Abstract
Background
The objective of this study was to examine the epidemiology of syphilis among high-risk socially marginalized populations in urban, coastal Peru, to quantify the prevalence of recent syphilis infection and identify risk factors.
Methods
Survey data and serologic specimens were collected from a population-based sample of 3 populations: men who have sex with only men (MSOM), socially marginalized heterosexual men, and socially marginalized women. Syphilis prevalence was determined for each population, and multivariate analysis was used to analyze risk factors for recent syphilis infection among the MSOM and among the socially marginalized men.
Results
The prevalence of recent syphilis infection was 10.5% among the MSOM, 1.5% among the socially marginalized men, and 2.0% among the women. Among both MSOM and the socially marginalized men, recent syphilis infection was significantly associated with Herpes simplex virus Type 2 infection (prevalence ratio = 1.96; 95% confidence interval, 1.03–3.74, and PR = 3.72; 95% CI, 2.12–6.53, respectively). Recent syphilis infection was also significantly associated with HIV infection among the socially marginalized men (PR = 11.13; 95% CI, 4.50 –27.51) and with the number of sexually active years among the MSOM (PR = 1.05, 95% CI, 1.01–1.10).
Conclusions
All 3 groups included in this study exhibited a high prevalence of recent syphilis infection, with recent infection being most prevalent among the MSOM. These findings demonstrate the need for more effective syphilis control services among those populations, to decrease syphilis-associated morbidity, transmission of syphilis, and the potential transmission of HIV.
Syphilis is one of several ulcerative sexually transmitted infections that increase the risk of acquisition and transmission of the human immunodeficiency virus (HIV).1,2 In populations with a high seroprevalence of HIV, a key strategy for preventing further transmission of HIV is the diagnosis, treatment, and management of ulcerative genital infections, which also include chancroid and Herpes simplex virus Type 2 (HSV-2). Enhanced sexually transmitted infection (STI) control programs have proven efficacious in decreasing HIV seroincidence in some populations.3
Previous research has found a relatively low prevalence of syphilis infection among the general population in Peru. In a large, population-based sample of low-income Peruvians in coastal cities, syphilis prevalence was estimated to be 0.5% among men and 1.0% among women.4 A study of pregnant women attending public maternity hospitals found 1.6% syphilis seropositivity among this mostly low-income population; the same prevalence, 1.6%, was reported among heterosexual men attending STI clinics in Lima.5,6 In contrast, the prevalence of syphilis and other ulcerative STIs is markedly higher among men who have sex with men (MSM), female sex workers, and other socially marginalized populations. Sentinel surveillance among MSM in Lima has yielded lifetime syphilis seroprevalence estimates of 17.5% in 1998 and 12.4% in 2002, suggesting a downward trend over that period.7,8 Reports of syphilis prevalence among Peruvian female sex workers between 1999 and 2005 have ranged between 3% in Lima and 5% in smaller Peruvian cities.9,10 The epidemiology of HIV infection in Peru mirrors that of syphilis, with a less than 1% prevalence in the general population and an elevated prevalence among high-risk groups, specifically MSM.11–13
Prior studies on syphilis prevalence have used a broad case definition including all seroreactive specimens, which may include latent and even treated syphilis infections in addition to recent infections. The studies analyzing early syphilis infection have reported seroprevalence between 1.7% and 8.6% among Peruvian MSM.7,8 Previous research has focused on the risk factors for HIV and HSV-2 infection in Peru,8,14 but risk factors for recent syphilis infection in MSM remain unexamined.
MATERIALS AND METHODS
Study Design and Participant Recruitment
The information included in this report is from a population-based survey of 3 socially marginalized populations. We enrolled participants from 20 low-income neighborhoods in 3 coastal Peruvian cities, Lima, Trujillo, and Chiclayo, between 2003 and 2005. The number of neighborhoods selected per city was approximately proportional to the city’s population, and we chose neighborhoods that were similar based on the criterion of unmet basic needs index—an indicator used internationally to classify economic resources. Communities with potentially high levels of sexual risk behaviors were identified and characterized using ethnographic methods, including social and spatial mapping, informal interviews, participant observation, in-depth interviews, and focus groups. After enrollment, we collected epidemiologic data and serologic specimens from participants. This study was a part of the National Institute of Mental Health Collaborative HIV/STD Prevention Trial; the details of the sample selection have been previously described.15,16
Ethnographic research identified 3 distinct populations at elevated risk for HIV infection and STIs, who were included in this study.16,17 The first group was composed of heterosexually identified men who were under- or unemployed and who often engaged in drug use and petty theft. These men spend their time in vacant lots and on street corners, and sometimes engage in sex with men in exchange for money, food, or clothing. For the purpose of this analysis, they were referred to as socially marginalized men. The second group of men were self-identified and publicly recognized as homosexual, and were socially stigmatized on the basis of their homosexuality. This group of men who have sex with only men (MSOM) encompasses men with various levels of feminized appearance, including transvestites. In the neighborhoods sampled, the MSOM tended to congregate at volleyball courts and hair salons, where some of them were employed. Their behavior was also characterized by drug, alcohol use, and transactional sex, often with socially marginalized men. The women included in the study contradicted cultural norms by socializing with the socially marginalized men in the street. Most of these women were unemployed and a majority report having a stable partner, although many report having multiple partners, further defying the social norms of Peru.
Recruitment of participants occurred in specified target venues such as soccer fields, hair salons, parks, bars, and street corners. Eligible participants were restricted to people between 18 and 40 years of age who reported having had sex in the past 6 months; who intended to stay in the neighborhood for at least 2 years; and who frequented the target venue at least twice a week.
Data Collection
Participants were invited to a temporary project office in their neighborhood, where we privately administered a 30-minute structured computer-assisted personal interview in Spanish; trained interviewers read questions to participants and entered their answers into a computer. The survey instrument collected information on sociodemographic factors, sexual behavior, and other risk behaviors.
Upon completing the survey, participants underwent pretest counseling for STIs, including HIV infection, administered by a trained counselor. Phlebotomists then collected a blood sample from each participant, which was linked to the participant through a 10-digit code assigned by the study. Participants returning for results underwent post-test counseling to ensure their understanding of the meaning of both positive and negative results. In the case of a positive result, participants received treatment for syphilis and referrals for care for HIV and HSV-2 infections. Participants with syphilis were also encouraged to share test results with recent sex partners, who could also come in for syphilis treatment. In accordance with reporting requirements, we gave the initials and date of birth of individuals testing positive for HIV infection to the Peruvian Ministry of Health. We compensated participants for their travel costs and time: they received 15 Peruvian soles (approximately $4) at their first visit and an additional 10 Peruvian soles (approximately $3) at their post-test visit.
Laboratory Methods
All blood specimens were transported to the US Naval Medical Research Center Detachment in Lima for testing. Protocols adhered to the College of American Pathology proficiency testing program. Lab personnel used test kits according to the manufacturer’s specifications. Syphilis testing was conducted using RPR-nosticon II Rapid Plasma Reagin kits (Biomerieux, Boxtel, Netherlands). All RPR reactive specimens were then titrated and were confirmed by Serodia-TPPA (Fujirebio Diagnostics Inc, Toyko, Japan). Specimens with RPR titers of 1:8 or greater were considered recent syphilis infections, in keeping with suggested diagnostic guidelines for delineation of primary and secondary syphilis from latent or cured syphilis.18 Two enzyme immunoassays (EIAs) were used for HIV antibody detection with a Western blot for confirmation (EIA: BioRad, Hercules, CA and Biomerieux, Boxtel, Netherlands; Western blot: BioRad, Hercules, CA). HSV-2 testing was performed using an EIA for detecting IgG antibodies (Focus Technologies, HerpeSelect 2 ELISA IgG, United States). An index value of >3.50 was considered positive, <0.91 negative, and 0.91 to 3.49 indeterminate.
Human Subjects
The study was approved by the Committee on Human Research of the University of California, San Francisco; University of California, Los Angeles; and Cayetano Heredia University of Peru. The protocol was also approved by the Naval Medical Research Center Institutional Review Board. We collected data only from participants who gave written informed consent to participate in the study.
Data Analysis
We analyzed recent syphilis infection, defined as RPR titer of 1:8 or greater, as the primary outcome; this was a dichotomous variable. Covariates collected through interview and serologic testing were analyzed as exposure variables and possible confounders. Age and number of sexually active years were both coded continuously. The following covariates were coded as dichotomous variables: HIV infection, HSV-2 infection, compensated sex in the last 3 months (defined as exchanged money or goods for sex), drug use (defined as any marijuana and/or cocaine use in the past month), and education (defined as completion of secondary education). Finally, number of sex partners in the last 3 months was coded as an ordinal categorical variable with the population partitioned 0 or 1 partner, 2 or 3 partners, and 4 or more partners. For the socially marginalized men and MSOM, we performed chi square analyses of categorical variables (using Fisher exact test where appropriate), and nonparametric tests for trend for ordinal variables.
Because odds ratios tend to overestimate the underlying prevalence ratios when prevalence is high (as was the case for some of the included subpopulations) and logistic regression yields estimates of log odds ratios, we opted to use Poisson regression with a robust variance estimator as a technique to model the associations for this cross-sectional binary outcome. 17,19,20 Therefore, the model output is interpretable as prevalence ratios rather than odds ratios. In all regression analyses, we adjusted the standard errors to account for intragroup correlation within site of recruitment. We fit bivariate models to calculate prevalence ratios between syphilis and each covariate. We then fit a multivariate model including the aforementioned covariates that were significant at the P < 0.1 level in the bivariate analyses to calculate adjusted prevalence ratios. We used Stata 9.2 (Stata Corp, College Station, TX) for all analyses.
RESULTS
Of the 3149 eligible participants we identified, 2959 agreed to participate (94.0%). Among participants, 2944 (99.5%) provided a blood sample. The median age (interquartile range) was 22 (range, 20–26) years for the socially marginalized men, 26 (22–32) years for the MSOM, and 25 (21–31) years for the socially marginalized women. In pairwise comparisons, all 3 groups varied significantly along all demographic, sexual risk, and biologic variables listed in Table 1 (all P < 0.05), except for HIV prevalence, which did not differ significantly between the socially marginalized men and the women (P = 0.470).
TABLE 1.
Demographic and Sexual Risk Characteristics of Socially Marginalized Men, Men Who Have Sex With Only Men, and Women in Low-Income Barrios of Urban, Coastal Peru, 2003–2005
| Socially Marginalized Men (n = 2136) |
MSOM (n = 513) |
Women (n = 295) |
||||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |
| Demographics | ||||||
| Age (yr) | ||||||
| 18–21 | 957 | 44.8 | 104 | 20.3 | 86 | 29.1 |
| 22–25 | 606 | 28.4 | 130 | 25.3 | 70 | 23.7 |
| 26–29 | 281 | 13.2 | 106 | 20.7 | 48 | 16.3 |
| 30–33 | 153 | 7.2 | 82 | 16.0 | 35 | 11.9 |
| 34+ | 139 | 6.5 | 91 | 17.7 | 56 | 19.0 |
| Marital status | ||||||
| Single | 1419 | 66.5 | 484 | 94.3 | 94 | 31.9 |
| Married | 607 | 28.4 | 26 | 5.1 | 161 | 54.6 |
| Divorced or widowed | 109 | 5.1 | 3 | 0.6 | 40 | 13.5 |
| High school education | ||||||
| No | 1091 | 51.1 | 174 | 33.9 | 180 | 61.0 |
| Yes | 1045 | 48.9 | 339 | 66.1 | 115 | 39.0 |
| Sexual risk variables | ||||||
| Drug use* | ||||||
| No | 1488 | 69.7 | 464 | 90.4 | 279 | 94.6 |
| Yes | 646 | 30.3 | 49 | 9.6 | 16 | 5.4 |
| No. partners in last 3 mo | ||||||
| 0–1 | 1306 | 61.1 | 160 | 31.3 | 234 | 79.3 |
| 2–3 | 636 | 29.8 | 175 | 34.3 | 39 | 13.2 |
| 4+ | 194 | 9.1 | 176 | 34.4 | 22 | 7.5 |
| Sexually active years | ||||||
| <5 | 773 | 36.3 | 67 | 13.1 | 86 | 29.1 |
| 6–10 | 769 | 36.1 | 132 | 25.7 | 87 | 29.5 |
| 11–15 | 371 | 17.4 | 123 | 24.0 | 56 | 19.0 |
| 16+ | 218 | 10.2 | 191 | 37.2 | 66 | 22.4 |
| Compensated sex† | ||||||
| No | 1772 | 83.9 | 350 | 69.3 | 261 | 88.5 |
| Yes | 340 | 16.1 | 155 | 30.7 | 34 | 11.5 |
| HIV-1 antibody status | ||||||
| Negative | 2121 | 99.3 | 452 | 88.1 | 294 | 99.7 |
| Positive | 15 | 0.7 | 61 | 11.9 | 1 | 0.3 |
| HSV-2 antibody status | ||||||
| Negative | 1644 | 77.0 | 123 | 24.0 | 153 | 51.9 |
| Positive | 492 | 23.0 | 390 | 76.0 | 142 | 48.1 |
Drug use defined as use of marijuana or cocaine in the last month.
Compensated sex defined as exchanging sex for money or goods in the last 3 months.
MSOM indicates men who have sex with only men.
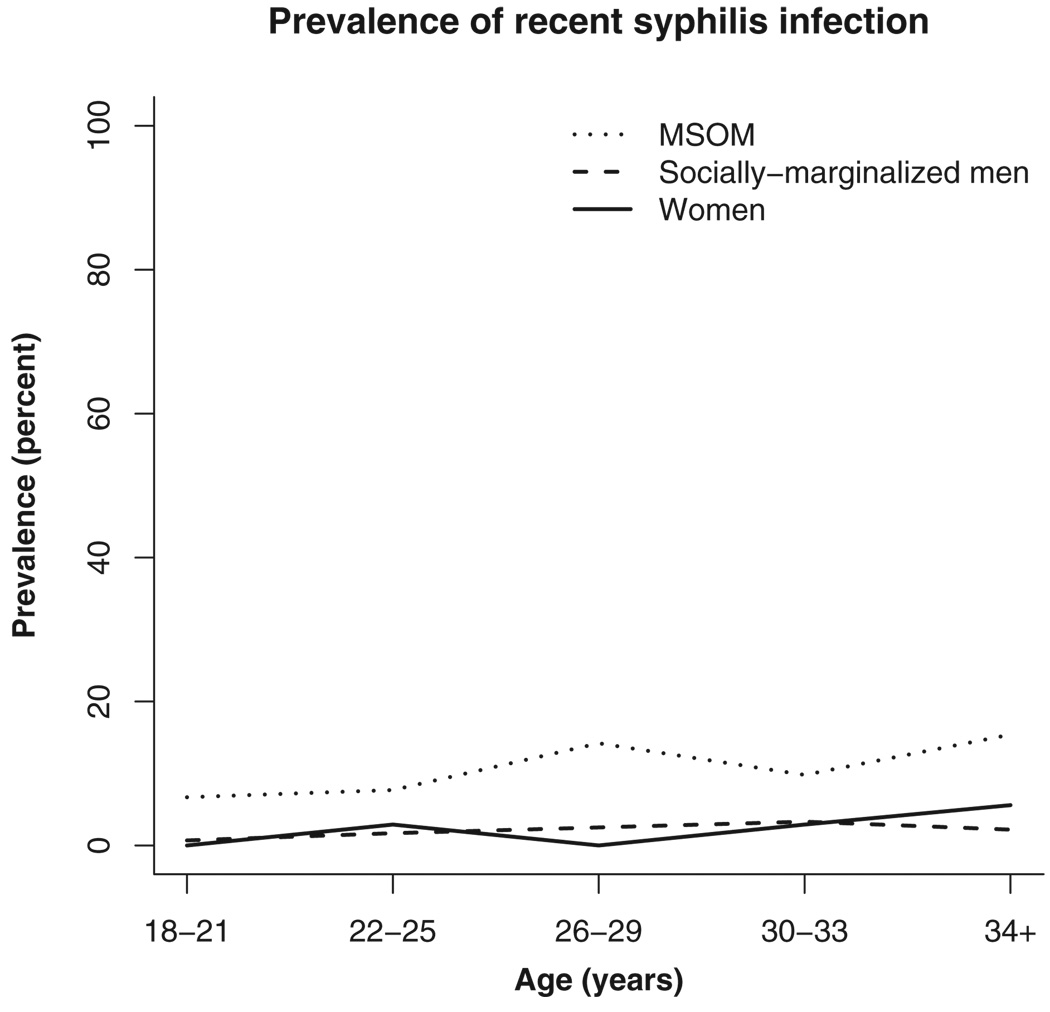
The prevalence of recent syphilis infection in each population is presented in Table 2. Prevalence was lowest among the socially marginalized men, at 1.5% (95% CI, 1.0%–2.1%). The prevalence of recent syphilis infection among the women was 2.0% (95% CI, 0.7%– 4.4%). Among MSOM, the prevalence was significantly higher, with 10.5% (95% CI, 8.0%–13.52%) testing positive. The prevalence of recent syphilis infection varied significantly between the MSOM population and both the socially marginalized men and the women (P < 0.001), but not between the socially marginalized men and the women (P = 0.487). The Figure 1 shows the trends of recent syphilis infection across age groups in all 3 populations.
TABLE 2.
Prevalence of Recent Syphilis Infection Among Socially Marginalized Men, Men Who Have Sex With Only Men, and Women in Low-Income Barrios of Urban, Coastal Peru, 2003–2005
| Socially Marginalized Men (n = 2,136) |
MSOM (n = 513) |
Women (n = 295) |
||||
|---|---|---|---|---|---|---|
| n/N | Recent Syphilis Prevalence* (%) |
n/N | Recent Syphilis Prevalence (%) |
n/N | Recent Syphilis Prevalence (%) |
|
| All | 32/2136 | 1.5 | 54/513 | 10.5 | 6/295 | 2.0 |
| Demographics | ||||||
| Age (yr) | ||||||
| 18–21 | 7/957 | 0.7 | 7/104 | 6.7 | 0/86 | 0.0 |
| 22–25 | 10/606 | 1.7 | 10/130 | 7.7 | 2/70 | 2.9 |
| 26–29 | 7/281 | 2.5 | 15/106 | 14.2 | 0/48 | 0.0 |
| 30–33 | 5/153 | 3.3 | 8/82 | 9.8 | 1/35 | 2.9 |
| 34+ | 3/139 | 2.2+ | 14/91 | 15.4† | 3/56 | 5.4 |
| Marital status | ||||||
| Single | 17/1419 | 1.2 | 52/484 | 10.7 | 2/94 | 2.1 |
| Married | 11/607 | 1.8 | 2/26 | 7.7 | 3/161 | 1.9 |
| Divorced or widowed | 4/109 | 3.7 | 0/3 | 0.0 | 1/40 | 2.5 |
| High school education | ||||||
| No | 18/1091 | 1.6 | 24/174 | 13.8 | 6/180 | 3.3 |
| Yes | 14/1045 | 1.3 | 30/339 | 8.8 | 0/115 | 0.0 |
| Sexual risk variables | ||||||
| Drug use* | ||||||
| No | 25/1488 | 1.7 | 48/464 | 10.3 | 6/279 | 2.2 |
| Yes | 7/646 | 1.1 | 6/49 | 12.2 | 0/16 | 0.0 |
| No. partners in last 3 mo | ||||||
| 0–1 | 16/1306 | 1.2 | 14/160 | 8.8 | 3/234 | 1.3 |
| 2–3 | 12/636 | 1.9 | 16/175 | 9.1 | 3/39 | 7.7 |
| 4+ | 4/194 | 2.1 | 23/176 | 13.1 | 0/22 | 0.0 |
| Sexually active years | ||||||
| <5 | 6/773 | 0.8 | 2/67 | 3.0 | 0/86 | 0.0 |
| 6–10 | 12/769 | 1.6 | 11/132 | 8.3 | 2/87 | 2.3 |
| 11–15 | 9/371 | 2.4 | 14/123 | 11.4 | 0/56 | 0.0 |
| 16+ | 5/218 | 2.3† | 27/191 | 14.1† | 4/66 | 6.1 |
| Compensated sex‡ | ||||||
| No | 26/1772 | 1.5 | 36/350 | 10.3 | 4/261 | 1.5 |
| Yes | 6/340 | 1.8 | 16/155 | 10.3 | 2/34 | 5.9 |
| HIV-1 antibody status | ||||||
| Negative | 28/2121 | 1.3 | 49/452 | 10.8 | 6/294 | 2.0 |
| Positive | 4/15 | 26.7§ | 5/61 | 8.2 | 0/1 | 0.0 |
| HSV-2 antibody status | ||||||
| Negative | 13/1644 | 0.8 | 6/123 | 4.9 | 1/153 | 0.7 |
| Positive | 19/492 | 3.9§ | 48/390 | 12.3† | 5/142 | 3.5 |
Recent syphilis infection defined as RPR titer ≥1:8.
P < 0.05.
Drug use defined as use of marijuana or cocaine in the last month; compensated sex defined s exchanging sex for money or goods in the last 3 months.
P < 0.001; among MSOM and socially-marginalized men, non-parametric test for trend performed on age, number of partners in the last 3 month, and sexually active years; chi square test, or Fisher exact test performed on all other variables.
n indicates the number of recent syphilis cases per stratum and N indicates the number of subjects per stratum (stratum sample size).
MSOM indicates men who have sex with only men.
Figure 1.
Early syphilis infection by age in years among persons in low-income neighborhoods in 3 coastal Peruvian cities, 2003 to 2005. MSOM indicates men who have sex with only men.
The relatively small sample size of female participants (n = 295) precluded regression analysis of risk factors for recent syphilis infection in this population, and so the risk factor analysis was confined to the 2 populations of men. Table 3 presents the results of the bivariate regression analysis, which calculated unadjusted prevalence ratios for risk factors. Age, HSV-2 infection, and HIV infection were found to be significantly associated with syphilis infection among the socially marginalized men (all P < 0.001). Among MSOM, the bivariate analyses demonstrated sexually active years (P = 0.001) and HSV-2 infection (P = 0.003) to be significant risk factors.
TABLE 3.
Factors Associated With Syphilis Among Socially Marginalized Men and Men Who Have Sex With Only Men in Low-Income Barrios of Urban, Coastal Peru, 2003–2005
| Bivariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Unadjusted PR | 95% CI | P | Adjusted PR | 95% CI | P | |
| Socially marginalized men* | ||||||
| Age (yr) | 1.07 | (1.03–1.11) | <0.001 | 1.04 | (0.99–1.09) | 0.093 |
| HSV-2 antibody status | ||||||
| Negative | Ref | — | — | Ref | — | — |
| Positive | 4.88 | (2.77–8.61) | <0.001 | 3.72 | (2.12–6.53) | <0.001 |
| HIV-1 antibody status | ||||||
| Negative | Ref | — | — | Ref | — | — |
| Positive | 20.20 | (10.27–39.72) | <0.001 | 11.13 | (4.50–27.51) | <0.001 |
| MSOM* | ||||||
| No. sexually active years | 1.06 | (1.03–1.10) | 0.001 | 1.05 | (1.01–1.10) | 0.027 |
| HSV-2 antibody status | ||||||
| Negative | Ref | — | — | Ref | — | — |
| Positive | 2.52 | (1.37–4.63) | 0.003 | 1.96 | (1.03–3.74) | 0.040 |
Bivariate and multivariate models also adjusted for intra-group correlation within venue of recruitment.
PR indicates prevalence ratio; MSOM, men who have sex with only men.
The results of the multivariate analysis are also presented in Table 3. After adjusting for all relevant demographic and sexual risk covariates, the following variables remained significantly associated with syphilis infection among the socially marginalized men HIV infection (adjusted PR = 11.13; 95% CI, 4.50 –27.51) and HSV-2 infection (adjusted PR = 3.72; 95% CI, 2.12–6.53). Age was no longer significant in the multivariate model.
The multivariate analysis indicated that HSV-2 infection was also significantly associated with syphilis among MSOM (adjusted PR = 1.96; 95% CI, 1.03–3.74). Among MSOM, sexually active years also remained a significant risk factor in the multivariate analysis (for each year, adjusted PR = 1.05; 95% CI, 1.01–1.10).
DISCUSSION
All 3 groups included in this population-based study exhibited a high prevalence of recent syphilis infection in comparison to the prevalence of all RPR/TPPA-reactive syphilis infection found in the general population (a broader definition that may include latent and treated cases of syphilis).4,5 However, recent syphilis infection was most prevalent among the MSOM. Among both socially marginalized men and MSOM, HSV-2 infection was associated with syphilis infection. This finding might be because HSV-2 and syphilis share transmission routes and risk factors. HIV infection was found to be significantly associated with recent syphilis infection among the socially marginalized men but not among the MSOM, though this finding was based on a small sample size due to the low prevalence of HIV infection among the socially marginalized men. Age was shown to be associated with recent syphilis infection in the socially marginalized men, but none of the sexual risk behaviors were associated with recent syphilis infection. Among the MSOM, the number of sexually active years remained significantly associated with recent syphilis infection in the multivariate model.
Currently, the Peruvian Ministry of Health encourages MSOM in these communities to be tested for STIs every 3 months and offers free or low cost treatment. These programs should include education on the behavioral risk factors for syphilis and the benefits of treatment, partner treatment, and frequent screening. Effective STI management—including syphilis treatment—might have the added benefit of diminishing HIV incidence in this high-risk population. Previous research on syphilis-HIV coinfection has demonstrated syphilis to increase HIV viral load, and this effect is counteracted by effective syphilis treatment.21,22
In an earlier report on syphilis reinfection 1 year after treatment in a similar population of low-income Peruvians, investigators reported that 42% were reinfected after cure.23 In that report, syphilis reinfection was strongly associated with prevalent HIV infection. Strategies to address reinfection with syphilis in these populations include partner notification.24 Future studies should explore other group-level sexual risk variables and environmental factors that might be associated with syphilis in these high-risk populations, especially because the individual-level sexual risk factors examined in the current study did not remain significant in the multivariate analysis.
Although the current study’s sample size precluded analysis of risk factors among women, the elevated risk of syphilis found among the women in this study merits further investigation with regard to their risk factors for syphilis infection. This research question is especially significant because syphilis infection among women carries the added risk of congenital syphilis in the case of pregnancy and birth among women of reproductive age.25
Some features of our study design merit consideration when interpreting the results. First, the current study focused on recent syphilis infection, as indicated by TPPA-confirmed infections with RPR titers of 1:8 or greater. The more expansive case definition of syphilis that is used in most other studies of these populations includes all RPR/TPPA-positive cases, and may capture long-term, latent infections and even treated syphilis infections, thereby preventing valid analysis of proximal risk factors for syphilis infection.18 The current study’s case definition restricts the analysis to recent syphilis infections, and therefore more accurately describes risk factors for syphilis transmission. Another limitation in interpreting our results is the nature of a cross-sectional study, which precludes inferring causality. A final aspect of this study that warrants consideration is the reliability of self-reported sexual risk behavior. A tendency to underreport perceived risky sexual behavior might introduce bias into the prevalence ratios calculated in this study, possibly causing the true associations between syphilis and risk factors to be underestimated.
In conclusion, this study among socially marginalized populations in coastal Peru demonstrated some important behavioral and epidemiologic cofactors associated with recent syphilis infection among both socially marginalized men and homosexual men. Especially among the MSOM, where syphilis prevalence is highest, these findings call renewed attention to the need for more effective syphilis control services among this population. Specifically, the current syphilis control program operated by the Peruvian Ministry of Health should be reevaluated to assess areas for further investment and potential improvement in reaching this population. As a highly prevalent ulcerative STI that is curable, syphilis is uniquely suitable for STI control programs aiming to interrupt the epidemiologic synergy between STIs and HIV infection in this population. Such STI control could decrease syphilis-associated morbidity and transmission of syphilis among the high-risk populations in this study.
Acknowledgments
Supported by NIH/NIMH grant number U10 MH61536 which is a five-country Cooperative Agreement being conducted in China, India, Peru, Russia, and Zimbabwe. Each site has selected a different venue and population with which to implement the prevention program entitled Community Public Opinion Leader (C-POL) Intervention. Additionally, supported by LP-CRADA NM-04–1787. Also, by the Fogarty AIDS International Training and Research Program/University of California, Berkeley (1-D43-TW00003) (to J.M.S.).
Footnotes
This article is based on a prebaseline study conducted in all the sites to prepare for initiation of the intervention.
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position or the Department of the Navy, Department of Defense, nor the US Government.
REFERENCES
- 1.Cohen MS. Sexually transmitted diseases enhance HIV transmission: No longer a hypothesis. Lancet. 1998;351 suppl 3:5–7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 2.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosskurth H, Mosha F, Todd J, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: Randomised controlled trial. Lancet. 1995;346:530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 4.NIMH Collaborative HIV/STD Prevention Trial Group. Sexually transmitted disease and HIV prevalence and risk factors in concentrated and generalized HIV epidemic settings. AIDS. 2007;21 suppl 2:S81–S90. doi: 10.1097/01.aids.0000266460.56762.84. [DOI] [PubMed] [Google Scholar]
- 5.Alarcon JO, Johnson KM, Courtois B, et al. Determinants and prevalence of HIV infection in pregnant Peruvian women. AIDS. 2003;17:613–618. doi: 10.1097/00002030-200303070-00017. [DOI] [PubMed] [Google Scholar]
- 6.Nelson A, Press N, Bautista CT, et al. Prevalence of sexually transmitted infections and high-risk sexual behaviors in heterosexual couples attending sexually transmitted disease clinics in Peru. Sex Transm Dis. 2007;34:344–361. doi: 10.1097/01.olq.0000240341.95084.da. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez J, Lama JR, Kusunoki L, et al. HIV-1, sexually transmitted infections, and sexual behavior trends among men who have sex with men in Lima, Peru. J Acquir Immun Defic Syndr. 2007;44:578–585. doi: 10.1097/QAI.0b013e318033ff82. [DOI] [PubMed] [Google Scholar]
- 8.Lama JR, Lucchetti A, Suarez L, et al. Association of herpes simplex virus type 2 infection and syphilis with human immunodeficiency virus infection among men who have sex with men in Peru. J Infect Dis. 2006;194:1459–1466. doi: 10.1086/508548. [DOI] [PubMed] [Google Scholar]
- 9.Trujillo L, Munoz D, Gotuzzo E, et al. Sexual practices and prevalence of HIV, HTLV-I/II, and Treponema pallidum among clandestine female sex workers in Lima, Peru. Sex Transm Dis. 1999;26:115–118. doi: 10.1097/00007435-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Campos PE, Buffardi AL, Chiappe M, et al. Utility of the Determine Syphilis TP rapid test in commercial sex venues in Peru. Sex Transm Infect. 2006;82 suppl 5:v22–v25. doi: 10.1136/sti.2006.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabet S, Sanchez J, Lama J, et al. HIV, syphilis and heterosexual bridging among Peruvian men who have sex with men. AIDS. 2002;16:1271–1277. doi: 10.1097/00002030-200206140-00010. [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS. Peru, Epidemiological Fact Sheets on HIV and Sexually Transmitted Infections, 2004 Update. Geneva, Switzerland: UNAIDS; 2004
- 13.Analisis de la situación epidemiológica del VIH/SIDA en el Perú. Lima, Perú: Ministry of Health; 2006. [Google Scholar]
- 14.Konda KA, Klausner JD, Lescano AG, et al. The epidemiology of herpes simplex virus type 2 infection in low-income urban populations in coastal Peru. Sex Transm Dis. 2005;32:534–541. doi: 10.1097/01.olq.0000175413.89733.ae. [DOI] [PubMed] [Google Scholar]
- 15.Methodological overview of a five-country community-level HIV/sexually transmitted disease prevention trial. AIDS. 2007;21 suppl 2:S3–S18. doi: 10.1097/01.aids.0000266453.18644.27. [DOI] [PubMed] [Google Scholar]
- 16.Selection of populations represented in the NIMH Collaborative HIV/STD Prevention Trial. AIDS. 2007;21 suppl 2:S19–S28. doi: 10.1097/01.aids.0000266454.26268.90. [DOI] [PubMed] [Google Scholar]
- 17.Cáceres CF, Salazar X, Rosasco AM, et al. Where the needs are: identifying those at higher risk for HIV to implement a prevention trial in lower-income communities in Peru: Implications for public health programming. Paper presented at: XV International AIDS Conference; Bangkok, Thailand. 2004. [Google Scholar]
- 18.Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1–21. doi: 10.1128/cmr.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J. Odds ratio or relative risk for cross-sectional data? Int J Epidemiol. 1994;23:201–203. doi: 10.1093/ije/23.1.201. [DOI] [PubMed] [Google Scholar]
- 20.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchacz K, Patel P, Taylor M, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18:2075–2079. doi: 10.1097/00002030-200410210-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kofoed K, Gerstoft J, Mathiesen LR, et al. Syphilis and human immunodeficiency virus (HIV)-1 coinfection: Influence on CD4 T-cell count, HIV-1 viral load, and treatment response. Sex Transm Dis. 2006;33:143–148. doi: 10.1097/01.olq.0000187262.56820.c0. [DOI] [PubMed] [Google Scholar]
- 23.Long CM, Klausner JD, Leon S, et al. Syphilis treatment and HIV infection in a population-based study of persons at high risk for sexually transmitted disease/HIV infection in Lima, Peru. Sex Transm Dis. 2006;33:151–155. doi: 10.1097/01.olq.0000204506.06551.5f. [DOI] [PubMed] [Google Scholar]
- 24.Clark JL, Long CM, Giron JM, et al. Partner notification for sexually transmitted diseases in Peru: Knowledge, attitudes, and practices in a high-risk community. Sex Transm Dis. 2007;34:309–313. doi: 10.1097/01.olq.0000240289.84094.93. [DOI] [PubMed] [Google Scholar]
- 25.Klausner JD, Hook EW. Current Diagnosis & Treatment of Sexually Transmitted Diseases. New York, NY: McGraw-Hill; 2007. [Google Scholar]