%) | Ref | |
| C. trachomatis | 0.05 | ||||
| Positive | 95 ( 4%) | 57 ( 5%) | 38 ( 4%) | 1.51 (0.99, 2.30) | |
| Negative | 2046 (96%) | 1019 (95%) | 1027 (96%) | Ref | |
| Sexual history with women | |||||
| Age at 1st sexual encounter | 16 (14-17; 5-24) | 16 (14-17; 5-23) | 16 (14-17; 5-24) | 0.99 (0.95, 1.02) | 0.81 |
| Use of condom last partner | 0.001 | ||||
| Yes | 1061 (49%) | 495 (46%) | 566 (53%) | 0.75 (0.64, 0.89) | |
| No | 1102 (51%) | 592 (54%) | 510 (47%) | Ref | |
| Years of sexual activity | 0.94 | ||||
| 7+ | 603 (29%) | 306 (30%) | 297 (28%) | 1.06 (0.82, 1.37) | |
| 5-6 | 551 (27%) | 275 (27%) | 276 (26%) | 1.03 (0.79, 1.33) | |
| 3-4 | 521 (25%) | 255 (25%) | 266 (26%) | 0.99 (0.76, 1.28) | |
| 0-2 | 402 (19%) | 198 (19%) | 204 (20%) | Ref | |
| Lifetime number of female partners | 0.04 | ||||
| 6+ | 690 (34%) | 356 (35%) | 334 (34%) | 1.54 (1.09, 2.17) | |
| 2-5 | 1145 (57%) | 584 (58%) | 561 (57%) | 1.50 (1.08, 2.09) | |
| 1 | 166 ( 8%) | 68 ( 7%) | 98 (10%) | Ref |
Sample sizes vary slightly from the number of randomized participants due to different data sources. Data are median (IQR; range) for ordinal data, or n (%) for categorical data. Percentages may not sum to 100% due to rounding.
Odds ratio (OR) estimates based on logistic regression; for age and age at first sexual encounter, OR based on one year increment in variable.
p-value for comparison between HPV positive and HPV negative men using either Wilcoxon ranksum or Pearson's chi-square test unless noted otherwise.
The study inclusion criteria required participants be 18-24 years old; there three protocol violations resulting in two 17 year-olds and one 28 year-old being enrolled.
Exact OR confidence intervals and Fisher's exact test p-value.
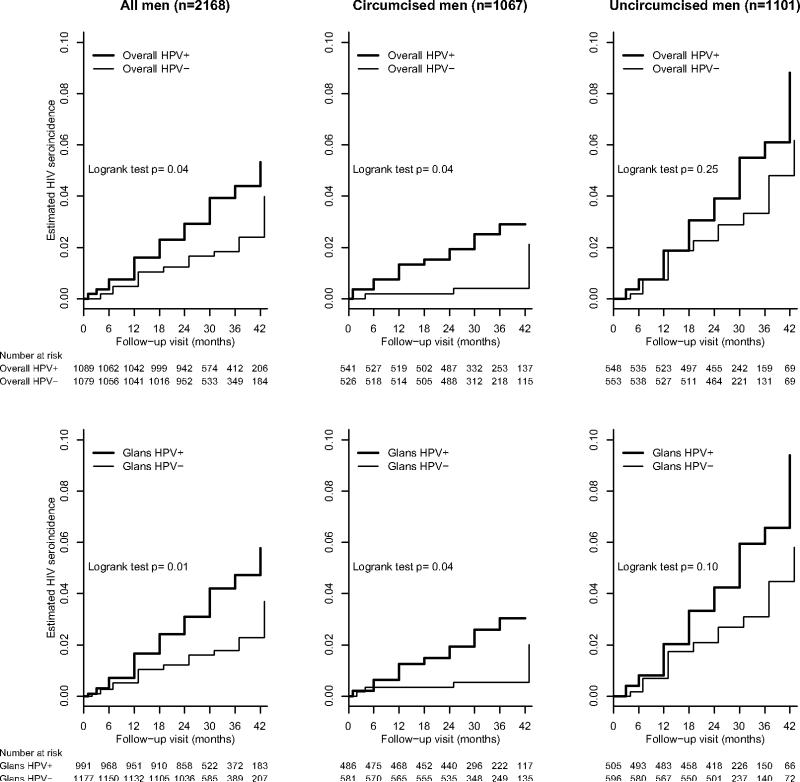
Figure 2 shows the Kaplan-Meier estimates of the cumulative incidence of HIV seroconversion stratified by baseline HPV and circumcision status. The 42-month cumulative incidence of HIV was 5.3% [95% CI 3.4, 7.2] in men with HPV detected in the glans or shaft, versus 4.0% [1.9, 6.1] in HPV-negative men (p=0.04). Similarly, there was greater risk of HIV sero-conversion by 42 months in men with HPV-positive glans specimens compared to men with HPV-negative glans specimens (5.8% [3.6, 7.9] versus 3.7% [1.8, 5.6%], p=0.01).
Figure 2.
Estimated cumulative incidence of HIV seroconversion across follow-up visits by baseline HPV positivity
Estimated HRs for different categories of baseline HPV positivity are presented in Table 2. For each categorization of HPV infection, adjusted HR estimates were obtained from multivariate proportional hazards models including circumcision status, age, employment status, and HSV-2 infection. Gonorrhea was marginally associated with risk of HIV, but was not significant at the 0.10 level in any multivariate model. Other variables in Table 1 were not associated with HIV risk in both unadjusted and adjusted analyses. Genital warts were not considered in the multivariate models since they were present at baseline in only 12 men (<1%). The adjusted hazard of HIV infection was 1.6 [1.0, 2.7] times higher in men with HPV in either the glans or shaft compared to HPV negative men (Table 2). Similarly, the adjusted HR estimate for men with HPV-positive glans specimens was higher (1.8 [1.1, 2.9]) compared to men without HPV in the glans. HIV infection rates did not differ significantly between men infected with multiple HPV types versus single HPV types (p=0.37), or between men infected with high-risk HPV types versus low-risk HPV types (p=0.59). In terms of HPV types included in current generation prophylactic vaccines, the hazard of HIV in men infected with type 16 or 18 was significantly greater than in HPV negative men, but differed only marginally from men infected with other HPV types (p=0.07; Table 2, Figure 3). The hazard of HIV infection in individuals infected with 16, 18, 6 or 11 was significantly greater than in HPV negative individuals, but did not differ significantly from HPV positive individuals not infected with 16, 18, 6 or 11 (p=0.20). There was no evidence of an interaction between the effects of circumcision status and HPV for any categorization of HPV infection (p-values ranged from 0.11-0.42; Table 2), indicating no effect modification. Comparison of the different multivariate models in Table 2 by AIC showed that categorization by HPV positivity in the glans provided the best fit. For this model (Table 3), higher rates of HIV seroconversion were associated with being younger, being employed, and being HSV-2 seropositive. There was no evidence of an interaction between HSV-2 and HPV infection in the glans (p=0.43) with risk of HIV acquisition. The HR estimate and corresponding p-value for HPV were virtually the same whether HSV-2 was included or excluded from the multivariate model. Multivariate analyses stratifying on circumcision status (not shown) yielded nearly identical results to those obtained including circumcision as a covariate. The multivariate model using circumcision as a time-dependent covariate also yielded results similar to those given in Table 3, with an adjusted hazard ratio estimate for HPV in the glans of 2.0 (95% CI 1.2, 3.3) and corresponding p-value 0.008. Restricting analyses to men with beta-globin positive specimens gave similar results to the analyses including all participants described above. For instance, of the 2,168 men included in the main analysis, 1,225 (56.5%) had beta-globin positive specimens from the glans. Fitting the final model described in Table 3 to data from these 1,225men only, the adjusted hazard of HIV infection was 2.3 [1.1, 4.8] times higher in men with HPV in the glans compared to men without HPV in the glans (p=0.03).
Table 2.
Estimated hazard ratios of HIV seroincidence by baseline HPV status
| HIV infections/Total men | Hazard ratio† (95% CI) | P-value** | |
|---|---|---|---|
| HPV overall | |||
| Positive glans or shaft | 40/1089 | 1.6 (1.0, 2.7) | 0.08 (0.23) |
| Negative | 23/1079 | Ref | |
| HPV in glans | |||
| Positive | 39/991 | 1.8 (1.1, 2.9) | 0.03 (0.38) |
| Negative | 24/1177 | Ref | |
| Risk type | |||
| High risk infection | 27/754 | 1.5 (0.9, 2.6) | 0.15 (0.11) |
| Low risk infection | 13/335 | 1.8 (0.9, 3.6) | 0.09 (0.93) |
| Negative | 23/1079 | Ref | |
| Number of types | |||
| Multiple | 26/628 | 1.8 (1.0, 3.1) | 0.04 (0.23) |
| Single | 14/461 | 1.3 (0.7, 2.6) | 0.41 (0.42) |
| Negative | 23/1079 | Ref | |
| Type 16/18 | |||
| Positive with 16 or 18 | 15/283 | 2.4 (1.2, 4.6) | 0.01 (0.17) |
| Positive without 16 and 18 | 25/806 | 1.3 (0.8, 2.4) | 0.32 (0.41) |
| Negative | 23/1079 | Ref | |
| Type 16/18/6/11 | |||
| Positive with 16, 18, 6, or 11 | 16/380 | 1.9 (1.0, 3.7) | 0.05 (0.25) |
| Positive without 16, 18, 6, and 11 | 24/709 | 1.5 (0.8, 2.6) | 0.20 (0.33) |
| Negative | 23/1079 | Ref |
Based on multivariate proportional hazards models including HPV, circumcision status, age, employment status, and HSV-2 as covariates
P-value for test whether hazard is the same as the referent category (parenthetical p-value for test of interaction between HPV and circumcision status)
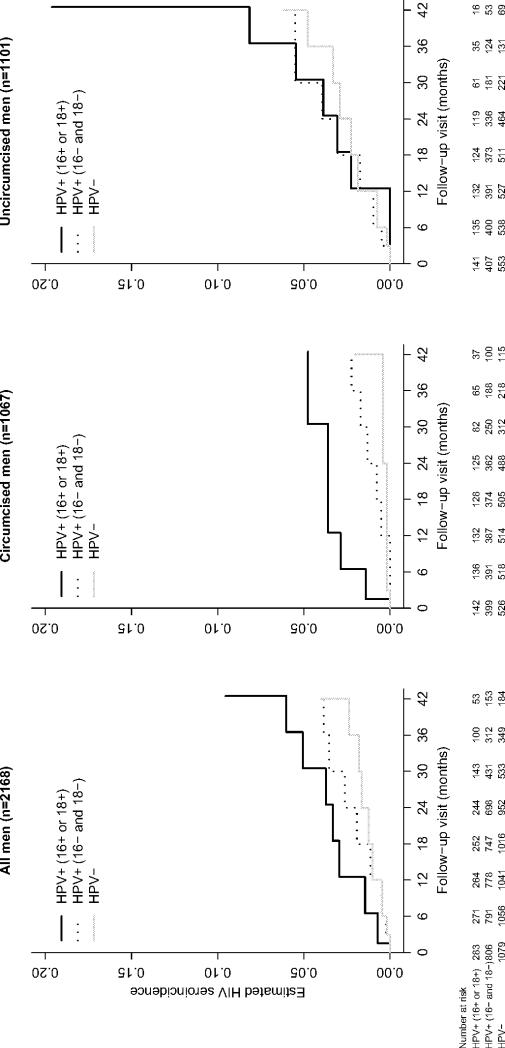
Figure 3.
Estimated cumulative incidence of HIV seroconversion across follow-up visits by baseline HPV positivity in glans or shaft with type 16 or 18
Table 3.
Multivariate proportional hazards model for HIV seroincidence
| HIV infections/Total men | Hazard ratio† (95% CI) | P-value* | |
|---|---|---|---|
| Age (years) | 63/2168 | 0.8 (0.7, 1.0) | 0.02 |
| Employment status | 0.05 | ||
| Employed with salary | 10/192 | 2.4 (1.2, 5.1) | |
| Self-employed | 20/583 | 1.4 (0.8, 2.5) | |
| Unemployed | 33/1393 | Ref | |
| HPV (in glans) | |||
| Positive | 39/991 | 1.8 (1.1, 2.9) | 0.03 |
| Negative | 24/1177 | Ref | |
| Herpes simplex virus 2 serostatus | |||
| Positive | 25/577 | 1.8 (1.1, 3.1) | 0.02 |
| Negative | 38/1585 | Ref | |
| Randomization group | |||
| Circumcised | 17/1067 | 0.3 (0.2, 0.6) | <.001 |
| Control | 46/1101 | Ref |
From multivariate proportional hazards model including all covariates in the table
P-value for test whether hazard is the same as the referent category
Similar results were also obtained when the final multivariate model was fit to data limited to the first 24 months of follow-up. The estimated adjusted HR of HIV infection in men with HPV-positive glans specimens was 1.7 [1.0, 3.2] (p=0.05). Age and employment also remained associated with risk of HIV infection (p=0.07 and p=0.006 respectively), although HSV-2 infection was no longer significant (p=0.34).
Discussion
Among 2,168 young men in Kenya, HPV infection was independently associated with an increased risk of HIV acquisition over 42 months of follow-up. Observed associations were largely due to HPV infection in the glans/coronal sulcus. Effect estimates were similar before and after adjustment for HSV-2 serostatus, gonorrhea, chlamydial infection, and other possible confounders. HIV risk was not associated with the presence of multiple type HPV infections or whether the HPV infection included high-risk types. HR estimates of HIV acquisition associated with baseline HPV glans positivity were virtually identical to those with baseline HSV-2 seropositivity. Associations of HSV-2 seropositivity with increased risk of HIV infection have been observed in previous studies, with similar magnitudes of increased risk(10). With adjustment for HSV-2 serostatus, the observed increased risk of HIV among HPV positive men was less likely to be due to residual confounding of sexual behavior, although this possibility cannot completely be ruled out. Within this population of young men at a high risk of HIV acquisition in Kenya, HPV infection was very common (50%), so preventing HPV infection could potentially be another tool for HIV prevention.
In terms of biological plausibility, HPV infection, unlike many other sexually transmitted infections, is localized to the epithelial cells, thus limiting exposure of the virus to the immune system(11). Although HPV infection alone is not generally characterized by the induction of notable inflammatory responses, an up-regulation of T-cells has been observed during the clearance of genital warts(12) and cervical lesions in women(13;14), thus resulting in a higher number of HIV susceptible cells. Alternatively, HPV-associated flat lesions and penile intraepithelial neoplasia in men are characterized by inflammatory infiltrates with hyperemic blood vessels, which may potentially enhance susceptibility to HIV infection by creating portals of HIV entry, analogous to genital tissue microtrauma (15;16). HPV and HPV-associated genital lesions may also induce the local production of specific cytokines (i.e. MIP-3, IL-8) which may, in turn, increase HIV susceptibility(17). One limitation of our present study is that associations of HIV with HPV were based on HPV infection ascertainment, rather than the detection of HPV-associated clinical outcomes, since HPV-associated flat penile lesions(16) were not ascertained at the baseline visit and the observed prevalence of genital warts was so low (<1%). Strengths of our study include a relatively large sample size, the ascertainment of a broad range of HPV types using the GP5+/6+ HPV DNA detection assay, and the comprehensive laboratory ascertainment of incident HIV acquisition. To our knowledge, only one other study has published prospective data of an increased risk of HIV among HPV-positive individuals(3); however, that study did not include heterosexual men nor control for HSV-2 seropositivity. Another study among 1,638 heterosexual men participating in a randomized clinical trial of male circumcision in South Africa reported a higher incidence of HIV among men with HPV positive results from urethral sampling, compared to men with HPV negative results (18). However, HPV DNA ascertainment was conducted at the last study visit, and some cases of HIV may have preceded HPV infection acquisition; thus, the temporal associations between HPV on HIV risk could not be established in that study.
Some caution is needed with interpretation of our results extending beyond the 24 months of the trial, as only a subset of the original participants were observed; uncircumcised men could self-select to be circumcised or remain uncircumcised, and there were only a moderate number of HIV infections after 24 months. Our main study findings were similar, however, when the data were restricted to 24 months. Further, sensitivity analyses of the effect of baseline HPV DNA status on HIV risk presented in Table 2 among stratified, non-overlapping HPV subgroups for comparison (e.g., HPV+ for 16, 18, 6 or 11) are limited, due to relatively small sample sizes. For example, we were not able to reliably examine the effect of HPV positivity in the penile shaft separately from that of the glans/coronal sulcus, given that most men (~90%) who were positive in the glans specimen were also positive in the shaft specimen. Although our results are based on a randomized clinical trial and none of the sexual behavior variables given in Table 1 were found to be associated with the risk of HIV infection, these analyses are limited to a cross-sectional assessment of HPV DNA status, HSV-2 seropositivity and sexual behavior at baseline, rather than a longitudinal assessment of these factors on HIV acquisition over study follow-up. Finally, we classified HPV infections as high-risk types based on their association with invasive cervical cancer among women, although it is currently not clear whether such a classification might be important in men.
If our results are confirmed by others, it would suggest that, in addition to male circumcision, vaccinating against HPV could be an effective way to prevent HIV infection. Two prophylactic HPV vaccines have the potential to prevent invasive cervical cancer and precancerous disease attributable to oncogenic HPV types 16 and 18(19,20). A quadrivalent vaccine can also prevent low risk HPV types 6 and 11 that cause genital warts(19). HR estimates from our analysis suggest that prophylactic vaccines providing protection against HPV 16 and 18 oncogenic types may reduce the hazard of HIV infection by half, assuming that vaccination prevents infections with types 16 or 18 when administered to young men prior to first sexual intercourse. In uncircumcised men, HPV vaccination and circumcision could lead to even greater reductions: for instance, in our study, the estimated 24-month cumulative incidence of HIV among uncircumcised men with HPV 16 or 18 was 3.9% [0.5, 7.2] compared to only 0.8% [0.2, 1.4%] in circumcised men without HPV 16 and 18 (Figure 3). While community-based randomized controlled trials have investigated the effect of the prevention of bacterial STIs on the incidence of HIV infection(21,22),no trials have examined the effectiveness of preventing HPV infection. Our results warrant the conduct of a randomized controlled trial to determine whether HPV prophylactic vaccination reduces HIV acquisition.
Acknowledgements
Foremost, we thank the young men of Kisumu who volunteered to participate in this study. We also thank the laboratory staff at University of Nairobi and the entire UNIM Project staff for their long hours of work and devotion to sound research.
Funding sources: This research was supported by the National Cancer Institute, National Institutes of Health (grant R01 CA114773-04). The RCT was supported by grant number AI50440 from the Division of AIDS, NIAID, NIH and by grant number HCT 44180 from the Canadian Institutes of Health Research. S Moses was supported by a CIHR Investigator Award.
Footnotes
Conflict of Interest: We declare that we have no conflict of interest.
Ethics Committee Approval: The study protocol was reviewed and approved by the Institutional Review Boards of the Universities of Illinois at Chicago, Manitoba, Nairobi, North Carolina and the VU University Medical Center.
Preliminary results of this study were presented as a Late Breaker Presentation at the IAS 2009 Conference in Cape Town, South Africa on July, 22 2009 (Abstract WELBC1).
Reference List
- 1.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007 September 8;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 2.Kahn JA. HPV vaccination for the prevention of cervical intraepithelial neoplasia. N Engl J Med. 2009 July 16;361(3):271–8. doi: 10.1056/NEJMct0806938. [DOI] [PubMed] [Google Scholar]
- 3.Chin-Hong PV, Husnik M, Cranston RD, et al. Anal human papillomavirus infection is associated with HIV acquisition in men who have sex with men. AIDS. 2009 June 1;23(9):1135–42. doi: 10.1097/QAD.0b013e32832b4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007 February 24;369(9562):643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 5.Smith JS, Moses S, Hudgens MG, et al. Human papillomavirus detection by penile site in young men from Kenya. Sex Transm Dis. 2007 November;34(11):928–34. doi: 10.1097/OLQ.0b013e318065b8ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snijders PF, van den Brule AJ, Jacobs MVeal. HPV DNA detection and typing in cervical scrapes by general primer GP5+/6+ PCR. Methods in Molecular Medicine: Human papillomaviruses- Methods and Protocols. Humana Press; Totowa: 2005. [DOI] [PubMed] [Google Scholar]
- 7.van den Brule AJ, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJ, Snijders PJ. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol. 2002 March;40(3):779–87. doi: 10.1128/JCM.40.3.779-787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieger JN, Bailey RC, Opeya J, et al. Adult male circumcision: results of a standardized procedure in Kisumu District, Kenya. BJU Int. 2005 November;96(7):1109–13. doi: 10.1111/j.1464-410X.2005.05810.x. [DOI] [PubMed] [Google Scholar]
- 9.Collett D. Modelling survival data in medical research. Chapman & Hall Ltd; New York: 2003. [Google Scholar]
- 10.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006 January 2;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 11.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008 May;109(2 Suppl):S15–S21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Coleman N, Birley HD, Renton AM, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994 December;102(6):768–74. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 13.de JA, van der Burg SH, Kwappenberg KM, et al. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 2002 January 15;62(2):472–9. [PubMed] [Google Scholar]
- 14.Nedergaard BS, Ladekarl M, Thomsen HF, Nyengaard JR, Nielsen K. Low density of CD3+, CD4+ and CD8+ cells is associated with increased risk of relapse in squamous cell cervical cancer. Br J Cancer. 2007 October 22;97(8):1135–8. doi: 10.1038/sj.bjc.6604001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleeker MC, Hogewoning CJ, van den Brule AJ, et al. Penile lesions and human papillomavirus in male sexual partners of women with cervical intraepithelial neoplasia. J Am Acad Dermatol. 2002 September;47(3):351–7. doi: 10.1067/mjd.2002.122198. [DOI] [PubMed] [Google Scholar]
- 16.Bleeker MC, Snijders PF, Voorhorst FJ, Meijer CJ. Flat penile lesions: the infectious “invisible” link in the transmission of human papillomavirus. Int J Cancer. 2006 December 1;119(11):2505–12. doi: 10.1002/ijc.22209. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009 April 23;458(7241):1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auvert B, Lissouba P, Cutler E, Zarka K, Puren A, Taljaard D. Association of Oncogenic and Non-Oncogenic Human papillomavirus with HIV incidence. JAIDS. doi: 10.1097/QAI.0b013e3181b327e7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007 May 10;356(19):1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 20.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006 April 15;367(9518):1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 21.Grosskurth H, Gray R, Hayes R, Mabey D, Wawer M. Control of sexually transmitted diseases for HIV-1 prevention: understanding the implications of the Mwanza and Rakai trials. Lancet. 2000 June 3;355(9219):1981–7. doi: 10.1016/S0140-6736(00)02336-9. [DOI] [PubMed] [Google Scholar]
- 22.Nicoll A, Johnson AM, Adler MW, Laga M. Preventing HIV-1: lessons from Mwanza and Rakai. Lancet. 1999 May 1;353(9163):1522–4. doi: 10.1016/S0140-6736(99)00078-1. [DOI] [PubMed] [Google Scholar]