Abstract
Objectives
To propose standardized methods for measuring concurrent adherence to multiple related medications and to apply these definitions to a cohort of patients with diabetes mellitus.
Study Design
Retrospective cohort study of 7567 subjects with diabetes prescribed 2 or more classes of oral hypoglycemic agents in 2005.
Methods
For each medication class, adherence for each patient was estimated using prescription-based and interval-based measures of proportion of days covered (PDC) from cohort entry until December 31, 2006. Concurrent adherence was calculated by applying these 2 measures in the following 3 ways: (1) the mean of each patient's average PDC, (2) the proportion of days during which patients had at least 1 of their medications available to them, and (3) the proportion of patients with a PDC of at least 80% for all medication classes. Because patients taking multiple related medications have distinct patterns of use, the analysis was repeated after classifying patients into mutually exclusive groups.
Results
Concurrent medication adherence ranged from 35% to 95% depending on the definition applied. Interval-based measures provide lower estimates than prescription-based techniques. Definitions that require the use of at least 1 drug class categorize virtually all patients as adherent. Requiring patients to have a PDC of at least 80% for each of their drugs results in only 30% to 40% of patients being defined as adherent. The variability in adherence is greatest for patients whose treatment regimen changed the most during follow-up.
Conclusions
The variability in adherence estimates derived from different definitions may substantially impact qualitative conclusions about concurrent adherence to related medications. Because the measures we propose have different underlying assumptions, the choice of technique should depend on why adherence is being evaluated.
Adherence (also called compliance) to prescribed medications is a key dimension of healthcare quality. Nonadherence is associated with poor health outcomes1 and with substantial economic cost2,3 and threatens the gains in quality that have been made by appropriate pharmacotherapy over the past several decades.4 Efforts to accurately measure and improve adherence have received increasing attention from patients, physicians, payers, and other healthcare stakeholders. Moreover, the National Committee for Quality Assurance has recently included adherence among the measures by which it evaluates the quality of care provided by healthcare plans.5
Adherence may be assessed in various ways, including surveying patients, directly observing medication taking, measuring drug or metabolite blood levels, and using electronic medication monitors.3 Pharmacy refill claims, which are highly correlated with these measures,6 provide an objective way to measure adherence for large populations of patients. Such data are frequently used by health services researchers and pharmacy benefit plan managers to calculate adherence by estimating the amount of medication available to a patient over a given interval.7
These measures were developed and have been most widely used to evaluate adherence to individual medications or medication classes. For example, Benner et al8 assessed statin adherence by measuring the proportion of days covered (PDC) for patients newly prescribed 1 of these agents. However, it is common for patients with chronic conditions (such as hypertension, coronary artery disease, or congestive heart failure) to switch between classes of medications or to receive multiple medications to treat a single disease. Such behavior is clinically sensible and formalized in practice guidelines but is inadequately captured by measuring adherence for a single agent or medication class.
Studies that have measured concurrent adherence to multiple medication classes have used various techniques,9-11 and unlike adherence to single medications or single medication classes,6,12 there are no published definitions or guidelines about how to structure these measurements. Understanding the variability in adherence estimates obtained from different measures will assist in selecting appropriate definitions. In this article, we propose standardized methods for measuring concurrent adherence, apply them to a cohort of patients with diabetes mellitus receiving treatment with oral hypoglycemic agents, and compare their performances.
Take-Away Points
Variability in adherence estimates derived from different definitions of concurrent adherence to related medications may lead to very different qualitative conclusions about adherence in a given population.
Therefore, it is important to establish how concurrent adherence to multiple related medications should be calculated.
This study found that concurrent medication adherence varied widely depending on the definitions applied because of the assumptions on which they are based.
The choice of adherence measurement technique should depend on the reason why adherence is being measured.
Methods
Study Cohort
We assembled a cohort of patients who received pharmacy benefits through Horizon Blue Cross Blue Shield of New Jersey and who were prescribed an oral hypoglycemic agent between January 1, 2005, and December 31, 2005. We considered only those patients who had 1 or more inpatient or outpatient claims with a diagnosis of diabetes (International Classification of Diseases, Ninth Revision, Clinical Modification code 250.x) and were prescribed 2 or more classes of oral hypoglycemic medications during this period. While all oral hypoglycemics may be considered members of 1 therapeutic class, we defined classes on a mechanistic basis (ie, sulfonylurea, metformin hydrochloride, glitazones, acarbose, and meglitinides), as many patients take agents belonging to these different classes concurrently. Furthermore, this strategy allows for the generalization of our methods to patients using noninterchangeable medication classes (eg, statins, angiotensin-converting enzyme inhibitors, antiplatelets, and β-blockers after myocardial infarction).
We excluded patients who lost eligibility or did not fill any prescriptions or use any medical services in 2006. We defined an index date for each oral hypoglycemic class prescribed to each patient as the first prescription date for any member of the class during the accrual period.
We combined filled prescription data for patients in our cohort with complete paid claims data and eligibility files to create a relational database consisting of data for all filled prescriptions, procedures, inpatient and outpatient physician encounters, hospitalizations, and deaths for the patients in our cohort. Prescription information in the claims data included drug name, dosage, date dispensed, quantity dispensed, and days supplied. All traceable person-specific identifying factors were transformed into anonymous coded study numbers. The institutional review board of Brigham and Women's Hospital approved the study.
Measures of Adherence
To measure adherence, we first created a supply diary for each patient-day by stringing together consecutive fills of each medication class being studied based on dispensing dates and reported days' supply. All drugs dispensed within a therapeutic class (eg, glyburide and glipizide in the sulfonylurea class) were considered interchangeable. When a dispensing occurred before the previous dispensing should have run out, utilization of the new medication was assumed to begin the day after the end of the old dispensing. If a patient accumulated more than 180 days' supply on a given day, the accumulated supply was truncated at 180 days.
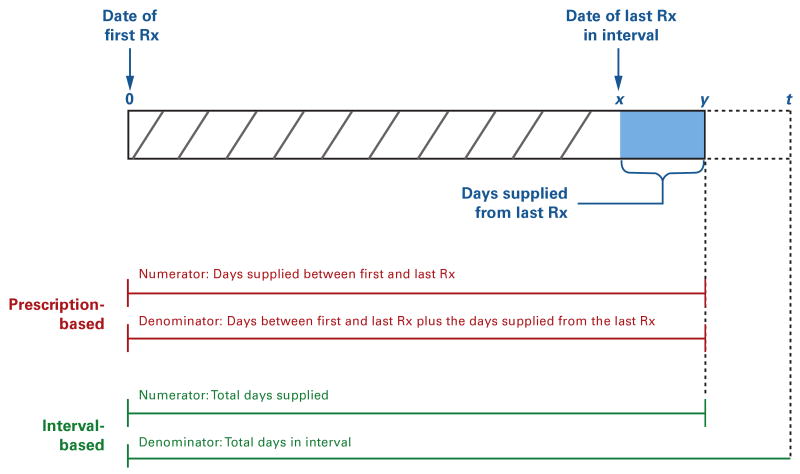
We estimated adherence by calculating the PDC for each drug class prescribed to each patient from the index date to the end of our assessment period (December 31, 2006) using 2 different methods that differ in how the denominator of the adherence measure is calculated (Figure 1). We defined prescription-based adherence based on medication possession for all drugs within a class during the time between 2 prescriptions; that is, the number of days of medication supplied between the first and last prescriptions in a given period (numerator) was divided by the number of days between these 2 prescriptions plus the accumulated days supplied from the last prescription (denominator). In contrast, we defined interval-based adherence based on medication possession during the interval from the index date to December 31, 2006. In this way, the number of days of medication supplied throughout the period (numerator) was divided by the number of days in it (denominator).
Figure 1. Measuring Adherence to Individual Medication Classes Using Prescription-Based and Interval-Based Approaches.
The figure shows a medication-filling pattern for a hypothetical patient. Prescription-based approaches measure adherence between 2 prescriptions within a class. The numerator is the number of days of medication supplied from all prescriptions in the class, including the first and last prescriptions, and the denominator is the number of days between the first and last prescriptions plus the days' supply from the last prescription (ie, x + y days). Interval-based approaches measure adherence during a given interval. The denominator is the number of days of medication supplied from all prescriptions (ie, x + y days) during the interval, and the denominator is the total number of days in the interval (ie, t days).
Adherence to Multiple Medications Concurrently
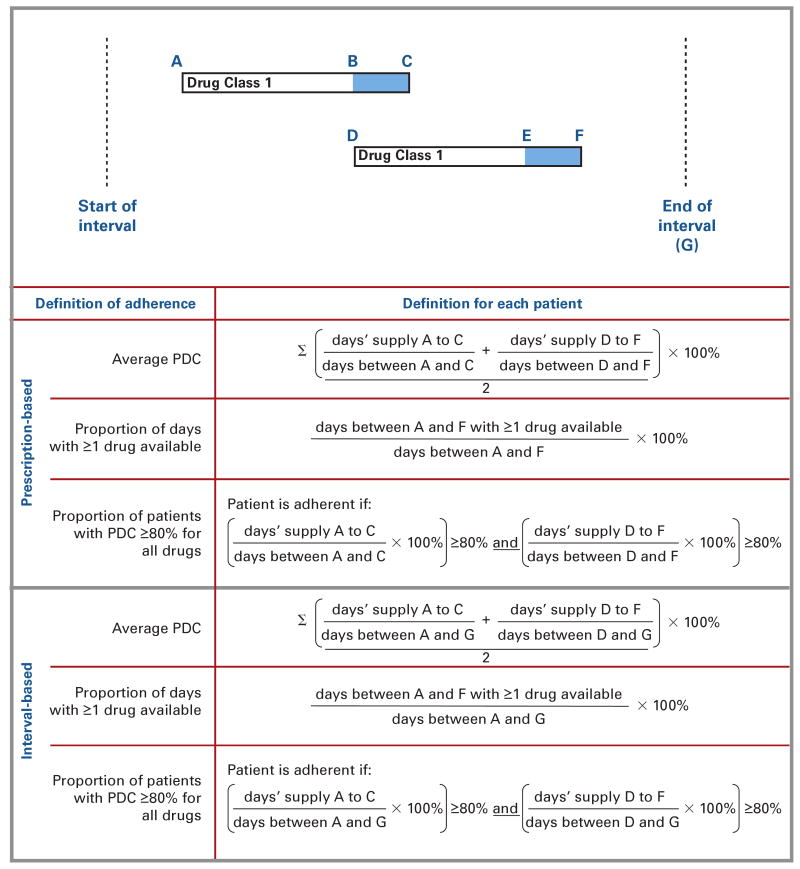
We estimated simultaneous adherence to multiple medication classes for each patient by using the prescription-based adherence estimated for each class in 3 distinct ways (Figure 2). First, we calculated an average of the prescription-based PDC for each patient and then generated a group mean of these averages for the entire cohort. For example, for patients taking 2 classes of oral hypoglycemics, the PDC for each was calculated and then averaged, and this average was used to calculate the mean group PDC. Second, we calculated the number of days during which patients had at least 1 of their prescribed medications available to them beginning from the date they filled their first prescription for any oral hypoglycemic (ie, their earliest index date) until their latest prescription date for any of the medications they were using. For example, for a patient being treated with metformin and glyburide, the numerator of the adherence measure was the number of days during which he or she had either metformin or glyburide available. Third, we estimated the proportion of patients who had a prescription-based PDC of at least 80% for each medication they were using. For example, for patients treated with glyburide and metformin, each patient was considered adherent if his or her prescription-based PDC for each was at least 80%.
Figure 2. Methods for Measuring Concurrent Adherence to Related Classes of Medications.
PDC indicates proportion of days covered.
The hypothetical example represents a patient receiving one medication class who then initiated treatment with a second class (1-drug add-on). Methods for calculating adherence for each individual patient using different definitions are shown and are described in greater detail in the text.
We repeated this process using interval-based adherence estimates. In this way, we determined adherence using 6 different methods (ie, 3 different techniques for each of the prescription-based and interval-based methods).
Complex Patterns of Medication Use
Patients taking multiple related medications may have many distinct patterns of medication-taking behavior, and measures of concurrent adherence may differ more substantially for some patients. Therefore, we classified patients into mutually exclusive groups and then applied our 6 adherence definitions to each group individually.
We created medication-taking groups by first generating clinically plausible scenarios of how patients may use multiple medications. For example, patients may initiate therapy with 1 medication and then may be started on a second agent at some point later for them to meet their therapeutic goals. We applied our schema to a subsample of 500 patients from our cohort and then created additional categories for patients who could not be categorized into 1 of our prespecified groups. We used 8 distinct medication-taking patterns generated in this way to categorize our entire cohort based on the definitions given in Table 1 and then applied our 6 different adherence definitions to each group. In all cases, follow-up began on the earliest prescription date for any oral hypoglycemic prescribed in 2005.
Table 1.
Descriptions of Different Patterns of Adherence to Multiple Medications
| Name | Description | Example | ||
|---|---|---|---|---|
| 1-Drug Switcher | Class A | Patient receives 2 (or more) classes of agents but prescriptions are not overlapping | Patient is begun on metformin and then after discontinuing this drug switches to glyburide | |
| Class B | ||||
| 1-Drug Add-On | Class A | Patient receives 1 agent and then after refilling ≥1 time begins a second class of medications | Patient is begun on metformin and then glyburide is added while metformin is continued | |
| Class B | ||||
| Polytherapy Consistent | Class A | Patient receives 2 (or more) classes simultaneously (ie, patient receives a prescription for 2 different classes on the same day or at any point before filling a second prescription). No further alterations to regimen (other than changes within a class) occur during follow-up | Patient is begun on metformin and then receives glyburide before receiving a second prescription for metformin | |
| Class B | ||||
| Polytherapy Add-On | Class A | Patient receives 2 classes simultaneously (as defined above) and then receives a third class after refilling both of the first 2 medications | Patient is begun on metformin and glyburide simultaneously and then after refilling both pioglitazone is added | |
| Class B | ||||
| Class C | ||||
| Polytherapy Drop-Off | Class A | Patient receives 2 (or more) classes simultaneously (as defined above), then after refilling these medications 1 (or more) is discontinued (and no other classes are subsequently started) | Patient is begun on metformin and glyburide simultaneously and then after refilling both metformin is discontinued | |
| Class B | ||||
| Polytherapy Switcher | Class A | Patient receives 2 classes simultaneously (as defined above), then discontinues 1 (or more) and starts a different class | Patient is begun on metformin and glyburide simultaneously and then after refilling both metformin is discontinued and acarbose is started | |
| Class B | ||||
| Class C | ||||
| 2-Drug Add-On | Class A | Patient receives 1 therapy only, then after refilling ≥1 time begins 2 additional different classes of medication simultaneously (ie, begins a third class at any point before refilling a prescription for the second class added) | Patient is begun on metformin, and then glyburide and pioglitazone are added, while metformin is continued | |
| Class B | ||||
| Class C | ||||
| Sequential Add-On | Class A | Patient receives 1 therapy only, then after refilling ≥1 time begins another class and then after refilling ≥1 prescription for the second class starts a third class | Patient is begun on metformin then glyburide added while metformin is continued; while metformin and glyburide are continued, pioglitazone is added | |
| Class B | ||||
| Class C | ||||
Sensitivity Analysis
We also explored how our adherence definitions affected the results in several subgroups of patients. First, because adherence is time varying, we restricted our cohort to patients who were new users, defined as not having used any oral hypoglycemic of any class in the 12 months before their earliest index date (ie, before the first oral hypoglycemic prescribed in 2005) and then reran our analyses. Second, because the differences between prescription-based and interval-based measures may be particularly large for patients who discontinued therapy altogether, we identified patients who did not fill any prescriptions for oral hypoglycemics in the last 6 months of 2006 and repeated our analyses in this subgroup of patients.
Results
Cohort
We identified 34,211 patients who filled prescriptions for at least 1 diabetes medication in 2005. From this group, we excluded 12,755 because they did not have a medical services claim for diabetes during this time, 2607 who lost eligibility or did not fill any prescriptions or use any medical services in 2006, and 11,292 who filled prescriptions for agents in only 1 oral hypoglycemic class. Our final cohort consisted of 7567 patients who filled prescriptions for 2 or more classes of oral hypoglycemic agents in 2005.
The mean (SD) age of the patients was 53.2 (7.6) years, and 56% of the cohort were female. Sixty-four percent of the cohort had been prescribed 2 different medication classes, and the remaining 36% had been prescribed 3 or more classes. Twenty-three percent of patients were new users, not having filled a prescription for any oral hypoglycemic during the 12 months before their index date, and 12% of patients filled no prescriptions for diabetes medications between July 1 and December 31, 2006 (ie, they discontinued therapy altogether during follow-up). The mean length of observation from cohort entry until December 31, 2006, was 665 days (range, 366-729 days).
Overall Concurrent Adherence
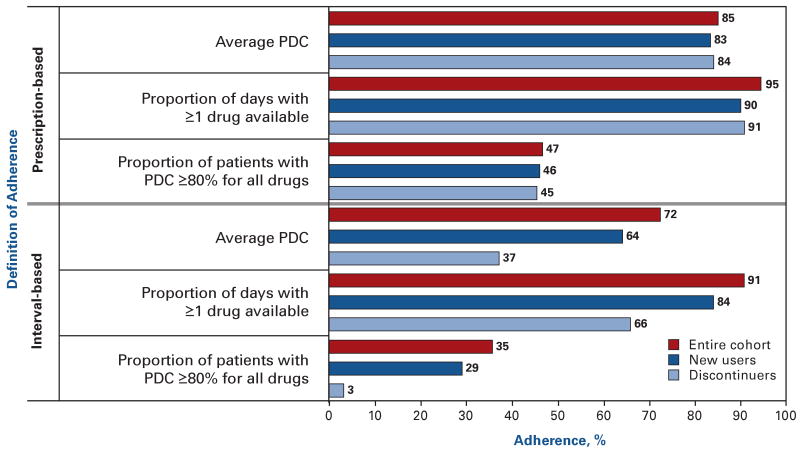
Concurrent medication adherence ranged from 35% to 95% depending on the definition applied (Figure 3). Interval-based measures provide lower estimates of adherence than the corresponding prescription-based techniques. Defining adherence based on whether patients have at least 1 medication available to them at all times results in most patients being defined as adherent. In contrast, requiring patients to have a PDC of at least 80% for each of their drugs results in less than half of the patients being adherent.
Figure 3. Adherence Rates for Oral Hypoglycemic Agents Among Patients Prescribed More Than 1 Class of Agents.
PDC indicates proportion of days covered.
The burgundy bars represent adherence estimates for the entire cohort (N = 7567). The dark blue and light blue bars represent subgroups of patients who newly initiated therapy and who discontinued therapy altogether, respectively.
Adherence estimates varied the most for patients who discontinued therapy altogether, particularly using interval-based methods (Figure 3). For example, the average PDC using an interval-based approach for the entire cohort was 72% but was only 37% in the subgroup of patients who discontinued treatment. Adherence rates for new users were slightly lower than those for the overall cohort, although not sufficiently to change qualitative interpretations of adherence.
Concurrent Adherence in Patients With Different Medication-Taking Patterns
The variability in adherence from the 6 definitions is particularly large for some subgroups of patients (Table 2). For example, adherence rates for patients who switched from one drug class to another (1-drug switcher) ranged from 2% (using an interval-based definition requiring a PDC of ≥80% for all drugs) to 86% based on the average prescription-based PDC. Similarly, for patients treated with 2 classes and who then initiated therapy with a third class (polytherapy add-on), adherence ranged from 19% (using a prescription-based definition requiring a PDC of ≥80% for all drugs) to 99% (using an “at least 1” prescription-based definition).
Table 2.
Adherence Rates to Oral Hypoglycemics for Different Patterns and Definitions of Adherence for Users of Multiple Medications
| Pattern of Medication Use, % | ||||||||
|---|---|---|---|---|---|---|---|---|
| Definition of Adherence | 1-Drug Switcher (n = 300) | 1-Drug Add-On (n = 3404) | Polytherapy Consistent (n = 871) | Polytherapy Add-On (n = 615) | Polytherapy Drop-Off (n = 577) | Polytherapy Switcher (n = 132) | 2-Drug Add-On (n = 177) | Sequential Add-On (n = 1491) |
| Interval-based Definition | ||||||||
| Average PDC | 43 | 75 | 82 | 77 | 59 | 52 | 77 | 70 |
| Proportion of days with ≥1 drug available | 54 | 91 | 94 | 98 | 87 | 87 | 97 | 95 |
| Proportion of patients with a PDC of ≥80% for all drugs | 2 | 44 | 53 | 24 | 18 | 22 | 15 | 27 |
| Prescription-based Definition | ||||||||
| Average PDC | 86 | 86 | 88 | 86 | 80 | 82 | 89 | 85 |
| Proportion of days with ≥1 drug available | 63 | 95 | 99 | 99 | 94 | 90 | 99 | 96 |
| Proportion of patients with a PDC of ≥80% for all drugs | 57 | 60 | 60 | 19 | 40 | 27 | 16 | 26 |
PDC indicates proportion of days covered.
Discussion
We propose standardized definitions of pharmacy refill–based adherence for patients receiving treatment with 2 or more related medications concurrently. Applying these definitions to a cohort of patients with diabetes demonstrates the enormous variability in adherence that each definition provides and the disparate qualitative conclusions that might be drawn from them. Requiring patients to have a PDC of at least 80% for each of their medication classes classifies few patients in our cohort as adherent to their treatment regimen. In contrast, defining adherence using the “at least 1” definition supports the conclusion that most patients are adherent.
These definitions result in such different interpretations of adherence because of the assumptions on which they are based. Requiring patients to have a PDC of at least 80% for each drug class they are using classifies patients as adherent who are very likely to be adherent but may erroneously identify patients who appropriately discontinue or substitute medications as nonadherent, especially using interval-based techniques. In contrast, the “at least 1” definition allows a patient who is fully adherent to one of their medications and largely nonadherent to another to nevertheless be considered adherent. Averaging the PDC provides an intermediate position to these extremes.
Therefore, the choice of adherence measure for patients using more than 1 related medication should depend on the reason why adherence is being measured. This is analogous to how the choice of a diagnostic test depends on whether it is being used for screening or confirmation. For example, in the case of using adherence to determine reimbursement, the limited penetration of electronic prescribing may make it difficult for prescribers and health systems to receive and provide feedback about their patients' adherence; thus, it may be politically and practically most reasonable to use a measure that is sensitive to nonadherence such as that provided by the “at least 1” definition. A more specific definition (ie, a PDC of ≥80% for all drugs) could be introduced as information systems improve or in situations where an adherence intervention is being evaluated. Similarly, in cases where adherence is being measured as a quality benchmark, a specific definition may yield greater behavior change.
There are several limitations to our analysis. First, although pharmacy refill claims are widely believed to be a valid method for assessing compliance,6 none of the techniques we propose indicate with certainty which medications a patient is actually taking. This concern applies generally to the use of pharmacy refill claims to assess adherence but may be particularly problematic in the case of multiple related medications, as patients whose medications are being titrated may inappropriately be considered noncompliant. While the “at least 1” definition that we propose provides for this possibility, further research is needed to correlate our measures with medical records and other techniques for assessing adherence.
Second, we estimated adherence to single drugs or classes using an interval-based PDC and a prescription-based PDC. Because our objective was to illustrate the variability in adherence estimates that could be obtained from different methods of measuring simultaneous adherence to multiple medications, we did not use the numerous other adherence measures that exist or any measure of persistence.
In conclusion, the use of multiple medications to treat various chronic diseases (such as diabetes, hypertension, congestive heart failure, coronary artery disease, and chronic obstructive pulmonary disease) is increasing, as is the interest in measuring adherence by payers and health services researchers. As a result, methods to measure simultaneous adherence to complex treatment regimens using routinely collected data are required. The definitions we propose should serve as a starting point for future validation studies.
Acknowledgments
Funding Source: This study was funded in part by an unrestricted research grant from GlaxoSmithKline to Brigham and Women's Hospital. Dr Shrank is supported by a career development award from the National Heart, Lung, and Blood Institute (K23HL090505-01). Dr Solomon receives salary support from the National Institutes of Health (K24AR055989).
Footnotes
Author Disclosure: Dr Choudhry reports receiving grants from Aetna, CVS/Caremark, and the Commonwealth Fund. Dr Shrank reports receiving grants from CVS/Caremark. Dr Brookhart reports receiving grants from Amgen. The other authors (RLL, JLL, SAJ, DHS) report no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (NKC, WHS, SAJ, MAB, DHS); acquisition of data (NKC, WHS, RLL, SAJ); analysis and interpretation of data (NKC, WHS, RLL, JLL, MAB, DHS); drafting of the manuscript (NKC, JLL); critical revision of the manuscript for important intellectual content (NKC, WHS, SAJ, MAB, DHS); statistical analysis (NKC, RLL); obtaining funding (NKC); administrative, technical, or logistic support (WHS, JLL); and supervision (NKC).
Contributor Information
Niteesh K. Choudhry, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women's Hospital, Harvard Medical School, Boston, MA.
William H. Shrank, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women's Hospital, Harvard Medical School, Boston, MA.
Raisa L. Levin, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women's Hospital, Harvard Medical School, Boston, MA.
Joy L. Lee, Divisions of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women's Hospital, Harvard Medical School, Boston, MA.
Saira A. Jan, Horizon Blue Cross Blue Shield of New Jersey, Newark, NJ.
M. Alan Brookhart, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women's Hospital, Harvard Medical School, Boston, MA.
Daniel H. Solomon, Division of Pharmacoepidemiology and Pharmacoeconomics, and Rheumatology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA.
References
- 1.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 2.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 3.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 4.Choudhry NK, Winkelmayer WC. Medication adherence after myocardial infarction: a long way left to go. J Gen Intern Med. 2008;23(2):216–218. doi: 10.1007/s11606-007-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee TH. Eulogy for a quality measure. N Engl J Med. 2007;357(12):1175–1177. doi: 10.1056/NEJMp078102. [DOI] [PubMed] [Google Scholar]
- 6.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 7.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records: description and validation. Med Care. 1988;26(8):814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 9.Choudhry NK, Setoguchi S, Levin R, Winkelmayer WC, Shrank WH. Trends in adherence to secondary prevention medications in elderly post-myocardial infarction patients. Pharmacoepidemiol Drug Saf. 2008;17(12):1189–1196. doi: 10.1002/pds.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman RH, Benner JS, Petrilla AA, et al. Predictors of adherence with antihypertensive and lipid-lowering therapy. Arch Intern Med. 2005;165(10):1147–1152. doi: 10.1001/archinte.165.10.1147. [DOI] [PubMed] [Google Scholar]
- 11.Chernew ME, Shah MR, Wegh A, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Aff (Millwood) 2008;27(1):103–112. doi: 10.1377/hlthaff.27.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care. 2008;46(11):1125–1133. doi: 10.1097/MLR.0b013e31817924d2. [DOI] [PubMed] [Google Scholar]