Abstract
Purpose
Sunburns are an important risk factor for melanoma and those occurring in childhood are often cited as posing the greatest risk. We conducted a meta-analysis to quantify the magnitude of association for melanoma and sunburns during childhood, adolescence, adulthood and over a lifetime.
Methods
After reviewing over 1300 article titles and evaluating 270 articles in detail, we pooled ORs from 51 independent study populations for “ever” sunburned and risk of cutaneous melanoma. Among these, 26 studies reported results from dose-response analyses. Dose-response analyses were examined using both fixed-effects models and Bayesian random-effects models.
Results
An increased risk of melanoma was seen with increasing number of sunburns for all time-periods (childhood, adolescence, adulthood and lifetime). In an attempt to understand how risk between life-periods compares, we also report these same linear models on a scale of 5 sunburns per decade for each life-period. The magnitude of risk for 5 sunburns per decade is highest for adult and lifetime sunburns.
Conclusions
Overall, these results show an increased risk of melanoma with increasing number of sunburns during all life-periods, not just childhood. Prevention efforts should focus on reducing sunburns during all life-periods.
Keywords: adolescent, adult, child, intermittent sun exposure, melanoma, meta-analysis, odds ratio, sunburn, sun sensitivity
INTRODUCTION
Ultraviolet radiation (UVR) is considered the foremost environmental cause of cutaneous melanoma (CM). Most dermatologists and melanoma researchers agree that sunburns are an important risk factor for CM. In particular, sunburns occurring during childhood are often cited as posing the greatest risk for CM. Armstrong (1) in a 1988 review outlined strong evidence for a hypothesis put forward by Elwood and others (2) that CM risk was associated with intermittent sun exposure. These early reviews found less clear evidence for cumulative sun exposure. Armstrong theorized that at low frequencies of sun exposure, a tan is not maintained (1). Tanned skin may be a mechanism to shield melanocytes from UVR, similar to the protection seen in naturally darker skin. With intermittent exposure to the sun, the skin is more vulnerable to the effects of UVR and exposure may result in a sunburn. Thus, we are examining sunburns as a marker for intermittent sun exposure.
Quantification of the magnitude of risk by life-period may help dispute that only childhood sunburns matter. Although there have been previous meta-analyses of sunburns and CM risk, none have examined the dose-response effects. We believe that pooling “ever” sunburned separately from dose-response analyses and using all categories from original studies in the dose-response analyses is more appropriate and offers more information about the association between sunburns and CM. The purpose of this meta-analytic review was to quantify the overall magnitude of association between CM and increasing number of sunburns for different life-periods of exposure.
METHODS
Literature Search
Analytic studies that measured sunburns in relation to CM were eligible for this meta-analysis. We repeatedly searched the PUBMED database through December 2007 for articles with keywords related to melanoma and sun exposure including sun, sunlight, tanbed, sunbed, artificial UV, and sunburn, along with references in relevant articles. Titles and abstracts from over 1,300 articles were screened to exclude case reports, commentaries or editorials, animal studies, therapies, biological aspects of melanoma and other irrelevant articles. If relevant or unclear, the article was reviewed in detail. An abstraction form summarizing study design, study population, relevant raw and adjusted data was completed for each article by at least two reviewers.
Study Populations
Since multiple articles reported on the same study populations, they were grouped, in order to prevent pooling of duplicate data. For each life-period of sunburns that was pooled, we chose data (from articles within the same study) with more detail on the number of sunburns. If the same categories of sunburns were reported, then we chose the data with adjustment for more potential confounders, specifically demographics and sun sensitivity factors other than nevi (which may be in the causal pathway). Thus, study data from different articles from the same study may have been selected for different life-periods (e.g., Autier 1994 for adult sunburns and Autier 1998 for childhood sunburns). Publications on the Nurses’ Health Study (NHS) (3) and on the NHS, NHS II and the Health Professionals Follow-up Study (4) were considered as the same population, but one article reported data for lifetime sunburns and the other reported adolescent and adult sunburns. We found several articles from the MD Anderson Cancer Center in Texas on subjects recruited during 1996–2001. Thus, we treated the study subjects from these articles as one population. Duplicate publications from other studies were more obvious based on their authors, study description and referencing of methods.
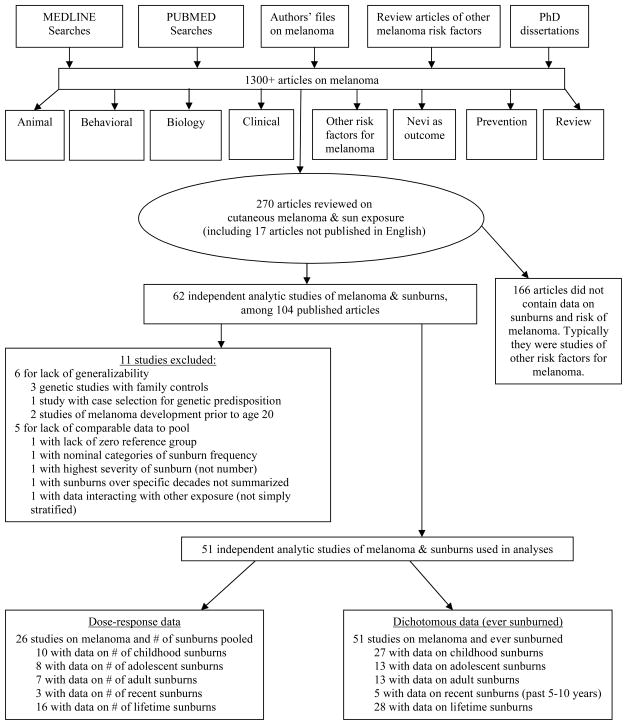
The search and review processes are as follows and are summarized in Figure 1. Among the 270 articles reviewed in detail (253 in English and 17 non-English), 104 articles from 62 independent populations had data on sunburns. Only 51 of the 62 study populations contributed to the meta-analysis. Eleven studies were excluded due to lack of generalizability (5–10) or lack of comparable data to pool (11–16). Among the 51 studies in this report, all either reported results for “ever” sunburned or contained data that made it possible to compute results for “ever” sunburned for at least one life-period. Twenty-six studies reported dose-response data on number of sunburns including one study that only reported recent sunburns (17).
Figure 1.
Literature search for articles on risk of cutaneous melanoma and sunburn history.
Non-English articles were reviewed by an experienced researcher fluent in the appropriate language to determine the relevance and if needed, they completed an abstraction form. Among the four non-English articles with data on sunburns; three had additional publications in English which were pooled since they provided more data (18–22). Translated information on the Argentinean study (23) published in Spanish was used.
Data Extraction
Two independent reviewers abstracted data for each article. Inconsistencies were re-reviewed until consensus was achieved or additional reviewers resolved disagreements. The senior epidemiologist and the dermatologist were responsible for final decisions on disagreements. Studies were classified as non-population-based case-control studies if hospital, clinic, hospital visitors, respondents to newspaper ads, friends, employees, professional organizations or unidentified control groups were used. We considered studies with neighborhood controls, community controls, general population controls, and cohort or nested data from a cohort to be population-based.
We abstracted data on reported sunburns during childhood, adolescence, adulthood, recent, and over a lifetime. These data included both “ever” sunburned and number of sunburns depending on the article. Covariate adjusted odds ratios (ORs) were utilized in the meta-analysis when reported. For each study and level of dose, the natural log of the OR and its variance were required for analyses. When available, the variances were calculated based on the reported confidence intervals (CIs) from adjusted models. Otherwise, we calculated the lnOR and its variance from reported data.
Statistical Analysis
For dichotomous factors (“ever” sunburned), we employed both fixed-effects and random-effects models to obtain pooled relative risk estimates (ORs) (24). Since fixed-effects models assume that risk is homogeneous across included studies and random-effects models assume that the risks are random draws from a larger universe of possible studies, we report only random-effects for “ever” sunburned. Statistical tests of heterogeneity (25) were performed.
For number of sunburns summarized as ordinal categories, we used a fixed-effects method to evaluate possible dose-response relationships in the ln(OR) as a linear function (25). Greenland and Longnecker (25) recommend the use of median scores to weight each exposure category, but few articles reported medians, thus we used the midpoints of the reported ranges. None of the studies provided an upper limit for the highest category (e.g., 6+ sunburns); therefore we derived their midpoint using several methods to estimate the upper limit. Upper bounds of 12 for childhood, 10 for adolescence, 6 for recent, 12 for adult, and 35 for lifetime sunburns were assumed for the final analysis based on clinical experience. Other choices for the upper bounds were examined in sensitivity analyses and found to have minimal effect on risk estimation. We also report the percent of total variability (I2) in risk estimates that is due to heterogeneity between studies (26).
Dose-response effects for number of sunburns were estimated using Bayesian random-effects models, where ORij is the published estimate, from study i, of the odds at exposure category j relative to the first category; xij is our derived numeric score for category j; s2ij is the reported variance for the ln(OR) and βi is a study-specific random slope parameter with overall mean β that represents a log-linear effect of exposure (27). Vague prior distributions were specified for the Bayesian model to reflect a lack of prior information. Bayesian meta-analysis was performed using the WinBUGS 1.4.2 (MRC Biostatistics Unit Cambridge, UK) software.
We chose to report an increase of 5 sunburns, since it is more clinically important than an increase of 1 sunburn. Nevertheless, we note that the choice of unit increase only affects the magnitude of the estimated effect and not its significance (p-value). We additionally report melanoma risk for a rate of 5 sunburns per decade. While this is the same model mathematically, converting each life-period to the same scale places adult and lifetime sunburns on a comparable scale with childhood and adolescence. Articles provided the number of years covered for childhood and adolescent sunburns, so rates per year were calculated per study then converted to rates per life-period (12 years for childhood, 7 for adolescence based on the averages across studies) and per decade. The number of years covered were not specified in articles reporting adult and lifetime sunburns. Since most studies had subjects ranging from age 20 to 80 (with most subjects in the middle of the age range), we estimated that lifetime sunburns typically covered an average of 50 years leaving about 30 years of adult exposure.
RESULTS
We pooled 51 studies in one or more analyses (3, 4, 17–23, 28–79). Among these, 16 were population-based studies. Often, recruitment included all cases (41% of studies) and randomly selected controls (53% of studies). Exclusion or inclusion of in-situ cases was often unreported (43%). Most studies (86%) used interviews to collect survey information, but few (10%) of the studies reported piloting their survey. Participation or response rates were frequently unreported (45%) and not consistently defined.
Table 1 describes the pooled ORs for “ever” sunburned during each life-period. For “ever” being sunburned the risk of CM for specific life-periods was highest in childhood (OR=1.9), followed by adolescence (OR=1.6), recent (OR=1.6) and adulthood (OR=1.4). For lifetime the overall odds ratio was 1.6 (CI 1.4–1.8), which is consistent with that for the specific life-period intervals. Since pooled recent sunburns are based on five heterogeneous studies, few conclusions can be made regarding risk of melanoma with “ever” having a recent sunburn. We also stratified sunburns by how they were defined. No differences by definitions or labels were seen for adolescent sunburns, whereas, the highest OR for adulthood was for blistering sunburns. Among childhood and lifetime sunburns, the studies that either provided no label or labeled sunburns as “severe” or “painful” had the highest pooled estimate, suggesting potential higher recall bias when sunburns were not defined as “so severe they cause blistering or pain for 2 or more days”. Such bias is further supported by the larger amounts of heterogeneity seen between studies lacking clear definitions of sunburns. Among the 28 studies reporting “ever” sunburned during a lifetime, the highest risk was seen for studies which either provided no label or labeled sunburns as severe or painful. When “ever” sunburned analyses were restricted to studies that also reported dose data, no consistent pattern was seen to suggest bias among studies reporting dose information.
Table 1.
A meta-analysis pooling relative risk estimates (OR) for melanoma and “ever” being sunburned during a specific time-period among 51 studies.
| N | OR* | 95% CI | Heterogeneity | |
|---|---|---|---|---|
| Childhood | 27 | 1.91 | 1.59–2.30 | <0.01 |
| Blistering | 9 | 1.71 | 1.40–2.09 | 0.17 |
| Painful† | 7 | 1.73 | 1.27–2.37 | <0.01 |
| Other‡ | 11 | 2.23 | 1.54–3.21 | <0.01 |
| Adolescent | 13 | 1.63 | 1.42–1.86 | 0.09 |
| Blistering | 7 | 1.66 | 1.30–2.12 | 0.05 |
| Painful† | 3 | 1.55 | 1.28–1.86 | 0.27 |
| Other‡ | 3 | 1.63 | 1.23–2.17 | 0.22 |
| Adult | 13 | 1.44 | 1.27–1.63 | 0.14 |
| Blistering | 6 | 1.62 | 1.35–1.94 | 0.56 |
| Painful† | 3 | 1.35 | 1.12–1.63 | 0.55 |
| Other‡ | 4 | 1.41 | 1.08–1.85 | 0.02 |
| Recent (past 5–10 years)§ | 5 | 1.62 | 0.99–2.65 | <0.01 |
| Lifetime | 28 | 1.59 | 1.37–1.83 | <0.01 |
| Blistering | 13 | 1.40 | 1.13–1.74 | <0.01 |
| Painful† | 6 | 1.65 | 1.40–1.96 | 0.25 |
| Other‡ | 9 | 1.74 | 1.32–2.31 | <0.01 |
Pooled estimates are based on random effects models.
Pain lasting 2 or more days
Other sunburns were not clearly defined: thus they may have been described as severe, or pain unspecified, or not defined at all
There were too few studies for recent sunburns to stratify by definition of sunburns.
Only 26 of the study populations reported data on the risk of CM with increasing numbers of sunburns (3, 4, 17, 19–23, 28–50). These studies are described in detail in Table 2, including their study design, location, diagnosis dates of cases (or study dates), number of subjects, and ORs for life-period of reported sunburns and adjustment factors. As can be seen in Table 2, several studies reported crude ORs or ORs adjusted only for age, sex, and other demographic characteristics. Few studies adjusted for measures of sun sensitivity (hair, skin and eye color, tendency to burn, ability to tan, and skin type). A few other studies adjusted for number of nevi, a precursor or marker of melanoma risk, which would adjust away some of the risk for melanoma.
Table 2.
Dose response data among 26 studies that reported risk of melanoma with increasing number of sunburns.
| Place Author (citation) | Case*/control Diagnosis dates [Data collection] | # of Sunburns under age 20† | # of Sunburns, adult or lifetime† | Adjustment factors | ||
|---|---|---|---|---|---|---|
| Population-based | ||||||
| Australia, Queensland | 183/183 | Lifetime, painful | Crude OR, calculated‡ (matching on age, sex & geography not preserved) | |||
| Green (28) | 1979–80 | 1 | 1.0 (0.6–1.8) | |||
| 2 | 2.6 (1.2–5.8) | |||||
| 3 | 1.7 (0.7–4.6) | |||||
| 4 | 2.5 (1.0–6.6) | |||||
| 5 | 3.1 (0.7–13.1) | |||||
| 6 | 6.2 (1.7–23.4) | |||||
| 7 | 0.9 (0.2–4.1) | |||||
| 8 | 14.0 (1.7–114) | |||||
| 9+ | 2.9 (1.3–6.3) | |||||
| Denmark, eastern | 474§/926 | Childhood, painful | Recent, painful | Sex, # of raised nevi, freckles, & hair color (frequency matched on age & sex) | ||
| Osterlind (29) | 1982–85 [1984+] | 1 | 1.2 (0.7–1.9) | 1 | 1.6 (0.9–2.8) | |
| 2–4 | 1.9 (1.3–2.9) | 2–4 | 1.1 (0.5–2.3) | |||
| 5+ | 2.7 (1.6–4.8) | 5+ | 3.0 (1.5–5.9) | |||
| Adolescent, painful | ||||||
| 1 | 1.5 (1.0–2.3) | |||||
| 2–4 | 1.8 (1.2–2.7) | |||||
| 5+ | 1.9 (1.2–3.1) | |||||
| Germany, France, Belgium | 420/447 | Childhooda | Adultb | a. Hair color | ||
| 1991–92 | 1–4 data not reported | 1–4 data not reported | b. Crude OR, reported (frequency matched on sex & geography) | |||
| 5+ | 1.5 (1.0–2.3) | 5+ | 1.9 (1.2–2.5) | |||
| Autier (30, 31) | ||||||
| Germany, France, Italy, Bulgaria, Estonia, Israel, Austria | 603/627 | Childhooda | Adulta | a. Age, sex, center | ||
| --- | 1–2 | 1.1 (0.8–1.4) | 1–2 | 1.1 (0.8–1.4) | b. Age, sex, center, ethnic origin (frequency matched on age & sex) | |
| [1994–97] | 3–9 | 1.3 (0.9–1.9) | 3–9 | 1.6 (1.1–2.2) | ||
| 10+ | 3.2 (1.5–7.1) | 10+ | 1.9 (1.1–3.3) | |||
| Pfahlberg (21) | Lifetimeb | |||||
| Kolmel (22) | 1–2 | 1.2 (0.9–1.6) | ||||
| 3–5 | 1.3 (0.9–1.9) | |||||
| 6–14 | 2.0 (1.3–3.1) | |||||
| 15+ | 3.1 (1.7–5.6) | |||||
| Italy, Florence | 131§/174 | Lifetime, blistering | Age, sex, & education | |||
| Carli (32) | 1990–93 | 1–2 | 0.8 (0.4–1.5) | |||
| 3–5 | 1.2 (0.5–2.6) | |||||
| 6+ | 1.8 (0.6–5.5) | |||||
| Italy, Turin | 260¶/416 | Lifetime, painful | Age & sex (frequency matched on age & sex) | |||
| Zanetti (19) | 1984–87 | 1 | 1.7 (1.1–2.6) | |||
| 2+ | 1.5 (0.8–2.7) | |||||
| Sweden, southern | 400||/640 | Childhood, painful | Adult, painful | Raised nevi, & hair color (matched on age, sex & geography) | ||
| Westerdahl (34) | 1988–90 [1988–90] | 1–5 | 1.4 (1.0–1.9) | 1–5 | 1.5 (1.1–2.1) | |
| 6+ | 1.6 (1.0–2.6) | 6+ | 1.9 (1.2–3.1) | |||
| Adolescent, painful | ||||||
| 1–5 | 1.4 (1.0–1.9) | |||||
| 6+ | 1.6 (1.0–2.5) | |||||
| UK, England, Birmingham | 58/151 | Lifetime | Age, sex, tendency to burn, # of nevi, & holidays abroad (ORs & # of cases reported; controls & CI estimated) | |||
| Sorahan (35) | 1980–82 [1982–84] | 1–4 | 3.0 (1,3–6.7) | |||
| 5+ | 4.2 (1.7–10.4) | |||||
| USA, California, San Francisco | 452/930 | Childhood, painful | Adult, painful | Crude OR, reported (females only; frequency matched on age & geography) | ||
| 1981–86 | 1–3 | 1.3 (1.0–1.7) | 1–3 | 1.0 (0.8–1.4) | ||
| Holly (36) | 4–6 | 2.2 (1.5–3.1) | 4–6 | 1.6 (0.9–2.6) | ||
| 7+ | 2.0 (1.4–2.9) | 7+ | 2.0 (1.1–3.8) | |||
| Adolescent, painful | Recent, painful | |||||
| 1–3 | 1.2 (0.9–1.5) | 1 | 1.3 (0.8–2.0) | |||
| 4–6 | 1.6 (1.2–2.3) | 2–3 | 1.6 (1.1–2.6) | |||
| 7+ | 2.4 (1.6–3.5) | 4+ | 1.9 (1.3–3.0) | |||
| USA, Hawaii, Oahu | 278||/278 | Childhood, blistering | Height, education, hair color, ability to tan & drinking status (matched on age & sex) | |||
| Males: | ||||||
| Le Marchand (37) | 1986–98 [1988–92] | 1–4 | 0.9 (0.4–2.2) | |||
| 5+ | 0.7 (0.4–1.5) | |||||
| Females: | ||||||
| 1–3 | 1.2 (0.3–4.4) | |||||
| 4+ | 1.2 (0.4–3.8) | |||||
| Adolescent, blistering | ||||||
| Males: | ||||||
| 1–4 | 1.2 (0.6–2.2) | |||||
| 5–12 | 1.2 (0.6–2.6) | |||||
| 13+ | 2.0 (0.9–4.6) | |||||
| Females: | ||||||
| 1–3 | 2.4 (0.9–6.2) | |||||
| 4–10 | 3.3 (1.0–10.0) | |||||
| 11+ | 1.9 (0.7–6.7) | |||||
| USA (NHS I, NHS II, HPFS) cohort | 535§/178,155 | Lifetime | Age, sex, family history of melanoma, # of nevi, & hair color | |||
| Cho (4) | --- | 1–2 | 1.4 (1.0–1.9) | |||
| [1986–00] | 3–5 | 1.6 (1.2–2.2) | ||||
| 6–9 | 2.0 (1.4–2.7) | |||||
| 10+ | 2.4 (1.7–3.2) | |||||
| USA (NHS I), 11 states, nested case-control within cohort | 130/300 | Adolescent, blistering | Adult, blistering | Adjusted for written questionnaire vs. telephone interview (females only; matched on age & cycle of questionnaire) | ||
| 1976–84 [1976–84 ] | 1 | 0.7 (0.4–1.6) | 1 | 0.8 (0.5–1.6) | ||
| 2 | 1.8 (1.0–3.4) | 2 | 1.7 (1.0–2.9) | |||
| Weinstock (3) | 3–4 | 1.7 (0.9–3.0) | 3–4 | 1.0 (0.5–2.0) | ||
| 5+ | 1.9 (1.1–3.4) | 5+ | 1.1 (0.6–2.3) | |||
| USA, Washington, western | 386§/727 | Childhood | Age & sex | |||
| 1997 | 1 | 1.4 (0.9–2.1) | ||||
| Shors (40) | 2 | 1.3 (0.8–2.1) | ||||
| 3+ | 2.4 (1.8–3.4) | |||||
| Adolescent | ||||||
| 1 | 1.3 (0.9–2.0) | |||||
| 2 | 1.3 (0.8–2.0) | |||||
| 3+ | 2.7 (2.0–3.7) | |||||
| Non-population | ||||||
| Argentina, Cordoba | 65/195 | Childhood | Crude OR, calculated‡ (matched on age & sex) | |||
| Ruiz Lascano (23) | 1998–01 | 1–3 | 4.4 (1.9–10.3) | |||
| 3+ | 46.0 (10.2–207) | |||||
| Brazil, southern | 103/206 | Lifetime, blistering | Light eye & hair color, ephelides & dysplastic nevi, # of nevi, sunscreen & physical protection (matching on age, sex & geography not preserved) | |||
| Bakos (41) | 1995–98 | 1–29 | 1.9 (1.0–3.6) | |||
| 30+ | 11.4 (2.6–50.5) | |||||
| France, southeast region | 207§/295 | Recent | Age, # of nevi (<5 mm, 5–10 mm), maximum suntan, hair color, social level, complexion | |||
| 1986–88 | 1–2 | 0.4 (0.2–1.0) | ||||
| Grob (17) | 3+ | 1.7 (0.6–4.6) | ||||
| Germany | 200||/200 | Lifetime | Crude OR, calculated‡ (matching on age & sex not preserved) | |||
| Garbe (42) | 1980–87 [1988–89] | 1–2 | 0.7 (0.4–1.4) | |||
| 3–4 | 0.5 (0.2–0.9) | |||||
| 5–10 | 0.4 (0.2–0.8) | |||||
| 11+ | 0.6 (0.3–1.3) | |||||
| Ireland | 100/100 | Lifetime, blistering | Crude OR, calculated‡ (matching on age, sex & geography not preserved) | |||
| 1 | 2.3 (1.0–5.2) | |||||
| Dunn-Lane (43) | --- | 2+ | 4.6 (1.9–11.1) | |||
| Italy, regions of Emilia-Romagna or Marche | [1985–86] | Lifetime, blistering | Age, sex, dysplastic nevi, skin & eye color, & tanning ability (frequency matched on age & sex) | |||
| 183||/179 | 1–5 | 0.8 (0.4–1.8) | ||||
| 1994–99 | 6+ | 2.5 (0.7–8.9) | ||||
| Landi (44) | ||||||
| Italy, region of Lazio | 287||/299 | Childhood, blistering | Adult, blistering | Age & sex (frequency matched on age & sex) | ||
| Fortes (50) | 2001–03 | 1 | 1.6 (0.7–3.0) | 1 | 1.5 (0.8–2.5) | |
| 2–5 | 2.8 (1.6–4.3) | 2–5 | 1.8 (1.1–2.8) | |||
| 6+ | 3.6 (1.9–6.9) | 6+ | 2.5 (1.4–4.4) | |||
| Adolescent, blistering | ||||||
| 1 | 1.6 (0.9–2.9) | |||||
| 2–5 | 2.1 (1.3–3.4) | |||||
| 6+ | 2.7 (1.4–5.2) | |||||
| Spain, Andalusia | 105||/138 | Lifetime, blistering | Age, skin color, & skin type | |||
| Rodenas (50) | 1988–93 | 1 | 1.7 (0.7–4.3) | |||
| 2+ | 4.1 (1.6–1.0) | |||||
| UK, England, northeast Thames region | 413||/416 | Lifetime, blistering | Age, sex, & skin type (frequency matched on age & sex) | |||
| 1989–93 | 1 | 1.1 (0.8–1.5) | ||||
| Bataille (45) | 2–3 | 0.8 (0.5–1.3) | ||||
| 4–5 | 0.6 (0.3–1.3) | |||||
| 6–9 | 1.8 (0.6–5.6) | |||||
| 10+ | 1.9 (1.0–3.8) | |||||
| UK, Scotland | 280§/280 | Lifetime (males) | Total nevi, freckling, atypical nevi, skin type, ultraviolet use, & tropical residence (matched on age & sex) | |||
| MacKie (46) | 1987 | 1–2 | 2.8 (1.3–5.6) | |||
| 3+ | 7.6 (1.8–32.0) | |||||
| Lifetime (females) | ||||||
| 1–2 | 1.5 (1.0–2.4) | |||||
| 3+ | 2.3 (0.9–5.6) | |||||
| USA, California, San Francisco | 121/139 | Lifetime, blistering | Age, # of nevi, dysplastic nevi, hair color, & skin cancers (frequency matched on age & sex) | |||
| 1984–85 | 1–10 | 1.4 (0.7–3.0) | ||||
| Holly (47) | 11–20 | 1.7 (0.7–4.4) | ||||
| 21+ | 3.8 (1.4–10.4) | |||||
| USA, Iowa | 368§/373 | Childhood, blistering | Adult, blistering | Age, sex, skin color, & history of skin cancer (frequency matched on age & sex) | ||
| Beane Freeman (48) | 1999–00 [2002–03] | 1–2 | 1.5 (1.0–2.3) | 1–2 | 1.2 (0.8–1.7) | |
| 3–5 | 1.5 (0.9–2.5) | 3–5 | 1.3 (0.8–2.2) | |||
| 6+ | 2.1 (1.3–3.6) | 6+ | 2.9 (1.7–5.0) | |||
| Adolescent, blistering | ||||||
| 1–2 | 1.6 (1.1–2.4) | |||||
| 3–5 | 1.6 (1.0–2.6) | |||||
| 6+ | 1.8 (1.0–3.2) | |||||
| USA, Pennsylvania, Philadelphia & California, San Francisco | 318§/395 | Lifetime | Crude OR, calculated‡ (frequency matched on age, sex & geography) | |||
| 1991–92 | 1–3 | 1.6 (1.1–2.4) | ||||
| 4+ | 2.0 (1.4–3.0) | |||||
| Lea (49) | ||||||
| USA, Texas | 289||/308 [Referred in 1994–01] | Lifetime, blistering | Crude OR, calculated‡ (frequency matched on age, sex & ethnicity) | |||
| Shen (38) | 1–5 | 1.6 (1.1–2.3) | ||||
| Wei (39) | 6+ | 3.2 (2.0–5.2) | ||||
OR= odds ratio; CI= confidence interval; #=number; NHS = Nurses’ Health Study; HPFS = Health Professionals Follow-Up Study.
Unless otherwise noted, the study did not state whether cases only included invasive cutaneous melanomas or also included in situ melanomas.
Data shown are the # of sunburns, OR and 95% CI based on a zero reference category of sunburns. Sunburns are labeled as: blistering=blistering sunburn; painful=pain lasting for 2+ days; or unspecified = other sunburns not clearly defined, but described as severe, or pain unspecified, or not defined at all.
OR and 95% CI were calculated from reported data (frequencies or dichotomized dose data)
The article stated that cases only included cutaneous malignant melanoma (invasive cases).
The article stated that cases included both invasive and in situ cutaneous melanoma cases.
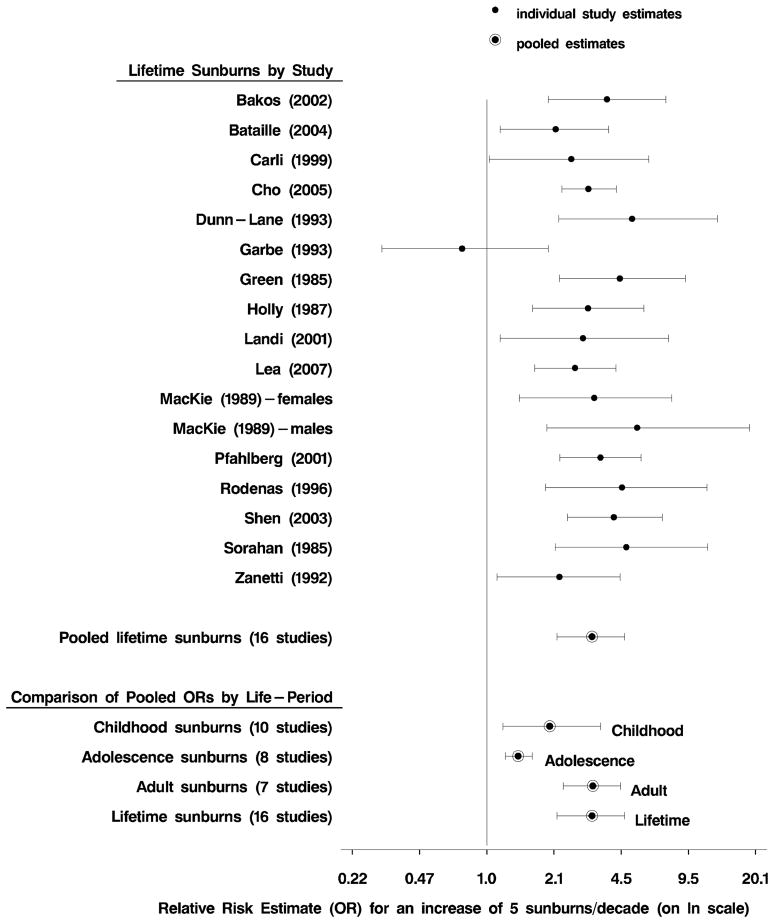
Pooled dose-response data are summarized in Table 3 by life-period of the sunburns. We reported ORs for a clinically important increase of 5 sunburns both per life-period and per decade. Pooled results show significant ORs for each life-period. The ORs for 5 sunburns per life-period were similar except for the lower OR for lifetime. When rates in the four life-periods were converted to decades, the highest risks were seen for number of adult and lifetime sunburns per decade (for both fixed- and random-effects models). The random-effects data for lifetime sunburns per decade are graphically shown in a forest plot (Figure 2). The number of lifetime sunburns is clearly important in CM risk. When comparing pooled ORs for 5 sunburns per decade across life-periods, the lower magnitude of risk for adolescence likely reflects that multiple sunburns experienced during adolescence typically occur in less than ten years.
Table 3.
A meta-analysis pooling relative risk estimates (OR) for melanoma and dose information for number of sunburns among 25 studies reporting on childhood, adolescent, adult or lifetime sunburns.
| Time-period | Increase modeled (# of sunburns)* | # of studies N | Fixed effects model OR (95% CI) | Hetero-geneity p-value | Heterogeneity between studies I2† | Random effects (Bayesian model) OR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| Per time period | ||||||||
| Childhood | 5 | 10 | 1.35 | 1.27–1.43 | <0.001 | 83% | 1.79 | 1.16–2.93 |
| Adolescent | 5 | 8 | 1.42 | 1.32–1.53 | <0.001 | 73% | 1.66 | 1.34–2.07 |
| Adult | 5 | 7 | 1.46 | 1.32–1.60 | 0.56 | -- | 1.48 | 1.31–1.67 |
| Lifetime | 5 | 16 | 1.22 | 1.18–1.26 | 0.013 | 49% | 1.26 | 1.17–1.36 |
| Per decade‡ | ||||||||
| Childhood | 5 | 10 | 1.43 | 1.33–1.54 | <0.001 | 83% | 2.02 | 1.20–3.56 |
| Adolescent | 5 | 8 | 1.28 | 1.21–1.35 | <0.001 | 73% | 1.42 | 1.23–1.66 |
| Adult | 5 | 7 | 3.08 | 2.31–4.11 | 0.56 | -- | 3.27 | 2.35–4.45 |
| Lifetime | 5 | 16 | 2.66 | 2.25–3.13 | 0.013 | 49% | 3.24 | 2.19–4.66 |
For each time-period, an upper limit of the number of sunburns was estimated from which the median of the upper category for each study was partially estimated. The upper limit of sunburn was 12 for childhood, 10 for adolescence, 12 for adult and 35 for lifetime sunburns.
The I2 statistic describes the proportion of total variability in risk estimates that is due to the heterogeneity between studies.
Estimates the pooled risk for an increase of five sunburns per decade, thus scales analyses for each time-period to the same number of sunburns per year in an attempt to show comparable risk estimates.
Figure 2.
Forest plots for melanoma and number of sunburns. Individual study’s linear dose-response estimates of the OR are for an increase of 5 sunburns per decade over a lifetime. Pooled odds ratios and confidence intervals are also shown for lifetime along with childhood, adolescence and adulthood for an increase of 5 sunburns per decade (in order to make the life-period scales comparable).
Little or no difference was seen by geography of the study population. We conducted sensitivity analyses in fixed-effects models by stratifying by population-based studies compared to other studies and found for childhood and adult sunburns that the non-population-based studies were somewhat higher that the population-based studies. Additional sensitivity analyses for dose restricted to studies that clearly defined sunburns as “blistering and/or pain lasting 2 or more days” showed negligible differences in the ORs. Surprisingly, very few studies (1–2 for childhood, adolescence and adulthood) adjusted for selected sun sensitivity factors important for CM (skin color or type, tendency to burn, ability to tan). This limited the ability to conduct restricted analyses. Lifetime sunburns contained 5 studies adjusted for sun sensitivity which gave a pooled fixed-effects OR for 5 sunburns very similar to the OR for all lifetime sunburns. When sun sensitivity factors either measured or adjusted for were examined as covariates in the Bayesian models, the pooled ORs reduced slightly. Our final sensitivity analyses pooled the four studies that reported childhood, adolescence, and adult sunburns, for comparable populations and confounders addressed. Pooled ORs were similar to each other and those in Table 3.
Funnel plots showed inconclusive evidence regarding possible publication bias, but Begg’s test (80) for publication bias using rank correlation based on Kendall’s tau for all time-periods combined suggested publication bias (p<0.01). It is unclear if this may be due to examining all time-periods together which have different magnitudes of their ORs. Thus, we examined the effect of possible missed studies by time-period. Since in theory, unpublished studies tend to be of smaller sample size with non-significant or protect effects, we conservatively examined how many studies with 100 cases and 100 controls with a dose-response OR of 0.8 it would take to reduce the results in each of the time-periods to non-significance. Under these assumptions, it would take 37 such studies to nullify the fixed-effect seen for childhood; 31 for adolescence; 21 for adult sunburns and 110 for lifetime sunburns.
DISCUSSION
This review and meta-analysis is the first to pool data on the number of sunburns in relation to cutaneous melanoma. Other analyses have considered sunburn to be a dichotomous exposure of either ever/never or lowest versus highest category of sunburns. Our pooled analyses of dose-response data on number of sunburns provide evidence for causality (81). Even with misclassification known to exist in ordered categories, an estimation of numbers of sunburns will give a better characterization of the true relationship between CM and sunburns. We found CM to be related to increasing numbers of sunburns during all life-periods. Traditional comparisons across time periods appear at first glance to suggest the lowest risk for lifetime sunburns. However, ORs per time period are comparing an increase of 5 sunburns across different lengths of times, thus it is not surprising that adolescence has one of the highest ORs because it covers the shortest time period, whereas lifetime has a lower OR for an increase of 5 sunburns over a very long time. When these pooled ORs are transformed to the same scale of 5 sunburns per decade, then the highest risks appear to be across adulthood and lifetime. While experts often focus on childhood sunburns as the primary risk factor for CM, our pooled analyses found increased risk of CM for sunburns experienced in adolescent, adult and lifetime number of sunburns as well as childhood. However, analyses per decade suggest a stronger association if individuals continue to experience sunburns at the same rate per decade into adulthood, and thus over a lifetime. Ultimately, these pooled data show that sunburns, regardless of timing, affect the risk of CM.
The conservative random-effects pooled dose-response ORs per life-period show high ORs for childhood (OR=1.8) and adolescent (OR=1.7) sunburns, but adult (OR=1.5) and lifetime (OR=1.3) sunburns also confer an increased risk of CM. When the 4 life-period models are all re-scaled to 5 sunburns per decade, higher ORs are seen for adult (OR=3.3) and lifetime (OR=3.2) sunburns compared to childhood (OR=2.0) and adolescence (OR=1.4). Since the models per life-period and per decade are identical models but on different scales, the OR for sunburns in adolescence decreases when rescaled from 5 sunburns per 7 years to 5 sunburns per 10 years. Although ORs for 5 sunburns per decade allow for comparison of risk across life-periods, it is unclear if the difference in number of sunburns between two individuals is likely to remain constant (5 sunburns) over each decade or between life-periods since sun behavior changes with age and responsibilities.
The magnitude of the pooled OR for lifetime number of sunburns varies depending on the scale (per lifetime or per decade). If sunburns during different life-periods were independent, then we would expect a comparable number of sunburns over a lifetime to be the sum of the individual life-periods. Estimates per decade are one way to address this. However, sunburns are not independent in that they are related to skin type and UV exposure. Individuals with fair skin type may learn from experience and in turn avoid UV exposure as suggested by Holly et al. (36). Therefore, after receiving multiple sunburns during childhood or adolescence individuals may receive less as adults imparting a low lifetime number of sunburns. Overall, each life-period shows a relationship between number of sunburns and risk of CM. While sunburns may be caused by acute UV exposure, which may interact with sun sensitivity, sunburns appear to represent real damage to the skin that may eventually cause CMs. Thus, sunburns are likely the mechanism through which sun sensitivity and sun exposure are related to CM.
Our dose-response analyses are robust as shown by the minimal differences seen in sensitivity analyses. However, several studies had ORs that were outliers in comparison to other studies. For lifetime number of sunburns, a German study (42) reported protective ORs likely due to a non-population control group that consisted of patients from the dermato-oncologic clinic with other skin diseases. This control group gives justification to removing Garbe et al. (42) from the pooled analyses. When Garbe et al. (42) was removed from the pooled analyses the heterogeneity between studies went away and the OR of 5 sunburns per decade increased from 2.7 to 2.9 (95% CI of 2.4–3.4; heterogeneity p=0.40, and 5% heterogeneity between studies). The three studies (19, 33, 43) that lumped lifetime sunburns into 0, 1, and 2+ sunburns had similar ORs for the linear dose-response analyses as other studies, as did the Brazilian study (41) that reported more than 30 lifetime sunburns. Among the 9 studies reporting adolescent sunburns, the study by Shors et al. (40) had a higher OR for fewer sunburns (3+); when it was removed from the pooled analyses the heterogeneity between studies went away (OR=1.2, 95% CI 1.2–1.3; heterogeneity p=0.36, and 9% heterogeneity between studies). This population-based study was conducted using the Seattle SEER program and random-digit dialing to recruit population based controls (40). Lacking obvious reasons this population may differ from other studies, the heterogeneity may have occurred due to lumping all sunburns > 2 together without reporting the mean or maximum number of sunburns in these subjects. Of all life-periods, the studies reporting on number of childhood sunburns were the most heterogeneous. The heterogeneity was only eliminated when three studies with high ORs (Argentina, Lazio Italy, Denmark) (23, 29, 50) and one study with a protective association (Hawaii males) (37) were eliminated (OR=1.4, 95% CI 1.3–1.5; heterogeneity p=0.37, and 8% heterogeneity between studies). While the Argentinean study (23) had the largest ORs it also had large variability due to a small sample size, whereas the studies in Denmark (29) and Italy (50) had small variances weighting these two studies more in the pooled analyses.
Only two studies reported risk for number of sunburns adjusted for other life-periods. One study (21) adjusted childhood and adult exposure for each other, whereas a second study (48) adjusted childhood, adolescent, and adult sunburns for one another. Pooling these two studies gave an OR for adult sunburns that decreased from 1.60 to 1.54 with adjustment for other life-period sunburns, whereas, the pooled OR for childhood only decreased from 1.56 to 1.49. These two studies would suggest that different life-periods do not confound other life-periods regarding sunburn and CM risk. However, more studies are needed to look at this relationship.
A few studies also reported data regarding recent sunburns. The five ORs for “any” sunburn in last 5–10 years (17, 29, 37, 55, 71) were heterogeneous, whereas, the three studies (17, 29, 36) reporting the number of recent sunburns appear to be homogeneous with a fixed-effects OR=2.1 (95% CI 1.6–2.8) for 5 sunburns per decade; this higher pooled OR for sunburns reported just prior to CM diagnosis may suggest recall bias. Recall bias would exist if cases reported more recent sunburns than they actually experienced, attempting to account for behavior that may have caused their CM. Alternatively, this higher OR for recent sunburns could indicate that progression to CM is accelerated by sunburns just prior to development. However, it is difficult to draw firm conclusions since few studies reported such data and there is a large potential for recall bias.
Pooled “ever” sunburned data had the strongest association for childhood sunburns and significant associations for all life-periods. While the ORs for vaguely defined sunburns were high, they tended to display the most heterogeneity, suggesting recall bias where cases may be more likely to over-report “undefined” sunburns than controls. Additionally, the poor categorization of sunburns into “ever sunburned” is difficult to interpret (regardless of definitions) given that such categorization lumps someone sunburned “once” with someone who has experienced repeated sunburns, thus causing severe heterogeneity among the “exposed” group. Future studies should focus on sunburns that are blistering or painful for 2 or more days.
Potential for Bias or Misclassification
Measurement of sunburns as “ever” or “never” sunburned as a child, adolescent, adult, or during one’s lifetime may appear to be simpler; however, such categorization loses valuable information and should not be over interpreted. The heterogeneity seen for “ever” sunburned during a specific life-period may reflect overly simplified dose data of different magnitudes of number of sunburns in different populations. Combining all number of sunburns into “ever” assumes the risk for CM of 1 sunburn is equivalent to 20 sunburns.
While in general retrospective studies may have reporting bias (non-differential) or recall bias (differential), sunburns are thought to be more easily recalled than other types of sun exposure since sunburns are frequently associated with memorable discomfort. Studies that reported reliability of “ever sunburned” used repeated measures more than 3 months apart, leaving questions as to whether differences were due to changes in sunburns or low reliability. “Ever sunburned” lumps those sunburned once with those who experienced many sunburns, causing severe heterogeneity among those “ever sunburned”. Dichotomous analyses are only stronger if “never” sunburned is measured completely accurately, but still would not quantify how many sunburns are important. Reliability studies (test-retest) examining sunburns (less than 3 months apart), showed consistency in reporting number of sunburns with Kappas ranging from 0.5 to 0.8 with the highest consistency among those defining sunburns as “blistering or pain lasting 2 or more days” (Kappas of 0.7 to 0.8) (82–85). This suggests that individuals can recall number of sunburns, particularly when sunburns are defined as severe with blisters or pain lasting 2+ days. But clear scientific evidence on how to best define sunburns is lacking. Among dose-response analyses, only 28% of studies did not clearly specify how they defined “sunburns” compared to 39% of those reporting “ever sunburned”. Since there is a difference in CM risk for sunburn susceptibility across skin-types, the heterogeneity in “ever” reporting childhood sunburns may reflect differences in sun sensitivity across those with a childhood sunburn.
Adjustment for Sun Sensitivity
The effect of UVR on CM risk may be strongly modified by sun sensitivity. Sun sensitivity characteristics are widely accepted as risk factors for CM, including eye, hair and skin color, tendency to sunburn and inability to tan (20, 76, 86). Lighter complexion as measured by both Fitzpatrick skin-type (21, 33, 41) and self-reported skin color (fair, medium, or dark) (87–89) appear to be the most consistent risk factors for CM. In theory, the same amount of UV exposure imparts a greater risk among individuals with fair complexions who sunburn easily than among those with darker complexions who tan. Therefore, sun sensitivity may modify or confound the association seen between CM and sunburns. However, only 14 of the 26 studies in dose-analyses adjusted for sun sensitivity. It is possible that studies examined measures of sun sensitivity as possible confounders but did not find that they confounded the data; however, this was not stated. Two studies adjusted for sunburns by Fitzpatrick skin-type (45, 46), one adjusted for both Fitzpatrick and skin color (33), and two additional studies adjusted for skin color (44, 48). Skin color should predict the skin’s acute (tendency to burn) and chronic reactions (ability to tan) to sun exposure. Three studies in the pooled dose-response analyses adjusted for acute or chronic reaction to the sun (35, 37, 44). While the risk of CM within light-skinned populations is also influenced by other pigmentary characteristics (adjusted for in the remaining seven (4, 17, 29, 30, 34, 41, 47) of 14 studies), such as hair color, eye color, and freckling, such factors often segregate with skin-type. Sunburn histories are clearly related to these measures of sun sensitivity. Adjustment for sun-sensitivity factors tells us the risk of CM for sunburns beyond host susceptibility. In Bayesian sub-analyses all studies were included with a covariate to identify which studies adjusted for sun sensitivity showing slightly lower ORs that remained significant. For fixed-effects, we restricted the models to only those studies that adjusted for sun sensitivity thus only lifetime had more than two studies to pool with a slightly higher OR. Overall, the reported articles on sunburns have not provided enough information to appropriately quantify the effect of adjustment for sun sensitivity. However, if sunburns initiate the development of CM, then the risk may be better described as crude risk stratified by sun sensitivity. Stratification would help in understanding if sunburns in darker skinned individuals are important.
The number of nevi as a potential confounder is more complex. Nevi are both genetically determined and influenced by sun exposure. Studies consistently show an increased number of nevi are related to an increased risk of CM. If nevi are precursors or markers of CM risk, it may be inappropriate to adjust for nevi when examining CM risk with sun exposure. Thus, for a study (21) reporting multiple models, we selected the model unadjusted for nevi (21). However, when nevi adjustment was examined as a covariate in the Bayesian model, minimal differences were seen in the magnitude of the OR for number of sunburns.
Prior meta-analyses
There have been previous meta-analyses on the association between sunburn history and CM that included 10–32 articles (90–93). We excluded one article included in the prior reviews because they only presented data on the highest severity of sunburn, but pooled additional articles published more recently or not previously included in other meta-analyses. In addition, our analyses differ in critical methodology. First, we pooled data separately for “ever” sunburned and for dose-response data. Prior meta-analyses pooled dissimilar categories: “ever” sunburned, the highest category reported from dose-response analyses, and severity of sunburns using simple methods for dichotomous exposures. The upper categories ranged from 2+ to 7+ sunburns. Pooling ever sunburned with 7+ sunburns and the highest severity of sunburns is likely to create misclassification and heterogeneity among such diverse measures. We used a method that considers the number of sunburns as a linear dose-response factor. Overall, the most important difference in this meta-analysis in comparison to previous publications is our linear dose-response analyses using fixed-effects and random-effects (Bayesian) models.
Limitations
Meta-analyses cannot overcome limitations of the original studies related to their data collection including misclassification, selection bias or recall bias. Publication bias is also an issue if significant studies are more likely to be published, however, we did not see much evidence of this since 40 of the 86 dichotomous ORs pooled for Table 1 had CIs that included 1.0.
Conclusions
This meta-analysis pooled ORs from 51 studies on CM and “ever” experiencing a sunburn. Our analyses further examined linear dose-response data among 26 studies, which had not previously been reported. These results suggest increasing number of sunburns increase the risk of CM regardless of when they are received. The large ORs seen per decade for adult and lifetime exposure suggest that it is the number of sunburns that increases melanoma risk not when they are received. More consistency may be seen in reported ORs for sunburns if clear definitions of sunburns are used to survey subjects. Future studies should focus on number of sunburns with blisters or pain lasting 2 or more days. A better understanding of the independence of sunburns and sun sensitivity would be gained if studies stratified their results for sun exposure by sun sensitivity factors such as skin type, skin color, or tendency to burn. Prevention efforts should focus on reducing all sunburns, regardless of what age they are acquired.
Acknowledgments
This research was supported in part by the National Cancer Institute (grant number: CA86221-01) and by the American Cancer Society (gr ant number: RSGPB CPPB-106999).
Abbreviations
- CM
cutaneous melanoma
- UVR
ultraviolet radiation
- OR
odds ratio
- CI
confidence interval
- NHS
Nurses’ Health Study
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong BK. Epidemiology of malignant melanoma: intermittent or total accumulated exposure to the sun? J Dermatol Surg Oncol. 1988;14(8):835–49. doi: 10.1111/j.1524-4725.1988.tb03588.x. [DOI] [PubMed] [Google Scholar]
- 2.Elwood JM, Hislop TG. Solar radiation in the etiology of cutaneous malignant melanoma in Caucasians. Natl Cancer Inst Monogr. 1982;62:167–71. [PubMed] [Google Scholar]
- 3.Weinstock MA, Colditz GA, Willett WC, Stampfer MJ, Bronstein BR, Mihm MC, Jr, et al. Nonfamilial cutaneous melanoma incidence in women associated with sun exposure before 20 years of age. Pediatrics. 1989;84(2):199–204. [PubMed] [Google Scholar]
- 4.Cho E, Rosner BA, Feskanich D, Colditz GAk. Risk factors and individual probabilities of melanoma for whites. J Clin Oncol. 2005;23(12):2669–75. doi: 10.1200/JCO.2005.11.108. [DOI] [PubMed] [Google Scholar]
- 5.Cockburn M, Black W, McKelvey W, Mack Tk. Determinants of melanoma in a case-control study of twins (United States) Cancer Causes & Control. 2001;12(7):615–25. doi: 10.1023/a:1011271117496. [DOI] [PubMed] [Google Scholar]
- 6.Chaudru V, Chompret A, Bressac-de Paillerets B, Spatz A, Avril MF, Demenais F. Influence of genes, nevi, and sun sensitivity on melanoma risk in a family sample unselected by family history and in melanoma-prone families. J Natl Cancer Inst. 2004;96(10):785–95. doi: 10.1093/jnci/djh136. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein AM, Falk RT, Fraser MC, Dracopoli NC, Sikorski RS, Clark WH, Jr, et al. Sun-related risk factors in melanoma-prone families with CDKN2A mutations. J Natl Cancer Inst. 1998;90(9):709–11. doi: 10.1093/jnci/90.9.709. [DOI] [PubMed] [Google Scholar]
- 8.Whiteman DC, Valery P, McWhirter W, Green AC. Risk factors for childhood melanoma in Queensland, Australia. Int J Cancer. 1997;70(1):26–31. doi: 10.1002/(sici)1097-0215(19970106)70:1<26::aid-ijc4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Youl P, Aitken J, Hayward N, Hogg D, Liu L, Lassam N, et al. Melanoma in adolescents: a case-control study of risk factors in Queensland, Australia. Int J Cancer. 2002;98(1):92–8. doi: 10.1002/ijc.10117. [DOI] [PubMed] [Google Scholar]
- 10.Matichard E, Verpillat P, Meziani R, Gerard B, Descamps V, Legroux E, et al. Melanocortin 1 receptor (MC1R) gene variants may increase the risk of melanoma in France independently of clinical risk factors and UV exposure. J Med Genet. 2004;41(2):e13. doi: 10.1136/jmg.2003.011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veierod MB, Weiderpass E, Thorn M, Hansson J, Lund E, Armstrong B, et al. A prospective study of pigmentation, sun exposure, and risk of cutaneous malignant melanoma in women. J Natl Cancer Inst. 2003;95(20):1530–8. doi: 10.1093/jnci/djg075. [DOI] [PubMed] [Google Scholar]
- 12.Siskind V, Aitken J, Green A, Martin N. Sun exposure and interaction with family history in risk of melanoma, Queensland, Australia. Int J Cancer. 2002;97(1):90–5. doi: 10.1002/ijc.1563. [DOI] [PubMed] [Google Scholar]
- 13.Beitner H, Norell SE, Ringborg U, Wennersten G, Mattson B. Malignant melanoma: aetiological importance of individual pigmentation and sun exposure. Br J Dermatol. 1990;122(1):43–51. doi: 10.1111/j.1365-2133.1990.tb08238.x. [DOI] [PubMed] [Google Scholar]
- 14.Holman CD, Armstrong BK, Heenan PJ. Relationship of cutaneous malignant melanoma to individual sunlight-exposure habits. J Natl Cancer Inst. 1986;76(3):403–14. [PubMed] [Google Scholar]
- 15.Holman CD, Armstrong BK, Heenan PJ, Blackwell JB, Cumming FJ, English DR, et al. The causes of malignant melanoma: results from the West Australian Lions Melanoma Research Project. Recent Results Cancer Res. 1986;102:18–37. doi: 10.1007/978-3-642-82641-2_3. [DOI] [PubMed] [Google Scholar]
- 16.Westerdahl J, Ingvar C, Masback A, Olsson H. Sunscreen use and malignant melanoma. Int J Cancer. 2000;87(1):145–50. doi: 10.1002/1097-0215(20000701)87:1<145::aid-ijc22>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Grob JJ, Gouvernet J, Aymar D, Mostaque A, Romano MH, Collet AM, et al. Count of benign melanocytic nevi as a major indicator of risk for nonfamilial nodular and superficial spreading melanoma. Cancer. 1990;66(2):387–95. doi: 10.1002/1097-0142(19900715)66:2<387::aid-cncr2820660232>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Zaridze D, Mukeria A, Duffy S. Risk factors for skin melanoma in Moscow. Int J Cancer. 1992;52(1):159–61. doi: 10.1002/ijc.2910520128. [DOI] [PubMed] [Google Scholar]
- 19.Zanetti R, Franceschi S, Rosso S, Colonna S, Bidoli E. Cutaneous melanoma and sunburns in childhood in a southern European population. Eur J Cancer. 1992;28A(6–7):1172–6. doi: 10.1016/0959-8049(92)90480-p. [DOI] [PubMed] [Google Scholar]
- 20.Rosso S, Zanetti R, Pippione M, Sancho-Garnier H. Parallel risk assessment of melanoma and basal cell carcinoma: skin characteristics and sun exposure. Melanoma Res. 1998;8(6):573–83. doi: 10.1097/00008390-199812000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Pfahlberg A, Kolmel KF, Gefeller O. Timing of excessive ultraviolet radiation and melanoma: epidemiology does not support the existence of a critical period of high susceptibility to solar ultraviolet radiation- induced melanoma. Br J Dermatol. 2001;144(3):471–5. doi: 10.1046/j.1365-2133.2001.04070.x. [DOI] [PubMed] [Google Scholar]
- 22.Kolmel KF, Pfahlberg A, Mastrangelo G, Niin M, Botev IN, Seebacher C, et al. Infections and melanoma risk: results of a multicentre EORTC case-control study. European Organization for Research and Treatment of Cancer. Melanoma Res. 1999;9(5):511–9. [PubMed] [Google Scholar]
- 23.Ruiz Lascano A, Kuznitzky R, Cuestas E, Mainardi C, Albertini R, Borello A, et al. Risk factors for cutaneous melanoma: case-control study in Cordoba, Argentina. Medicina (B Aires) 2004;64(6):504–8. [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med. 1995;14(24):2685–99. doi: 10.1002/sim.4780142408. [DOI] [PubMed] [Google Scholar]
- 28.Green A, Siskind V, Bain C, Alexander J. Sunburn and malignant melanoma. Br J Cancer. 1985;51(3):393–7. doi: 10.1038/bjc.1985.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. II. Importance of UV-light exposure. Int J Cancer. 1988;42(3):319–24. doi: 10.1002/ijc.2910420303. [DOI] [PubMed] [Google Scholar]
- 30.Autier P, Dore JF, Lejeune F, Koelmel KF, Geffeler O, Hille P, et al. Recreational exposure to sunlight and lack of information as risk factors for cutaneous malignant melanoma. Results of an European Organization for Research and Treatment of Cancer (EORTC) case-control study in Belgium, France and Germany. The EORTC Malignant Melanoma Cooperative Group. Melanoma Res. 1994;4(2):79–85. doi: 10.1097/00008390-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Autier P, Dore JF. Influence of sun exposures during childhood and during adulthood on melanoma risk. EPIMEL and EORTC Melanoma Cooperative Group. European Organisation for Research and Treatment of Cancer. Int J Cancer. 1998;77(4):533–7. doi: 10.1002/(sici)1097-0215(19980812)77:4<533::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Carli P, Massi D, Santucci M, Biggeri A, Giannotti B. Cutaneous melanoma histologically associated with a nevus and melanoma de novo have a different profile of risk: results from a case-control study. J Am Acad Dermatol. 1999;40(4):549–57. doi: 10.1016/s0190-9622(99)70436-6. [DOI] [PubMed] [Google Scholar]
- 33.Rodenas JM, Delgado-Rodriguez M, Herranz MT, Tercedor J, Serrano S. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case-control study in a Mediterranean population. Cancer Causes Control. 1996;7(2):275–83. doi: 10.1007/BF00051303. [DOI] [PubMed] [Google Scholar]
- 34.Westerdahl J, Olsson H, Ingvar C. At what age do sunburn episodes play a crucial role for the development of malignant melanoma. Eur J Cancer. 1994;30A(11):1647–54. doi: 10.1016/0959-8049(94)00337-5. [DOI] [PubMed] [Google Scholar]
- 35.Sorahan T, Grimley RP. The aetiological significance of sunlight and fluorescent lighting in malignant melanoma: a case-control study. Br J Cancer. 1985;52(5):765–9. doi: 10.1038/bjc.1985.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holly EA, Aston DA, Cress RD, Ahn DK, Kristiansen JJk. Cutaneous melanoma in women. I. Exposure to sunlight, ability to tan, and other risk factors related to ultraviolet light. Am J Epidemiol. 1995;141(10):923–33. doi: 10.1093/oxfordjournals.aje.a117359. [DOI] [PubMed] [Google Scholar]
- 37.Le Marchand L, Saltzman BS, Hankin JH, Wilkens LR, Franke AA, Morris SJ, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164(3):232–45. doi: 10.1093/aje/kwj115. [DOI] [PubMed] [Google Scholar]
- 38.Shen H, Liu Z, Strom SS, Spitz MR, Lee JE, Gershenwald JE, et al. p53 codon 72 Arg homozygotes are associated with an increased risk of cutaneous melanoma. J Invest Dermatol. 2003;121(6):1510–4. doi: 10.1046/j.1523-1747.2003.12648.x. [DOI] [PubMed] [Google Scholar]
- 39.Wei Q, Lee JE, Gershenwald JE, Ross MI, Mansfield PF, Strom SS, et al. Repair of UV light-induced DNA damage and risk of cutaneous malignant melanoma. J Natl Cancer Inst. 2003;95(4):308–15. doi: 10.1093/jnci/95.4.308. [DOI] [PubMed] [Google Scholar]
- 40.Shors AR, Solomon C, McTiernan A, White E. Melanoma risk in relation to height, weight, and exercise (United States) Cancer Causes & Control. 2001;12(7):599–606. doi: 10.1023/a:1011211615524. [DOI] [PubMed] [Google Scholar]
- 41.Bakos L, Wagner M, Bakos RM, Leite CS, Sperhacke CL, Dzekaniak KS, et al. Sunburn, sunscreens, and phenotypes: some risk factors for cutaneous melanoma in southern Brazil. Int J Dermatol. 2002;41(9):557–62. doi: 10.1046/j.1365-4362.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 42.Garbe C, Weiss J, Kruger S, Garbe E, Buttner P, Bertz J, et al. The German melanoma registry and environmental risk factors implied. Recent Results in Cancer Research. 1993;128:69–89. doi: 10.1007/978-3-642-84881-0_6. [DOI] [PubMed] [Google Scholar]
- 43.Dunn-Lane J, Herity B, Moriarty MJ, Conroy R. A case control study of malignant melanoma. Ir Med J. 1993;86(2):57–9. [PubMed] [Google Scholar]
- 44.Landi MT, Baccarelli A, Calista D, Pesatori A, Fears T, Tucker MA, et al. Combined risk factors for melanoma in a Mediterranean population. British Journal of Cancer. 2001;85(9):1304–10. doi: 10.1054/bjoc.2001.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bataille V, Winnett A, Sasieni P, Newton Bishop JA, Cuzick J. Exposure to the sun and sunbeds and the risk of cutaneous melanoma in the UK: a case-control study. Eur J Cancer. 2004;40(3):429–35. doi: 10.1016/j.ejca.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 46.MacKie RM, Freudenberger T, Aitchison TC. Personal risk-factor chart for cutaneous melanoma. Lancet. 1989;2(8661):487–90. doi: 10.1016/s0140-6736(89)92097-7. [DOI] [PubMed] [Google Scholar]
- 47.Holly EA, Kelly JW, Shpall SN, Chiu SH. Number of melanocytic nevi as a major risk factor for malignant melanoma. J Am Acad Dermatol. 1987;17(3):459–68. doi: 10.1016/s0190-9622(87)70230-8. [DOI] [PubMed] [Google Scholar]
- 48.Beane Freeman LE. dissertation. Univ. of Iowa; Iowa City (IA): 2003. Arsenic exposure, artificial tanning and melanoma in Iowa. [Google Scholar]
- 49.Lea CS, Holly EA, Hartge P, Lee JS, Guerry Dt, Elder DE, et al. Reproductive risk factors for cutaneous melanoma in women: a case-control study. Am J Epidemiol. 2007;165(5):505–13. doi: 10.1093/aje/kwk040. [DOI] [PubMed] [Google Scholar]
- 50.Fortes C, Mastroeni S, Melchi F, Pilla MA, Alotto M, Antonelli G, et al. The association between residential pesticide use and cutaneous melanoma. Eur J Cancer. 2007;43(6):1066–75. doi: 10.1016/j.ejca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Loria D, Matos E. Risk factors for cutaneous melanoma: a case-control study in Argentina. Int J Dermatol. 2001;40(2):108–14. doi: 10.1046/j.1365-4362.2001.01132.x. [DOI] [PubMed] [Google Scholar]
- 52.Wolf P, Quehenberger F, Mullegger R, Stranz B, Kerl H. Phenotypic markers, sunlight-related factors and sunscreen use in patients with cutaneous melanoma: an Austrian case-control study. Melanoma Res. 1998;8(4):370–8. doi: 10.1097/00008390-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Nijsten T, Leys C, Verbruggen K, Verlinden V, Drieghe J, Stas M, et al. Case-control study to identify melanoma risk factors in the Belgian population: the significance of clinical examination. J Eur Acad Dermatol Venereol. 2005;19(3):332–9. doi: 10.1111/j.1468-3083.2005.01196.x. [DOI] [PubMed] [Google Scholar]
- 54.de Vries E, Boniol M, Severi G, Eggermont AM, Autier P, Bataille V, et al. Public awareness about risk factors could pose problems for case-control studies: the example of sunbed use and cutaneous melanoma. Eur J Cancer. 2005;41(14):2150–4. doi: 10.1016/j.ejca.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 55.Walter SD, King WD, Marrett LD. Association of cutaneous malignant melanoma with intermittent exposure to ultraviolet radiation: results of a case-control study in Ontario, Canada. International Journal of Epidemiology. 1999;28(3):418–27. doi: 10.1093/ije/28.3.418. [DOI] [PubMed] [Google Scholar]
- 56.Elwood JM, Gallagher RP, Davison J, Hill GB. Sunburn, suntan and the risk of cutaneous malignant melanoma--The Western Canada Melanoma Study. Br J Cancer. 1985;51(4):543–9. doi: 10.1038/bjc.1985.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Breitbart M, Garbe C, Buttner P, Weiss J, Soyer HP, Stocker U, et al. Ultraviolet light exposure, pigmentary traits and the development of melanocytic naevi and cutaneous melanoma. A case-control study of the German Central Malignant Melanoma Registry. Acta Derm Venereol. 1997;77(5):374–8. doi: 10.2340/0001555577374378. [DOI] [PubMed] [Google Scholar]
- 58.Kaskel P, Sander S, Kron M, Kind P, Peter RU, Krahn G. Outdoor activities in childhood: a protective factor for cutaneous melanoma? Results of a case-control study in 271 matched pairs. British Journal of Dermatology. 2001;145(4):602–9. doi: 10.1046/j.1365-2133.2001.04432.x. [DOI] [PubMed] [Google Scholar]
- 59.Lasithiotakis K, Kruger-Krasagakis S, Ioannidou D, Pediaditis I, Tosca A. Epidemiological differences for cutaneous melanoma in a relatively dark-skinned Caucasian population with chronic sun exposure. Eur J Cancer. 2004;40(16):2502–7. doi: 10.1016/j.ejca.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 60.Naldi L, Altieri A, Imberti GL, Gallus S, Bosetti C, La Vecchia C. Sun exposure, phenotypic characteristics, and cutaneous malignant melanoma. An analysis according to different clinico-pathological variants and anatomic locations (Italy) Cancer Causes Control. 2005;16(8):893–9. doi: 10.1007/s10552-005-2300-4. [DOI] [PubMed] [Google Scholar]
- 61.Fargnoli MC, Piccolo D, Altobelli E, Formicone F, Chimenti S, Peris K. Constitutional and environmental risk factors for cutaneous melanoma in an Italian population. A case-control study. Melanoma Res. 2004;14(2):151–7. doi: 10.1097/00008390-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 62.Carli P, Biggeri A, Giannotti B. Malignant melanoma in Italy: risks associated with common and clinically atypical melanocytic nevi. J Am Acad Dermatol. 1995;32(5 Pt 1):734–9. doi: 10.1016/0190-9622(95)91451-x. [DOI] [PubMed] [Google Scholar]
- 63.Zanetti R, Rosso S, Martinez C, Nieto A, Miranda A, Mercier M, et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case-case-control study. Br J Cancer. 2006;94(5):743–51. doi: 10.1038/sj.bjc.6602982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cristofolini M, Franceschi S, Tasin L, Zumiani G, Piscioli F, Talamini R, et al. Risk factors for cutaneous malignant melanoma in a northern Italian population. Int J Cancer. 1987;39(2):150–4. doi: 10.1002/ijc.2910390205. [DOI] [PubMed] [Google Scholar]
- 65.Nelemans PJ, Groenendal H, Kiemeney LA, Rampen FH, Ruiter DJ, Verbeek AL. Effect of intermittent exposure to sunlight on melanoma risk among indoor workers and sun-sensitive individuals. Environ Health Perspect. 1993;101(3):252–5. doi: 10.1289/ehp.93101252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kennedy C, Bajdik CD, Willemze R, De Gruijl FR, Bouwes Bavinck JN. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120(6):1087–93. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- 67.Dabkowski J, Omulecki A, Zalewska A. Identification of melanoma risk factors in the Polish population. Dermatol Surg. 1997;23(11):1039–42. doi: 10.1111/j.1524-4725.1997.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 68.Westerdahl J, Olsson H, Masback A, Ingvar C, Jonsson N, Brandt L, et al. Use of sunbeds or sunlamps and malignant melanoma in southern Sweden. Am J Epidemiol. 1994;140(8):691–9. doi: 10.1093/oxfordjournals.aje.a117317. [DOI] [PubMed] [Google Scholar]
- 69.Elwood JM, Whitehead SM, Davison J, Stewart M, Galt M. Malignant melanoma in England: risks associated with naevi, freckles, social class, hair colour, and sunburn. Int J Epidemiol. 1990;19(4):801–10. doi: 10.1093/ije/19.4.801. [DOI] [PubMed] [Google Scholar]
- 70.Elwood JM, Williamson C, Stapleton PJ. Malignant melanoma in relation to moles, pigmentation, and exposure to fluorescent and other lighting sources. Br J Cancer. 1986;53(1):65–74. doi: 10.1038/bjc.1986.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.MacKie RM, Aitchison T. Severe sunburn and subsequent risk of primary cutaneous malignant melanoma in scotland. Br J Cancer. 1982;46(6):955–60. doi: 10.1038/bjc.1982.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly JW, Holly EA, Shpall SN, Ahn DK. The distribution of melanocytic naevi in melanoma patients and control subjects. Australas J Dermatol. 1989;30(1):1–8. doi: 10.1111/j.1440-0960.1989.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 73.Cress RD, Holly EA, Ahn DK. Cutaneous melanoma in women. V. Characteristics of those who tan and those who burn when exposed to summer sun. Epidemiology. 1995;6(5):538–43. doi: 10.1097/00001648-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 74.Moore DH, 2nd, Patterson HW, Hatch F, Discher D, Schneider JS, Bennett D, et al. Case-control study of malignant melanoma among employees of the Lawrence Livermore National Laboratory. Am J Ind Med. 1997;32(4):377–91. doi: 10.1002/(sici)1097-0274(199710)32:4<377::aid-ajim9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 75.Berwick M, Begg CB, Fine JA, Roush GC, Barnhill RL. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88(1):17–23. doi: 10.1093/jnci/88.1.17. [DOI] [PubMed] [Google Scholar]
- 76.Lew RA, Sober AJ, Cook N, Marvell R, Fitzpatrick TB. Sun exposure habits in patients with cutaneous melanoma: a case control study. J Dermatol Surg Oncol. 1983;9(12):981–6. doi: 10.1111/j.1524-4725.1983.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 77.Dubin N, Pasternack BS, Moseson M. Simultaneous assessment of risk factors for malignant melanoma and non-melanoma skin lesions, with emphasis on sun exposure and related variables. Int J Epidemiol. 1990;19(4):811–9. doi: 10.1093/ije/19.4.811. [DOI] [PubMed] [Google Scholar]
- 78.Millen AE, Tucker MA, Hartge P, Halpern A, Elder DE, Guerry Dt, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13(6):1042–51. [PubMed] [Google Scholar]
- 79.Fernandez L, Milne R, Bravo J, Lopez J, Aviles J, Longo M, et al. MC1R: three novel variants identified in a malignant melanoma association study in the Spanish population. Carcinogenesis. 2007;28(8):1659–64. doi: 10.1093/carcin/bgm084. [DOI] [PubMed] [Google Scholar]
- 80.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 81.Breslow NE, Day NE. Statistical methods in cancer research: volume 1 - analusis of case-control studies. Lyon: International Agency for Research on Cancer; 1980. p. v. [PubMed] [Google Scholar]
- 82.Beane Freeman LE, Dennis LK, Lynch CF, Lowe JB, Clarke WR. Test-retest of self-reported exposure to artificial tanning devices, self-tanning creams, and sun sensitivity showed consistency. J Clin Epidemiol. 2005;58(4):430–2. doi: 10.1016/j.jclinepi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Branstrom R, Kristjansson S, Ullen H, Brandberg Y. Stability of questionnaire items measuring behaviours, attitudes and stages of change related to sun exposure. Melanoma Res. 2002;12(5):513–9. doi: 10.1097/00008390-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 84.Glanz K, Schoenfeld E, Weinstock MA, Layi G, Kidd J, Shigaki DM. Development and reliability of a brief skin cancer risk assessment tool. Cancer Detect Prev. 2003;27(4):311–5. doi: 10.1016/s0361-090x(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 85.van der Mei IA, Blizzard L, Ponsonby AL, Dwyer T. Validity and reliability of adult recall of past sun exposure in a case-control study of multiple sclerosis. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1538–44. doi: 10.1158/1055-9965.EPI-05-0969. [DOI] [PubMed] [Google Scholar]
- 86.Dubin N, Moseson M, Pasternack BS. Sun exposure and malignant melanoma among susceptible individuals. Environ Health Perspect. 1989;81:139–51. doi: 10.1289/ehp.8981139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han J, Colditz GA, Hunter DJ. Risk factors for skin cancers: a nested case-control study within the Nurses’ Health Study. Int J Epidemiol. 2006;35(6):1514–21. doi: 10.1093/ije/dyl197. [DOI] [PubMed] [Google Scholar]
- 88.Graham S, Marshall J, Haughey B, Stoll H, Zielezny M, Brasure J, et al. An inquiry into the epidemiology of melanoma. Am J Epidemiol. 1985;122(4):606–19. doi: 10.1093/oxfordjournals.aje.a114140. [DOI] [PubMed] [Google Scholar]
- 89.Dubin N, Moseson M, Pasternack BS. Epidemiology of malignant melanoma: pigmentary traits, ultraviolet radiation, and the identification of high-risk populations. Recent Results Cancer Res. 1986;102:56–75. doi: 10.1007/978-3-642-82641-2_5. [DOI] [PubMed] [Google Scholar]
- 90.Nelemans PJ, Rampen FH, Ruiter DJ, Verbeek AL. An addition to the controversy on sunlight exposure and melanoma risk: a meta-analytical approach. J Clin Epidemiol. 1995;48(11):1331–42. doi: 10.1016/0895-4356(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 91.Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73(2):198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 92.Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12(1):69–82. doi: 10.1023/a:1008980919928. [DOI] [PubMed] [Google Scholar]
- 93.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41(1):45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]