Abstract
In this 2-part review, I will focus on emerging virus infections of the central nervous system (CNS). Part 1 will introduce the basic features of emerging infections, including their definition, epidemiology, and the frequency of CNS involvement. Important mechanisms of emergence will be reviewed, including viruses spreading into new host ranges as exemplified by West Nile virus (WNV), Japanese encephalitis (JE) virus, Toscana virus, and enterovirus 71 (EV71). Emerging infections also result from opportunistic spread of viruses into known niches, often resulting from attenuated host resistance to infection. This process is exemplified by transplant-associated cases of viral CNS infection caused by WNV, rabies virus, lymphocytic choriomeningitis, and lymphocytic choriomeningitis–like viruses and by the syndrome of human herpesvirus 6 (HHV6)–associated posttransplantation acute limbic encephalitis. The second part of this review begins with a discussion of JC virus and the occurrence of progressive multifocal leukoencephalopathy in association with novel immunomodulatory therapies and then continues with an overview of the risk of infection introduced by imported animals (eg, monkeypox virus) and examples of emerging diseases caused by enhanced competence of viruses for vectors and the spread of vectors (eg, chikungunya virus) and then concludes with examples of novel viruses causing CNS infection as exemplified by Nipah and Hendra viruses and bat lyssaviruses.
Emerging viral infections can be defined as diseases that have infected new hosts, have spread into new geographic ranges, have altered characteristics of their pathogenesis, or are caused by agents not previously recognized as pathogenic.1 The number of emerging infectious diseases (EIDs) and the magnitude of their threat to global health is increasing.2 In a review of risk factors for human disease emergence, Taylor and colleagues3 identified 1415 species of infectious organisms known to be pathogenic to humans. Of this group, 175 organisms were considered to be emerging, and by far the largest taxonomic group of emerging pathogens were viruses and prions, which accounted for 77 (44%) of the total.3 It appears that among taxonomic divisions of microorganisms, the highest relative risk for emergence is among viruses.
Of the EID viruses identified by Taylor et al,3 approximately 80% had a primary nonhuman animal source (“zoonotic”). Among pathogens at large, it has been suggested that the majority of EID events (60%) are also caused by zoonotic pathogens.2 This strongly suggests that factors that enhance contact between humans and animals, particularly wild animals, are significant risk factors for EIDs generally and viral EIDs particularly. Similarly, approximately 40% of the viral zoo-notic EIDs are vectorborne,2,3 suggesting that increased human exposure to mosquitoes and other common arthropod vectors is another major factor in disease emergence. Climate change and climatic anomalies, including global warming, are likely key drivers in expanding vector ranges and thereby enhancing opportunities for vector-human contact.4 In addition to these pathogen-related factors, a number of host factors are likely to play a key role in EIDs. One of the most frequently cited is the increased susceptibility of humans to infection, resulting in substantial part from the human immunodeficiency virus/AIDS pandemic and to a lesser degree, from immunosuppression resulting from cancer chemotherapy, antirejection regimens in transplant recipients, and drugs used to treat autoimmune and immune-mediated disorders.
Emerging infections are often thought of as occurring predominantly in exotic geographical areas. A recent analysis contradicts this view and instead suggests that the highest concentration of EID events per million kilometers squared occurs between 30° and 60° north and 30° and 40° south latitude, encompassing the United States, Europe, Japan, and southeast Asia.2 However, a predictive model designed to identify “hotspots” where new EIDs may occur suggests that EIDs due to zoonotic and vectorborne pathogens are likely to occur in lower-latitude developing countries, especially in tropical Africa, Latin America, and Asia.2
Olival and Daszak1 recently tried to quantify the proportion of viral EIDs that involved the nervous system. Their analysis suggested that 39% of viral EIDs commonly produced severe neurological symptoms, including encephalitis, and that an additional 10% rarely or occasionally did so.1 This review highlights this series of emerging CNS viral infections.
POLIOMYELITIS
Although attention is naturally focused on the emergence of “new” viruses and their contribution to disease, it is equally important to recognize that viruses can also disappear as causes of human disease (“submergence”), either as a result of human interventions, such as vaccination (eg, polio, measles, mumps, rabies, varicella), or independently (eg, western equine encephalitis virus [WEEV]). Polio provides a striking example of the submergence of a human neurotropic viral infection due to human efforts. The last outbreak of poliomyelitis caused by wild virus (type 1) in the United States occurred in 1979 and involved 13 cases of paralytic disease in unvaccinated members of Amish communities in 3 US states and in Ontario, Canada. Polio has been the target of a massive worldwide eradication campaign.5 In 2007, for example, more than 400 million children were immunized against polio. Despite concerted efforts to fully eradicate the disease, several pockets of active wild-type poliovirus infection remain. In 2007, 1315 cases of poliomyelitis were reported worldwide, the majority occurring in India (66%) and Nigeria (22%), with smaller numbers elsewhere in Africa (Congo, Chad, Niger, Somalia, Angola, Sudan, Cameroon) and in Pakistan, Afghanistan, Myanmar, and Nepal (Figure 1).Unfortunately, the number of cases of polio reported worldwide in 2008 increased to 1652, with Nigeria (48.5%) and India (34%) again accounting for the bulk of cases, but with disease also occurring in 16 additional countries in Africa and Asia (Pakistan, Nepal, Afghanistan).5 This is still a dramatic reduction for a disease that during its peak in the early 1950s was responsible for 35 cases of paralysis per 100 000 population in the United States alone. However, even in areas where polio has been eradicated, imported cases in travelers from endemic areas may still appear, and lack of familiarity with the disease may delay diagnosis.6
Figure 1.
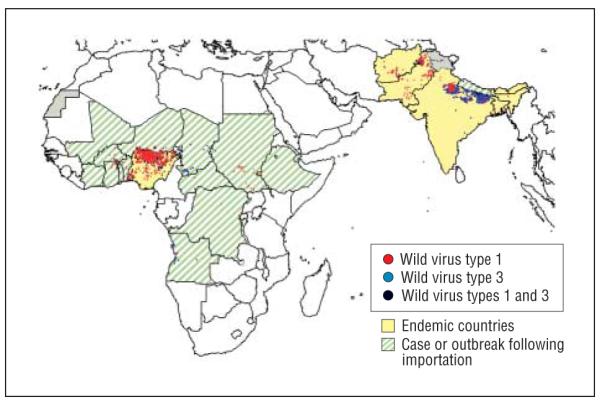
Distribution of wild-type poliomyelitis cases worldwide 2008. Source: World Health Organization.5
Unfortunately, there has been an unexpected down-side to the use of the Sabin live attenuated trivalent oral polio vaccine as the primary tool for polio eradication. Viruses circulating in nature that were originally derived from Sabin polio strains are shed in stool by vaccinated hosts (“vaccine-derived circulating polioviruses”) and may undergo mutations that allow reversion to virulence and can also recombine with other circulating nonpolio enteroviruses. Based on studies suggesting that the degree of divergence of the gene encoding the viral capsid protein VP1 can be used as a “molecular clock” to estimate how long viruses have been circulating, it is clear that these vaccinederived poliovirus strains can continue to circulate for years after oral vaccination has ceased.
The first polio outbreak attributed to circulating vaccinederived poliovirus occurred in 2000 in Hispaniola.7 Subsequent outbreaks occurred in the Philippines, Indonesia, Cambodia, China, Egypt, and Madagascar.8,9 Even though the use of oral polio vaccine ended in the United States in 2000, a small outbreak of polio infection resulting from vaccinederived poliovirus occurred in 2005, resulting in infections, but fortunately no paralytic disease, in 4 unvaccinated Amish children in Minnesota.10 The vaccine-derived type 1 poliovirus responsible had likely been circulating in the Amish community for several months before the index case was identified and contained neurovirulent genetic determinants, although it was not known to have caused paralytic disease. The largest vaccine-derived poliovirus outbreak, involving 46 confirmed cases of paralytic disease, occurred on the Indonesian island of Madura in 2005.8 The patients were children with a mean age of 2 years (range, 0.5-14 years) who presented with typical polio, including fever (95%) and asymmetrical paralysis (46%). Paralysis persisted at least 60 days in about two-thirds of cases.8
In an outbreak in China, it was found that the vaccinederived polioviruses causing paralytic disease had undergone recombination with human enterovirus C (HEV-C) species. In the 2005 Madagascar outbreak, there were 5 reported cases of type 2 vaccine-derived paralytic poliomyelitis. Careful studies of circulating vaccine-derived polioviruses also identified viruses that had recombined with HEV-C. The genomes were often complex mosaic structures of poliovirus and HEV-C with regions of HEV-C genome occurring at the 3′- and 5′- noncoding ends of the genome, in the P2 region encoding the 2A, 2B, and 2C nonstructural proteins, and in the P3 region encoding the 3D nonstructural protein.9
Although the submergence of poliovirus is clearly the result of concerted human efforts aimed at virus eradication, the submergence of WEEV remains mysterious. Western equine encephalitis virus is a mosquito-borne alphavirus that was initially identified in 1930 and first described as a cause of human encephalitis in 1938. Following a peak period in the 1950s, WEEV has declined dramatically in importance as a human pathogen. In the 20-year period from 1964 to 1985, there were only 587 Centers for Disease Control and Prevention–confirmed human cases of western encephalitis in the United States.11 There have only been 5 confirmed human cases of western encephalitis in the United States in the last 20 years (Centers for Disease Control and Prevention) and none in nearly a decade since the last reported case in 1999 in Minnesota.11 Western equine encephalitis virus circulates enzootically between birds and mosquitoes. However, the submergence of WEEV as a human pathogen does not appear to be due to changes in the capacity of contemporary viral isolates to infect their principal mosquito vector (Culex tarsalis) or to produce viremia and disease in birds or mice.12,13 It remains possible that the drop in disease incidence simply reflects less human exposure to virus or changes in virulence specific for humans. Western equine encephalitis virus continues to circulate in the United States and, like dormant volcanoes, submergent viruses have the potential to re-erupt. For example, after California encephalitis virus was first identified as a cause of human disease in 1952, it was not found to be responsible for another human case of encephalitis in California for half a century14 (although related members of the California encephalitis serogroup, such as La Crosse virus, continue to be important causes of encephalitis).15
VIRUSES SPREADING INTO NEW HOST RANGES
West Nile Virus
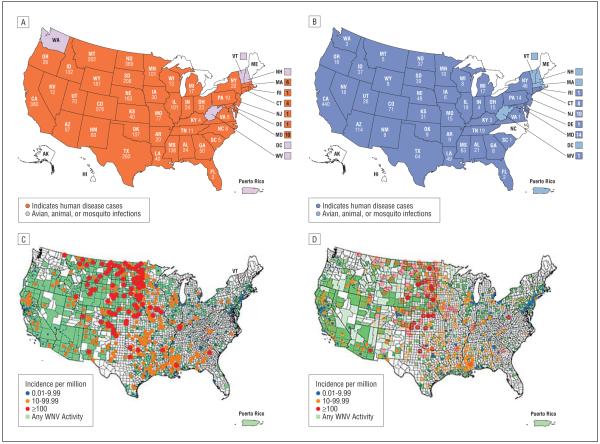
Perhaps the most dramatic recent example in the United States of a virus spreading into a new host range is WNV (Figure 2). The virus was initially isolated in the West Nile province of Uganda in 1937. The first cases of naturally occurring WNV encephalitis in the western hemisphere occurred in the New York City, New York, area in 1999.16 The number of cases in 1999 to 2001 was small and they were confined to the East Coast. In 2002 and 2003, WNV disease exploded, expanding westward in the United States and producing nearly 3000 confirmed cases of neuroinvasive disease (encephalitis, meningitis, acute flaccid paralysis) in each of these years.17 These represented the largest epidemics of arboviral meningoencephalitis in US history and the largest epidemics of WNV neuroinvasive disease reported to date.18
Figure 2.
Number of cases of West Nile virus (WNV) infection reported to the Centers for Disease Control and Prevention (CDC) in 2007 (A) and 2008 (B) and incidence per million population in 2007 (C) and 2008 (D). Reproduced with permission from the CDC.
The mechanism by which WNV initially spread to North America remains unknown. Sequence analysis of the initial New York isolates (NY1999) indicated the virus was closely related to a virus isolated from the brain of a goose in Israel in 1998.19-21 Closely related viruses were also circulating in Rumania and Eastern Europe at the time. It has been suggested that individuals arriving in New York City via airplane from Tel Aviv, Israel, in which a WNV outbreak was ongoing, introduced the virus into the United States.18,22 This seems unlikely, as the magnitude of WNV viremia in humans is not likely to have been sufficient to allow mosquito infection.
Culex species of mosquitoes are the predominant vectors of WNV, and birds serve as the principle vertebrate host during natural cycles of infection. It has been suggested that the rapid spread of virus across the United States was facilitated by migratory birds with disease spreading along bird flyways,23 although more recent models suggest dissemination by spread of resident rather than migratory bird species is a more likely explanation.24 Migratory birds, however, could conceivably have been responsible for WNV entry into the United States. The East Atlantic migratory bird flyway, for example, connects the East Coast of the United States with Europe and the west coast of Africa.
Unlike humans, many species of birds develop prolonged high-titer WNV viremia following infection.25 Following experimental inoculation of WNV, some Passeriforme species develop viremias in excess of 1011 plaque-forming units (PFU)/mL of serum that can persist for a week or longer.25 This is nearly 10 billion–fold higher than median viral loads of approximately 5×103 copies/mL (approximately 1.25×101 PFU/mL) reported in viremic human blood donors.26,27 The degree of host viremia also influences the likelihood of mosquito infection. For example, a recent study in Fox squirrels suggested that more than 56% of biting mosquitoes were infected when feeding on squirrels with viremia of 104.4 PFU/mL or greater.28 Taken together with the data cited on levels of human viremia, this would seem to make it unlikely that an infected person served as the initial source of WNV infection and perhaps more plausible that introduction resulted from an infected bird that subsequently was bitten by a susceptible mosquito, or even by an infected mosquito that traveled to North America from a WNV endemic area in a boat, plane, or other conveyance.
The basic epidemiology, clinical features, diagnosis, and treatment of WNV CNS infection have been reviewed elsewhere and are only briefly reviewed here.29-34 The majority of WNV infections are asymptomatic. Approximately 20% of infected individuals develop an acute febrile flulike illness (West Nile fever) and only approximately 1 in 150 infected individuals develop neuroinvasive disease. The major categories of neuroinvasive disease include meningitis, encephalitis, and acute flaccid paralysis/poliomyelitis, although there is often substantial overlap between these syndromes. Patients who develop encephalitis are typically substantially older (mean age, 60 years) than those with meningitis (mean age, 46 years) or acute flaccid paralysis (mean age, 56 years). Additional risk factors for development of encephalitis in addition to increasing age include immunosuppression, hypertension, diabetes mellitus, and liver disease.35,36 Host genetic factors also contribute to risk of developing severe neuroinvasive disease, including single-nucleotide polymorphisms in oligoadenylate synthetase L37 and deletions in the chemokine receptor CCR5.38 The former is likely involved with the interferon-induced host antiviral response, whereas the later influences lymphocyte trafficking into the CNS.
West Nile virus meningitis is clinically similar to other forms of “aseptic” meningitis and is characterized by fever, headache, nuchal rigidity, and photophobia. Cranial nerve palsies, particularly of the facial nerve, occur in about 20% of cases. The cerebrospinal fluid (CSF) shows a pleocytosis with a mean of about 200 cells/mm3 (median, approximately 100 cells/mm3), an elevated protein level in 70% (mean, 76 mg/dL; to convert to grams per liter, multiply by 10), and a normal glucose level.39 Almost half the patients will have an initial polymorphonuclear pleocytosis rather than the lymphocytic pleocytosis generally characteristic of viral meningitis. Neuroimaging study findings are usually normal.
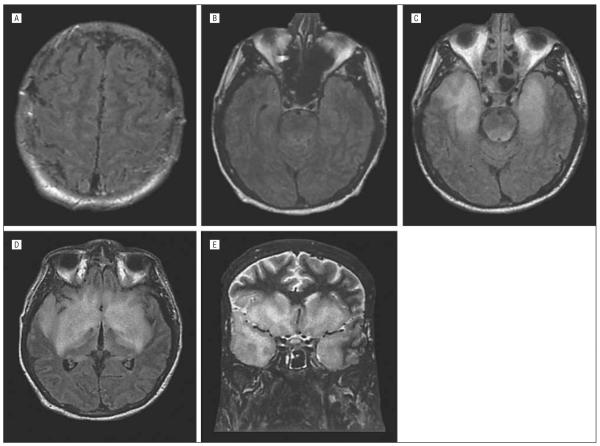
Patients with encephalitis have clinical or laboratory evidence of involvement of the brain parenchyma. Signs may include fever, headache, mental status changes, and movement disorders, including myoclonus, tremor, parkinsonism, ataxia, and weakness. The CSF findings are similar to those seen in meningitis.39 Magnetic resonance imaging (MRI) is more sensitive than computed tomography, with abnormalities found in about half the patients.40,41 An additional 25% may have abnormalities seen only on diffusion-weighted imaging sequences. When present, abnormalities typically consist of areas of increased signal on T2 and fluid-attenuated inversion recovery (FLAIR) sequences that occur in the thalamus, basal ganglia, and upper brainstem (Figure 3).
Figure 3.
Axial proton density magnetic resonance image (left) and coronal fluid-attenuated inversion recovery magnetic resonance image of patients with West Nile virus encephalitis showing increased signal in the upper brainstem, thalamus, and basal ganglia (A) and substantia nigra of the midbrain (B).
Acute flaccid paralysis is a poliomyelitislike illness that occurs in perhaps 5% to 10% of patients with neuroinvasive disease.42,43 More than half the affected patients have associated encephalitis with concomitant findings of parkinsonism, myoclonus, or tremor. Approximately 90% have associated fever and headache. In 1 large series, 44% had respiratory impairment requiring intubation. The CSF findings resemble those seen in meningoencephalitis. Magnetic resonance imaging may show increased signal on T2 sequences in the anterior horns of the spinal cord. Electromyogram/nerve conduction velocity studies show reduced or absent compound muscle action potentials with relatively preserved sensory neural action potentials. Electromyogram abnormalities develop after several weeks and include evidence of denervation (fasciculations, increased insertional activity, positive sharp waves).
Diagnosis of WNV disease is typically based on serologic testing.44,45 Serum IgG antibodies likely persist for life and are indicative of past exposure. IgM antibodies develop acutely and are still present at 3 months postinfection in almost all patients and at 6 months postinfection in 60% to 70%.45 By 18 months postinfection, 20% of cases still have detectable serum IgM antibodies. Detection of CSF WNV IgM antibodies is diagnostic of neuroinvasive WNV disease, as the large size of IgM molecules means they only cross the blood-brain barrier poorly and, as a result, their presence in CSF is generally indicative of intrathecal synthesis. Reverse transcriptase–polymerase chain reaction (RT-PCR) of CSF is highly specific but relatively insensitive in diagnosis of neuroinvasive disease.
There is no known specific treatment of proven benefit for WNV infection. A randomized placebocontrolled clinical trial of an Israeli intravenous immunoglobulin preparation (Omr-IgG-am) has recently been completed, but the results are not yet available (ClinicalTrials.gov Identifier: NCT00068055). A phase 1 clinical trial of a humanized monoclonal antibody directed against an epitope on the envelope glycoprotein has been completed (ClinicalTrials.gov Identifier: NCT00515385) and this antibody is now being evaluated in phase 2/3 trials. There is also an ongoing trial of the use of interferon alfa, although this agent was not found to be effective in a placebo-controlled randomized trial in JE.46 Several “chimeric” vaccines in which the genes encoding the WNV PrM and E proteins are inserted into the backbone of other attenuated related viruses, such as the vaccine strain of yellow fever virus or dengue, have successfully undergone phase 1 clinical trials and are now being evaluated in phase 2 trials.
Toscana Virus
In Europe, an emergence nearly as dramatic as that of WNV in the United States involved Toscana virus. Toscana virus is an arbovirus belonging to the family Bunyaviridae that is transmitted by the bite of sandflies (Phlebotomus perniciosus and Phlebotomus perfiliewi). The virus was first isolated in 1971 from a sandfly in central Italy. The sandfly may be both the vector and the reservoir, with virus persisting and spreading in sandflies through both venereal and transovarial transmission.47 No bird or small mammal reservoir is known. The virus appears to have a tropism for the CNS, and Toscana virus is now the most common cause of summertime (May-October) viral meningitis in central Italy, with a frequency eclipsing that of enteroviruses.47 The virus has spread within Europe to Spain, Portugal, and France and cases have been reported in several other European countries in travelers returning from endemic areas.48 Most infections are mild or self-limited febrile illnesses. Symptoms of meningitis include headache (100%), meningeal signs (50%-95%), fever (75%-95%), and nausea and/or vomiting (65%-90%).47 Tremors, myalgia, and weakness can also occur. Glasgow Coma Scale score was normal in about 70%, with no patient having a score less than 12 in 1 small case series.48 Cerebrospinal fluid shows a lymphocytic (60%-90%) pleocytosis (30-900 cells/mm3), elevated protein level (67-183 mg/dL), and normal glucose level.48 In patients with meningitis, neuroimaging results are typically normal, although electroencephalogram abnormalities have been found in about 60%.48 There are rare reports of severe encephalitis and meningoencephalitis.49,50 Diagnosis is typically made by detection of IgM antibodies in serum or CSF or by RT-PCR amplification of viral RNA. Most infected individuals recover fully.
JE Virus
Japanese encephalitis remains the single most important cause of acute viral encephalitis on a worldwide basis, accounting for 30 000 to 50 000 cases and 10 000 to 15 000 deaths annually.51 Transmission occurs primarily via Culex tritaeniorrhyncus and Culex vishnui mosquitoes, with water birds, including herons and egrets, serving as natural reservoirs and domestic pigs also serving as an important amplifying host.51,52 Infected humans are dead-end hosts as the level of viremia and its generally short duration make mosquito infection unlikely. After JE was first identified in Japan in the 1870s, it spread gradually but progressively to the Korean peninsula (1933), the Chinese mainland (1940), the Philippines (1950), Singapore and Malaysia (1952), India (1955), and Southeast Asia (Cambodia, Thailand) (1964-1965). More disturbing has been the recent extension within the Indian subcontinent (Bangladesh, 1977; Pakistan, 1983) and the recent reports of cases of JE from Papua New Guinea and the Torres Strait in Northern Australia (1995)52(Figure 4).
Figure 4.
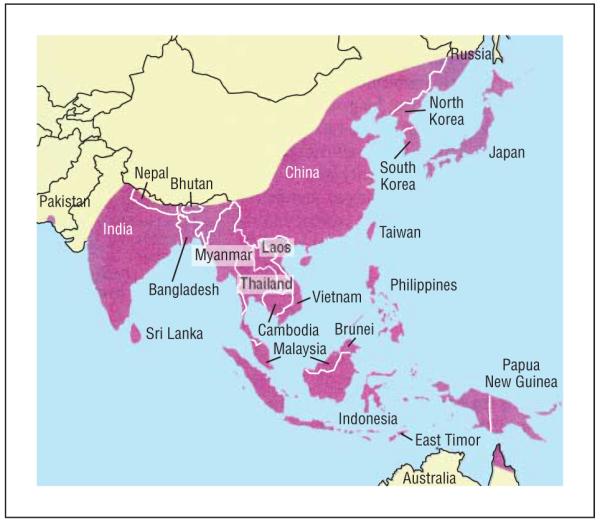
Approximate geographic distribution of Japanese encephalitis virus. Source: copyright 2006, Massachusetts Medical Society51; all rights reserved.
The expansion of JE host range has been fueled by dramatic population growth in endemic areas; the increased exposure of humans to vectors and amplifying hosts, such as pigs because of occupational pig rearing; and the expansion in area and intensity of rice farming.52 It has been estimated that nearly half the world’s population currently lives in countries where JE occurs.52
The largest recent major outbreak of JE occurred in the Utter Pradesh province of India in 2005 affecting more than 5000 people and causing 1300 deaths.51 In a recent study from Malaysia, the acute mortality was 8%, with 57% of survivors having moderate or severe neurological sequelae.53 Common clinical features include fever (>95%), altered consciousness (about 80%), seizures(50%-80%), headache (about 70%), and focal limb weakness (about 25%).53 No effective therapy for JE virus exists, with neither ribavirin54 nor interferon alfa46 having shown efficacy in treatment. An effective live attenuated vaccine (SA 14-14-2) has been available for several decades, and 2 new candidate vaccines are in advanced clinical development55; however, economic issues will likely significantly affect the ability to disseminate these vaccines into underdeveloped areas.
Enterovirus 71
Enterovirus 71 (EV71), like JE virus, represents another emerging pathogen whose geographic range has steadily expanded. The virus was first isolated in 1969 and is a member of the Enterovirus genus of the family Picornaviridae. Clinical illness occurs predominantly in children, usually taking the form of hand-foot-and-mouth disease (HFMD). Since 1997, there have been numerous outbreaks of EV71 disease in the Southeast Asia and Pacific region (Malaysia, Taiwan, Singapore, Japan, Vietnam, Australia), resulting in hundreds of thousands of cases.56 The incidence of neurological sequelae and the form of neurological disease has varied considerably across outbreaks for reasons that remain poorly understood. Common neurological manifestations include aseptic meningitis, encephalitis, and poliomyelitislike acute flaccid paralysis, but there are also reports of EV71-associated cerebellitis, brainstem encephalitis, opsoclonus-myoclonus syndrome, Guillain-Barre syndrome, and transverse myelitis.57 A recent review56 found an approximately 30% incidence of CNS disease among patients with positive EV71 isolates during an HFMD epidemic in Sarawak, Malaysia. Within this group, 52% of those with CNS disease had evidence of HFMD (rash on palm or soles, mouth ulcers/vesicles or herpangina) and 48% had aseptic meningitis without HFMD. Patients with aseptic meningitis typically had a CSF pleocytosis (mean, 75 cells/mm3), with lymphocytic predominance (78%) and a normal glucose level (90%). Common signs and symptoms in those with HFMD and CNS disease included fever (98%), mouth ulcers (88%), reduced consciousness (58%), and irritability (40%) and cough, coryza, and vomiting (37%42%). Diagnosis usually depends on virus isolation (throat, ulcer/vesicle, or rectal swabs; serum, CSF) or demonstration of seroconversion. Enterovirus CSF polymerase chain reaction (PCR) using “group-specific” primers may fail to reliably detect EV71.58
The explanation for the variations in the incidence and type of neurological disease associated with EV71 are unknown.59 Like other RNA viruses, EV71 has an extremely high spontaneous mutation rate and new EV71 viruses are constantly emerging. Phylogenetic studies based on sequence analysis of the viral VP1 gene sequence have identified 3 genotypes (A, B, and C) and numerous subtypes (C1-3, B1-5). It has been suggested that viruses belonging to some genotypes are less neurovirulent. For example, in the series of cases from Sarawak, there was a 15% incidence of CNS disease in patients infected with the B4 genotype as compared with about 28% in those infected with other genotypes.56 Additional factors influencing incidence and severity of CNS disease may include coinfection with other viruses60 and host immunity.61
NEW NICHES FOR OLD AGENTS
The immunosuppressant state created by antirejection therapy in patients receiving solid organ and bone marrow transplants has opened new niches for infections caused by a variety of neurotropic viruses, including rabies, lymphocytic choriomeningitis virus (LCMV), HHV6, and JC virus.
There are more than 28 000 solid organ transplants performed annually in the United States using organs derived from nearly 15 000 individual donors. The most commonly transplanted organs include kidneys (about 17 000), liver (about 6000), heart (about 2000), lung (about 1400), and pancreas (about 400). Transplants are performed at more than 250 sites nationally with organs from a single donor typically going to multiple recipients at geographically dispersed sites.62 Recognition of infections, including those involving the CNS, is more difficult in transplant recipients because of a variety of confounding factors, including immunosuppression-related attenuation in the signs and symptoms of infection, allograft rejection, and toxic effects from antirejection therapy.63 When infection does occur, it may be donor derived, recipient derived, nosocomial, or community acquired. Donors are routinely screened for human immunodeficiency virus, syphilis, herpesviruses (herpes simplex virus, varicella zoster virus, Epstein-Barr virus, cytomegalovirus), and hepatitis viruses (hepatitis B virus, hepatitis C virus)63 but not routinely for other microbial pathogens. Clusters of cases of CNS disease in transplant recipients have resulted from infection with WNV,64 rabies virus,65,66 and LCMV/LCMV-like viruses.67,68
West Nile Virus
In the WNV transplant-associated cases, 4 individuals received 2 kidneys, a liver, and a heart from a 20-year-old, previously healthy organ donor who died of trauma.64 Pretransplant serum from the donor was negative for WNV nucleic acid by RT-PCR and for WNV IgM antibodies, although her posttransfusion serum was culture and RT-PCR positive for WNV. As part of an unsuccessful resuscitation effort, the donor received 53 U of blood components, including 31 U of packed red blood cells, 17 U of fresh frozen plasma, and 5 U of platelets as well as a 10-U pool of antihemophilic factor. It subsequently was discovered that 1 of the transfused plasma units contained detectable WNV RNA by PCR. The donor of this unit had developed symptoms 2 to 3 weeks before donation consistent with WNV infection but was asymptomatic at the time of blood donation. This donor was tested postdonation and found to have WNV IgM antibodies. Three of the 4 organ recipients (those receiving the kidneys and heart) developed WNV encephalitis, with 1 dying of this disease and another dying of an unrelated cause. In these patients, symptoms began 10 to 17 days after they received transplanted organs. The fourth recipient (liver) developed WNV fever 7 days after transplant. Diagnosis of infection in the organ recipients was based on positive CSF and serum serologic test results (cases 1 and 3) and brain detection of antigen and viral nucleic acid at autopsy in a patient with negative serologic test results (case 2).
Rabies
In 2004, 4 patients died of rabies after receiving kidney, liver, and iliac artery segment transplants from a donor who died of encephalitis of unknown cause.65,66 In retrospect, it was discovered that a bat had bitten the organ donor, and his serum was later shown to have IgG and IgM antirabies virus antibodies at the time of his death. Prior to death, he had complained of nausea, vomiting, and dysphagia and then developed a progressive neurological disease characterized by altered mental status and seizures. His diagnosis was complicated by the presence of both active substance abuse (cocaine, marijuana) and detection of a subarachnoid hemorrhage on cranial computed tomography. The organ recipients developed rabies within a month of transplant. In all cases, the clinical disease was characteristic and included signs and symptoms of altered mental status, agitated delirium, seizures, respiratory failure, and CSF pleocytosis (mean, 18 cells/mm3) and MRI abnormalities, including increased signal on T2 and FLAIR sequences involving the hippocampi, brainstem, temporal lobes, and basal ganglia (Figure 5).
Figure 5.
Axial fluid-attenuated inversion recovery magnetic resonance images at day 22 (A and B) and day 28 (C and D) in a patient with posttransplant rabies encephalitis. Day 22 images show subtle leptomeningeal and pontomesencephalic junction increased signal. Day 28 images show increased signal in anteromedial temporal lobes including hippocampi. Coronal fast spin-echo image at day 28 (E) shows diffuse anterior temporal and anterior and inferior frontal increased signal. Source: copyright 2005, American Medical Association65; all rights reserved.
At autopsy, all patients showed Negri bodies (intracytoplasmic viral inclusions in neurons) and had tissue that stained positive for rabies antigen. One patient had rhabdovirus particles seen by electron microscopy. Three of 4 patients were rabies virus antibody positive at the time of their death, which occurred 7 to 23 days (mean, 13 days) after onset of neurological symptoms.
Lymphocytic Choriomeningitis Virus
The first 2 clusters of transplant-associated transmission of LCMV occurred in 2003 and 2005.67 A subsequent report described an additional cluster of cases attributed to an arenavirus closely related to LCMV.68 In all instances, the donor did not have clinical evidence suggestive of LCMV infection, and in only 1 of 3 clusters was there even epidemiological evidence of exposure to an infected rodent. The neurological components of the illnesses in the recipients are summarized briefly later; more details on the nonneurological components of their illness can be found in the original reports.
In the 2003 cluster, 4 recipients of kidneys, liver, and a lung developed fatal illnesses and died 9 to 76 days posttransplant. The donor was a 51-year-old man who had died of a subdural hematoma. Neurological signs and symptoms were prominent in the 2 kidney recipients but minimal or absent in the other 2 recipients. In the first kidney transplant recipient, neurological symptoms began 40 days posttransplant and included seizures, polymyoclonus, and chorioretinitis leading to death 13 days later. A lumbar puncture showed a markedly elevated protein level (720 mg/dL) with a normal cell count and MRI showed bilateral subdural fluid collections and dural thickening but no intraparenchymal abnormalities. The CSF culture was positive for LCMV, and viral antigen was detected in leptomeningeal and brain tissue at biopsy. Seroconversion did not occur. Case 2, a second kidney recipient, developed altered mental status, seizures, and myoclonus 31 days posttransplant. Death occurred on day 76 and was preceded by further deterioration in mental status with worsening meningeal signs (photophobia and nuchal rigidity). The CSF revealed a mild lymphocytic pleocytosis (12 cells/mm3) and markedly elevated protein level (620 mg/dL). The MRI showed bilateral subdural fluid collections and dural enhancement. The CSF cultures were positive for LCMV, and the patient developed LCMV-specific IgM antibodies. In the second cluster of cases in 2005, a 45-year-old woman who died of complications of a stroke donated kidneys, liver, and a lung. The lung recipient developed headache, fever, and seizures and ultimately died 26 days posttransplant. Epidemiological investigation uncovered the fact that a pet hamster had been introduced into the donor’s household 3 weeks before her death. The hamster was subsequently found to have been LCMV infected. By contrast, no history of rodent exposure was identified in the donor in the 2003 cluster. Regardless of the epidemiology, neither donor had clinical or laboratory evidence of acute LCMV infection.
The most recent cluster of transplant-associated infection with an LCMV-like arenavirus was reported in 2008.68 Diagnosis required the application of sophisticated high-throughput molecular biological techniques. A 57-year-old man died of cerebral hemorrhage and served as a liver and kidney donor. All 3 organ recipients developed a febrile illness with encephalopathy leading to death 29 to 36 days after transplant. Viral RNA was amplified from CSF and brain tissue of the first kidney recipient. Diagnosis in the other cases was based on seroconversion (1) and amplification of viral RNA from multiple tissues or serum. Although there was no clear history of rodent exposure or LCMV-like illness in the donor, he was subsequently found to have both IgG and IgM antibodies against LCMV.
In the cases of transplant-associated illness due to WNV, rabies, and LCMV described here, the transplanted organs served as the source of infection in the recipients. A far more common scenario is for the transplant recipients to be infected by viruses in the environment or latent in their own tissues. Antirejection therapy not only depresses the likelihood of graft rejection but also depresses the ability of the host to control reactivation of some latent viruses. Several recent articles have described transplant-associated cases of limbic encephalitis due to HHV6 and an increased risk of development of progressive multifocal leukoencephalopathy, a disease associated with reactivation of JC virus (see part 2 of this review).
Human Herpevirus 6
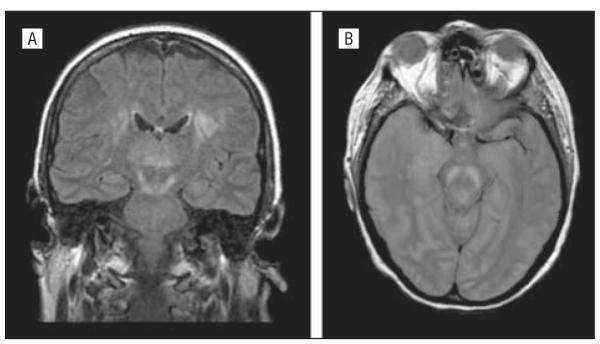
Human herpesvirus 6 is a member of the β-herpesvirus subfamily of Herpesviridae, a group also including cytomegalovirus. Two variants with 65% to 95% nucleotide sequence identity occur (HHV6A and HHV6B). In addition to causing the childhood disease exanthema subitum (roseola infantum) and being associated with febrile seizures (HHV6B), these viruses can cause encephalitis in immunocompetent children and adults.69-71 In 1 recent study based on a subset of cases of encephalitis of unknown etiology referred to the California Encephalitis Project for diagnostic evaluation, cell-free HHV6 DNA was amplified by PCR from 14 of 35 CSF specimens (40%). Human herpesvirus 6–specific IgG was detected in 37% and IgM in 28% of CSF specimens examined, although the results were not always concordant with DNA amplification. Prior studies have suggested that HHV6 accounted for about 7% of cases of acute focal encephalitis in immunocompetent adults.69 The higher prevalence of HHV6 as a cause of encephalitis in the California Encephalitis Project study may reflect the patient referral bias intrinsic to this project, with physicians failing to refer cases of encephalitis in which diagnosis can be easily established. Additional studies will be needed before the importance of HHV6 as a cause of encephalitis in immunocompetent adults can be more precisely established. Fatal HHV6B encephalitis in a hematopoietic stem cell transplant recipient was first described in 1994,72 and several series have now been reported in both children73 and adults.74 The virus appears to be the major cause of the syndrome of “posttransplant acute limbic encephalitis.”74 A series of 9 cases from a total of 584 hematopoietic stem cell transplants performed during a 3½-year period at the Dana Farber Cancer Center is consistent with a cumulative incidence of about 1.5% for posttransplant acute limbic encephalitis among hematopoietic stem cell transplant recipients.74 The mean age of patients was 41 years (range, 22-60 years); surprisingly, all were male, and all experienced graft-vs-host disease either before (5 cases) or after (4 cases) limbic encephalitis. Neurological symptoms began a month after transplant (range, 14-61 days). The core clinical features included the acute onset (range, 1-3 days) of dense anterograde and patchy retrograde amnesia often preceded by a confusional state and clinically evident seizures. Eight of the 9 patients had hyponatremia due to syndrome of inappropriate antidiuretic hormone secretion.
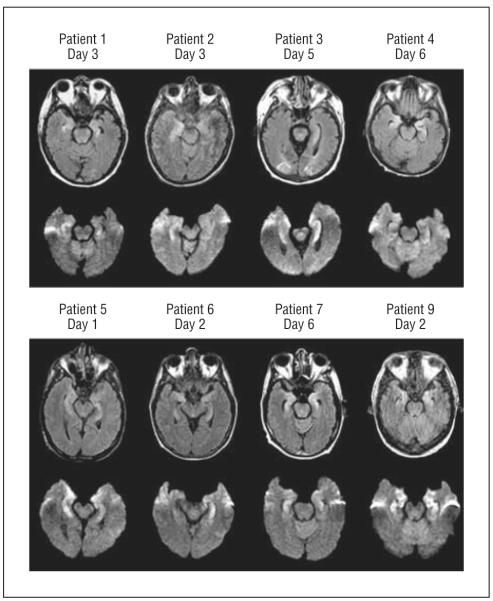
Magnetic resonance imaging showed bilateral focal medial temporal abnormalities in all patients that were hyperintense on T2 and FLAIR sequences and had restricted diffusion on diffusion-weighted images (Figure 6). Areas involved typically included the uncus, amygdala, hippocampal body, entorhinal cortex, and subiculum, with sparing of the parahippocampal gyrus.74,75 Seven of 9 examined patients had focal electroencephalogram abnormalities in temporal and frontotemporal areas, epileptiform activity, and periodic lateralized epileptiform discharges (3 cases). The CSF showed a mild lymphocytic pleocytosis (1-41 cells/ mm3) and a mildly elevated protein level (median, 48 mg/dL). Seven of 9 patients had at least 1 CSF specimen from which HHV6 DNA was amplified by PCR. The efficacy of antiviral treatment for HHV6 CNS infections including posttransplant acute limbic encephalitis is unclear, although recent guidelines recommend use of ganciclovir or foscarnet sodium either singly or in combination in immunocompromised patients with HHV6 encephalitis.76
Figure 6.
Axial fluid-attenuated inversion recovery (top rows) and diffusion-weighted (bottom rows) sequences in patients with human herpesvirus 6–associated posttransplant acute limbic encephalitis showing abnormal increased signal in the limbic system, including the uncus, amygdala, and anterior hippocampus in all patients. Reproduced with permission from Wolters Kluwer Health.74
Acknowledgments
Funding/Support: Dr Tyler is supported by grants from the National Institutes of Health National Institute of Neurological Disorders and Stroke and the Department of Veterans Affairs and by the Reuler-Lewin Family Professorship in Neurology.
Footnotes
Financial Disclosure: Dr Tyler has received compensation as an expert consultant to MacroGenics, a company developing a humanized monoclonal antibody for treatment of WNV infection, and from Genentech, Biogen Idec, and Sanofi Pasteur for expert consulting related to JC virus infections and progressive multifocal leukoencephalopathy.
Previous Presentation: This article was based in part on a plenary lecture presented at the 132nd Annual Meeting of the American Neurological Association; October 8, 2007; Washington, DC.
Additional Information: This article is the first of a 2-part series. The second part will appear next month.
REFERENCES
- 1.Olival KJ, Daszak P. The ecology of emerging neurotropic viruses. J Neurovirol. 2005;11(5):441–446. doi: 10.1080/13550280591002450. [DOI] [PubMed] [Google Scholar]
- 2.Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature. 2005;438(7066):310–317. doi: 10.1038/nature04188. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed May 1, 2009];Global Polio Eradication Initiative Web site. Wild poliovirus weekly update. http://www.polioeradication.org/casecount.asp.
- 6.Stewardson AJ, Roberts JA, Beckett CL, et al. Imported case of poliomyelitis, Melbourne, Australia, 2007. Emerg Infect Dis. 2009;15(1):63–65. doi: 10.3201/eid1501.080791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kew O, Morris-Glasgow V, Landaverde M, et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296(5566):356–359. doi: 10.1126/science.1068284. [DOI] [PubMed] [Google Scholar]
- 8.Estívariz CF, Watkins MA, Handoko D, et al. A large vaccine-derived poliovirus out-break on Madura Island-Indonesia, 2005. J Infect Dis. 2008;197(3):347–354. doi: 10.1086/525049. [DOI] [PubMed] [Google Scholar]
- 9.Rakoto-Andrianarivelo M, Gumede N, Jegouic S, et al. Reemergence of recombinant vaccine-derived poliovirus outbreak in Madagascar. J Infect Dis. 2008;197(10):1427–1435. doi: 10.1086/587694. [DOI] [PubMed] [Google Scholar]
- 10.Alexander JP, Ehresmann K, Seward J, et al. Vaccine-Derived Poliovirus Investigations Group. Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J Infect Dis. 2009;199(3):391–397. doi: 10.1086/596052. [DOI] [PubMed] [Google Scholar]
- 11. [Accessed February 1, 2009];CDC Web site. Arboviral encephalitis cases reported in humans, by type, United States, 1964-2007. http://www.cdc.gov/ncidod/dvbid/arbor/arbocase.htm.
- 12.Reisen WK, Fang Y, Brault AC. Limited interdecadal variation in mosquito (Diptera: Culicidae) and avian host competence for Western equine encephalomyelitis virus (Togaviridae: Alphavirus) Am J Trop Med Hyg. 2008;78(4):681–686. [PubMed] [Google Scholar]
- 13.Forrester NL, Kenney JL, Deardorff E, Wang E, Weaver SC. Western equine encephalitis submergence: lack of evidence for a decline in viral virulence. Virology. 2008;380(2):170–172. doi: 10.1016/j.virol.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eldridge BF, Glaser C, Pedrin RE, Chiles RE. The first reported case of California encephalitis in more than 50 years. Emerg Infect Dis. 2001;7(3):451–452. doi: 10.3201/eid0703.010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McJunkin JE, de los Reyes EC, Irazuzta JE, et al. La Crosse encephalitis in children. N Engl J Med. 2001;344(11):801–807. doi: 10.1056/NEJM200103153441103. [DOI] [PubMed] [Google Scholar]
- 16.Nash D, Mostashari F, Fine A, et al. 1999 West Nile Outbreak Response Working Group. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344(24):1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 17. [Accessed February 1, 2009];CDC Web site. West Nile virus. http://www.cdc.gov/ncidod/dvbid/westnile/index.htm.
- 18.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Emerg Infect Dis. 2007;45(8):1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 19.Lanciotti RS, Roehrig JT, Deubel V, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286(5448):2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 20.Lanciotti RS, Ebel GD, Deubel V, et al. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002;298(1):96–105. doi: 10.1006/viro.2002.1449. [DOI] [PubMed] [Google Scholar]
- 21.Jia X-Y, Briese T, Jordan I, et al. Genetic analysis of West Nile New York 1999 encephalitis virus. Lancet. 1999;354(9194):1971–1972. doi: 10.1016/s0140-6736(99)05384-2. [DOI] [PubMed] [Google Scholar]
- 22.Giladi M, Metzkor-Cotter E, Martin DA, et al. West Nile encephalitis in Israel in 1999, the New York connection. Emerg Infect Dis. 2001;7(4):659–661. doi: 10.3201/eid0704.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson AT, Vieglais DA, Andreasen JK. Migratory birds modeled as critical transport agents for West Nile virus in North America. Vector Borne Zoonotic Dis. 2003;3(1):27–37. doi: 10.1089/153036603765627433. [DOI] [PubMed] [Google Scholar]
- 24.Rappole JH, Compton BW, Leimgruber P, Robertson J, King DI, Renner SC. Modeling movement of West Nile virus in the Western hemisphere. Vector Borne Zoonotic Dis. 2006;6(2):128–139. doi: 10.1089/vbz.2006.6.128. [DOI] [PubMed] [Google Scholar]
- 25.Komar N, Langevin S, Hinten S, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9(3):311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stramer SL, Fang CT, Foster GA, Wagner AG, Brodsky JP, Dodd RY. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353(5):451–459. doi: 10.1056/NEJMoa044333. [DOI] [PubMed] [Google Scholar]
- 27.Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis. 2008;198(7):984–993. doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]
- 28.Platt KB, Tucker BJ, Halbur PG, et al. Fox squirrels (Sciurus niger) develop West Nile virus viremias sufficient for infecting select mosquito species. Vector Borne Zoonotic Dis. 2008;8(2):225–233. doi: 10.1089/vbz.2007.0182. [DOI] [PubMed] [Google Scholar]
- 29.Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11(8):1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis LE, DeBiasi RL, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60(3):286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 31.Debiasi RL, Tyler KL. West Nile virus meningoencephalitis. Nat Clin Pract Neurol. 2006;2(5):264–275. doi: 10.1038/ncpneuro0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sejvar JJ, Marfin AA. Manifestations of West Nile neuroinvasive disease. Rev Med Virol. 2006;16(4):209–224. doi: 10.1002/rmv.501. [DOI] [PubMed] [Google Scholar]
- 33.Kramer LD, Li J, Shi PY. West Nile virus. Lancet Neurol. 2007;6(2):171–181. doi: 10.1016/S1474-4422(07)70030-3. [DOI] [PubMed] [Google Scholar]
- 34.Beckham D, Tyler KL. Clinical manifestations of neurological disease. In: Diamond MS, editor. West Nile Encephalitis Virus Infection. Springer; New York NY: 2009. pp. 69–95. [Google Scholar]
- 35.Bode AV, Sejvar JJ, Pape WJ, Campbell GL, Marfin AA. West Nile virus disease: a descriptive study of 228 patients hospitalized in a 4-county region of Colorado in 2003. Clin Infect Dis. 2006;42(9):1234–1240. doi: 10.1086/503038. [DOI] [PubMed] [Google Scholar]
- 36.Murray K, Baraniuk S, Resnick M, et al. Risk factors of encephalitis and death from West Nile virus infection. Epidemiol Infect. 2006;134(6):1325–1332. doi: 10.1017/S0950268806006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yakub I, Lillibridge KM, Moran A, et al. Single nucleotide polymorphisms in genes for 2′-5′-oligoadenylate synthetase and RNase L inpatients hospitalized with West Nile virus infection. J Infect Dis. 2005;192(10):1741–1748. doi: 10.1086/497340. [DOI] [PubMed] [Google Scholar]
- 38.Glass WG, McDermott DH, Lim JK, et al. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203(1):35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyler KL, Pape J, Goody RJ, Corkill M, Kleinschmidt-DeMasters BK. CSF findings in 250 patients with serologically confirmed West Nile virus meningitis and encephalitis. Neurology. 2006;66(3):361–365. doi: 10.1212/01.wnl.0000195890.70898.1f. [DOI] [PubMed] [Google Scholar]
- 40.Ali M, Safriel Y, Sohi J, Llave A, Weathers S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26(2):289–297. [PMC free article] [PubMed] [Google Scholar]
- 41.Petropoulou KA, Gordon SM, Prayson RA, Ruggierri M. West Nile virus meningoencephalitis: MR imaging findings. AJNR Am J Neuroradiol. 2005;26(8):1986–1995. [PMC free article] [PubMed] [Google Scholar]
- 42.Sejvar JJ, Bode AV, Marfin AA, et al. West Nile virus-associated flaccid paralysis. Emerg Infect Dis. 2005;11(7):1021–1027. doi: 10.3201/eid1107.040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sejvar JJ, Bode AV, Marfin AA, et al. West Nile Virus-associated flaccid paralysis outcome. Emerg Infect Dis. 2006;12(3):514–516. doi: 10.3201/eid1203.050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tardei G, Ruta S, Chitu V, Rossi C, Tsai TF, Cernescu C. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection. J Clin Microbiol. 2000;38(6):2232–2239. doi: 10.1128/jcm.38.6.2232-2239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roehrig JT, Nash D, Maldin B, et al. Persistence of virus-reactive serum immunoglobulin m antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis. 2003;9(3):376–379. doi: 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon T, Dung NM, Wills B, et al. Interferon alfa-2a in Japanese encephalitis: a randomised double-blind placebo-controlled trial. Lancet. 2003;361(9360):821–826. doi: 10.1016/s0140-6736(03)12709-2. [DOI] [PubMed] [Google Scholar]
- 47.Charrel RN, Gallian P, Navarro-Mari J-M, et al. Emergence of Toscana virus in Europe. Emerg Infect Dis. 2005;11(11):1657–1663. doi: 10.3201/eid1111.050869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Nicuolo G, Pagliano P, Battisti S, et al. Toscana virus central nervous system infections in southern Italy. J Clin Microbiol. 2005;43(12):6186–6188. doi: 10.1128/JCM.43.12.6186-6188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dionisio D, Valassina M, Ciufolini MG, et al. Encephalitis without meningitis due to sandfly fever virus serotype Toscana. Clin Infect Dis. 2001;32(8):1241–1243. doi: 10.1086/319759. [DOI] [PubMed] [Google Scholar]
- 50.Baldelli F, Ciufolini MG, Francisci D, et al. Unusual presentation of life threatening Toscana virus meningoencephalitis. Clin Infect Dis. 2004;38(4):515–520. doi: 10.1086/381201. [DOI] [PubMed] [Google Scholar]
- 51.Solomon T. Control of Japanese encephalitis—within our grasp? N Engl J Med. 2006;355(9):869–871. doi: 10.1056/NEJMp058263. [DOI] [PubMed] [Google Scholar]
- 52.Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present and future of Japanese encephalitis. Emerg Infect Dis. 2009;15(1):1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ooi MH, Lewthwaite P, Lai BF, et al. The epidemiology, clinical features, and long term prognosis of Japanese encephalitis in central Sarawak, Malaysia 1997-2005. Clin Infect Dis. 2008;47(4):458–468. doi: 10.1086/590008. [DOI] [PubMed] [Google Scholar]
- 54.Kumar R, Tripathi P, Baranwal M, Singh S, Tripathi S, Banerjee G. Randomized, controlled trial of oral ribavirin for Japanese encephalitis in children in Uttar Pradesh, India. Clin Infect Dis. doi: 10.1086/596309. [published online ahead of print January 14, 2009] doi: 10.1086/596309. [DOI] [PubMed] [Google Scholar]
- 55.Tauber E, Dewasthaly S. Japanese encephalitis vaccines—needs, flaws and achievements. Biol Chem. 2008;389(5):547–550. doi: 10.1515/bc.2008.062. [DOI] [PubMed] [Google Scholar]
- 56.Ooi MH, Wong SC, Podin Y, et al. Human enterovirus 71 disease in Sarawak, Malaysia: a prospective clinical, virological, and molecular epidemiological study. Clin Infect Dis. 2007;44(5):646–656. doi: 10.1086/511073. [DOI] [PubMed] [Google Scholar]
- 57.McMinn P, Stratov I, Nagarajan L, Davis S. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin Infect Dis. 2001;32(2):236–242. doi: 10.1086/318454. [DOI] [PubMed] [Google Scholar]
- 58.Pérez-Vélez CM, Anderson MS, Robinson CC, et al. Outbreak of neurologic entero virus type 71 disease: a diagnostic challenge. Clin Infect Dis. 2007;45(8):950–957. doi: 10.1086/521895. [DOI] [PubMed] [Google Scholar]
- 59.Dolin R. Enterovirus 71: emerging infections and emerging questions. N Engl J Med. 1999;341(13):984–985. doi: 10.1056/NEJM199909233411309. [DOI] [PubMed] [Google Scholar]
- 60.Cardosa MJ, Krishnan S, Tio PH, Perera D, Wong SC. Isolation of subgenus B adenovirus during a fatal outbreak of enterovirus 71-associated hand, foot, and mouth disease in Sibu, Sarawak. Lancet. 1999;354(9183):987–991. doi: 10.1016/S0140-6736(98)11032-2. [DOI] [PubMed] [Google Scholar]
- 61.Yang KD, Yang MY, Li CC, et al. Altered cellular but not humoral reactions in children with complicated enteroviral 71 infections in Taiwan. J Infect Dis. 2001;183(6):850–856. doi: 10.1086/319255. [DOI] [PubMed] [Google Scholar]
- 62.2007 Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1997-2006. Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: 2007. [Google Scholar]
- 63.Fishman JA. Infection in solid organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 64.Iwamoto M, Jernigan DB, Guasch A, et al. West Nile Virus in Transplant Recipients Investigation Team. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348(22):2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 65.Burton EC, Burns DK, Opatowsky MJ, et al. Rabies encephalomyelitis: clinical, neuroradiological, and pathological findings in 4 transplant recipients. Arch Neurol. 2005;62(6):873–882. doi: 10.1001/archneur.62.6.873. [DOI] [PubMed] [Google Scholar]
- 66.Srinivasan A, Burton EC, Kuehnert MJ, et al. Rabies in Transplant Recipients In vestigation Team. Transmission of rabies virus from an organ donor to four trans plant recipients. N Engl J Med. 2005;352(11):1103–1111. doi: 10.1056/NEJMoa043018. [DOI] [PubMed] [Google Scholar]
- 67.Fischer SA, Graham MB, Kuehnert MJ, et al. LCMV in Transplant Recipients In vestigation Team. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;354(21):2235–2249. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- 68.Palacios G, Druce J, Du L, et al. A new arenavirus in a cluster of fatal transplant associated diseases. N Engl J Med. 2008;358(10):991–998. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- 69.McCullers JA, Lakeman FD, Whitley RJ. Human herpesvirus 6 is associated with focal encephalitis. Clin Infect Dis. 1995;21(3):571–576. doi: 10.1093/clinids/21.3.571. [DOI] [PubMed] [Google Scholar]
- 70.Isaacson E, Glaser CA, Forghani B, et al. Evidence of human herpesvirus 6 infection in 4 immunocompetent patients with encephalitis. Clin Infect Dis. 2005;40(6):890–893. doi: 10.1086/427944. [DOI] [PubMed] [Google Scholar]
- 71.Yao K, Honarmand S, Espinosa A, Akhyani N, Glaser C, Jacobson S. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol. 2009;65(3):257–267. doi: 10.1002/ana.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drobyski WR, Knox KK, Majewski D, Carrigan DR. Brief report: fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N Engl J Med. 1994;330(19):1356–1360. doi: 10.1056/NEJM199405123301905. [DOI] [PubMed] [Google Scholar]
- 73.Wainwright MS, Martin PL, Morse RP, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001;50(5):612–619. doi: 10.1002/ana.1251. [DOI] [PubMed] [Google Scholar]
- 74.Seeley WW, Marty FM, Holmes TM, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007;69(2):156–165. doi: 10.1212/01.wnl.0000265591.10200.d7. [DOI] [PubMed] [Google Scholar]
- 75.Provenzale JM, vanLandingham KE, Lewis DV, Mukundan S, Jr, White LE. Extrahippocampal involvement in human herpesvirus 6 encephalitis depicted at MR imaging. Radiology. 2008;249(3):955–963. doi: 10.1148/radiol.2492071917. [DOI] [PubMed] [Google Scholar]
- 76.Tunkel AR, Glaser CA, Bloch KC, et al. Infectious Diseases Society of America. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47(3):303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]