Abstract
Purpose
Development of new treatments is critical to effective protection against radiation-induced injury. Here we investigate the potential of developing small molecule inhibitors of GSK-3β, SB216763 or SB415286, as radioprotective agents to attenuate intestinal injury.
Methods and Materials
Survival study was done using C57/BL6 mice to evaluate radioprotective effect of GSK-3β inhibitors. TUNEL assay and immunohistochemical staining for Bax and Bcl-2 were used to assess apoptosis in small intestines of the treated mice. Clonogenic survival study, apoptosis assays (staining with Annexin V or DAPI), and immunoblot analysis of β-catenin, Bcl-2, Bax, and caspase-3 were done using IEC-6 cells.
Results
Pretreatment with SB415286 significantly improved survival of mice irradiated with 8 and 12 Gy. Mice pretreated with SB216763 or SB415286 showed significant reduction in TUNEL- and Bax-positive and an increase in Bcl-2-positive cells in intestinal crypts at 4 and/or 12 h after radiation with 4 and/or 8 Gy compared to radiation alone. Pretreatment of irradiated IEC-6 cells with GSK-3β inhibitors significantly increased clonogenic survival compared to cells treated with radiation alone. This increase was due to the attenuation of radiation-induced apoptosis, as demonstrated by Annexin V and DAPI assays, and immunoblot analysis of Bcl-2, Bax, and caspase-3.
Conclusion
GSK-3β small molecule inhibitors protect mouse intestines from radiation-induced damage in cell culture and in vivo and improve survival of mice. Molecular mechanisms of this protection involve attenuated radiation-induced apoptosis regulated by Bcl-2, Bax and caspase-3. Therefore, GSK-3β inhibitors reduce deleterious consequences of intestinal irradiation and thereby improve quality of life during radiation therapy.
Keywords: Ionizing irradiation, intestinal injury, radioprotection, GSK-3β inhibitors, apoptosis
Introduction
Patients undergoing pelvic radiotherapy will develop acute and chronic symptoms that impair quality of life (1). At least 50% of patients will develop bile salt malabsorption (2) and/or carbohydrate malabsorption (3). In gastrointestinal system, ionizing radiation causes a dose-dependent increase in apoptosis in the small intestinal crypts within hours of exposure (4-6). With a dose increasing beyond 1 Gy, this leads to a major denudation of the gastrointestinal mucosa (7, 8). Mice that receive higher doses of radiation die due to injury to the small intestine (7).
The sensitivity of the intestine to radiation is in part due to continuously renewing cells within the crypt of Lieberkühn (9, 10). Radiation-induced apoptosis occurs predominantly within the stem cell region. However, the molecular determinants of the intestinal radiosensitivity are not clearly understood. Several pathways have been implicated in regulating radiation-induced apoptosis in the crypt cells, including those of the tumor suppressor p53 (11, 12) and p53-dependent targets, such as PUMA (13), ATM (14) and Bcl-2 family proteins (15-17).
Molecular targeted radioprotectants could serve as pharmacologic prophylaxis to protect the normal tissues during radiotherapy of cancer patients. Various radioprotective strategies have been explored including compounds that scavenge free radical and modulate the DNA repair process, or growth factors and cytokines that function through receptor mediated mechanisms (18-20).
Glycogen-synthase kinase-3β (GSK-3β) signaling is a key regulator of radiation-induced apoptosis, and small molecule inhibitors of GSK-3β were shown to protect irradiated hippocampal neurons from apoptosis and improve cognitive performances in irradiated mice (21). GSK-3β belongs to a family of GSK-3, a multifunctional serine/threonine kinase which is implicated in multiple biological processes including embryonic development, cell differentiation, and apoptosis (22, 23). Direct overexpression of wild-type GSK-3β induces apoptosis in various cell types in culture, and specific inhibitors of GSK-3β are able to ameliorate apoptosis (21, 24-26). In addition, GSK-3β is a major component of Wnt signaling that plays an important role in the development and renewal of the intestinal epithelium by maintaining stem/progenitor cells and controlling migration and localization of epithelial cells along the crypt-villus axis (27). Specifically, GSK-3β phosphorylates β-catenin and targets it to ubiquitination and subsequent degradation, while β-catenin accumulation and activation promote cell cycle entry and progression of stem cells within intestinal crypts.
Here we study the protection of the small intestine from radiation-induced damage by specific small molecule inhibitors of GSK-3β, SB-216763 and SB-415286, which are structurally distinct maleimides that inhibit GSK-3α/β in vitro with Ki of 9 nM and 31 nM respectively, in an ATP competitive manner (28). To establish GSK-3β inhibitors as a new class of molecular targeted radioprotectors, we extend our studies from animal survival experiments to the study of putative molecular mechanisms of radioprotection of GSK-3β inhibitors in cell culture.
Methods and Materials
Chemicals
SB415286 (3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione) and SB216763 [3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione] were purchased from Tocris Biosciences.
Mice and treatment
All animal procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee. C57/BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Indicated doses of SB216763 and SB415286 dissolved in DMSO were administered to 10-week-old animals via intraperitoneal injection for 2 consecutive days. Whole-body irradiations were carried out using a Therapax DXT 300 X-ray machine (Pantak) delivering 2.04 Gy/min at 80 kVP. Mice were immobilized in a holder and irradiated with 4.0 Gy to 15.0 Gy.
Mouse survival study
Mice treated with DMSO or SB415286 and/or irradiated with 8 or 12 Gy were studied by the survival analysis. Each treatment group included 9-10 animals. Over the course of 30 days, mice were weighed daily and observed closely for the signs of premorbid state. These signs included hypoactivity, shallow, rapid and/or labored breathing, failure to groom, failure to respond to stimuli, hunched posture, dehydration and weight loss. Once these signs were present, mice were euthanatized. Surviving animals were euthanatized at the end of experiment (30th days after irradiation). Survival (%) was calculated using Kaplan-Meyer analysis. The average animal weight (+/- SD) was also calculated.
TUNEL assay and immunohistochemistry for Bax and Bcl-2
Mice were sacrificed at 4 and 12 h after irradiation by cervical dislocation under isoflurane anesthesia. The jejunum was fixed in 10% formalin, cut into 5 segments which were embedded vertically and sectioned. Five μm sections were placed on Superfrost Gold Plus slides (Erie Scientific, Portsmouth, NH).
For the TUNEL assay, tissue sections were stained as described previously (19). The average number of TUNEL-positive cells (TPC) per crypt (+/- SEM) was calculated. Crypts were identified by the presence of defined Paneth cells and 10 or more healthy looking chromophilic non-Paneth cells (29).
For immunohistochemical analysis, tissue sections were stained with antibody to Bax or Bcl-2 (SantaCruz Biotechnology, 1:100), counterstained with hematoxylin and eosin, and photographed under light microscopy as previously described (15).
Cell culture and treatment
Rat small intestine epithelium cells IEC-6 (CRL-1592) were obtained from ATCC and maintained in DMEM with 1.5 g/L sodium bicarbonate, 10% FBS, and 1% penicillin/streptomycin (Life Technologies, Gaithersburg, MD). Cells were treated with 10 μM SB216763 or 25 μM SB415286 in DMSO for 16 hours and then irradiated using Therapax DXT 300 X-ray machine (21).
Clonogenic survival
Colony-forming assay and clonogenic survival analysis were performed as previously described (26). Radiation doses of 0, 2, 4, 6 or 8 Gy were used.
Apoptosis assays for cultured cells
Apoptosis was determined by Annexin V-APC/propidium iodide staining using Apoptosis Detection Kit (BD PharMingen, San Diego, CA) as previously described (19, 21, 26). Alternatively, apoptotic nuclei were counted after 4′,6-diamidino-2-phenylindole (DAPI) staining as previously described (19, 21, 26).
Western immunoblot analysis
Cells were lysed and subjected to Western immunoblot analysis as previously described (19, 21, 26). Antibodies for the detection of β-catenin, caspase-3 (Cell Signaling Technologies, Danvers, MA), Bcl-2, Bax (SantaCruz Biotechnology), and actin (Sigma, St. Louis, MO) were used. Relative protein levels were determined by densitometry using Image Quant TL (Amersham Biosciences), normalized to actin and calculated as the ratio of treated samples to sham-irradiated controls.
Statistical analyses
The mean and standard error of the mean (SEM) of each treatment group were calculated for all experiments. The number of samples is indicated in the description of each experiment. Statistical analysis was performed using Kruskal-Wallis One Way Analysis of Variance (ANOVA). All pairwise comparison procedures including calculation of P value were done using Student-Newman-Keuls method. A P value of <0.05 was considered significant.
Results
GSK-3β inhibition increases survival of mice treated with whole body radiation
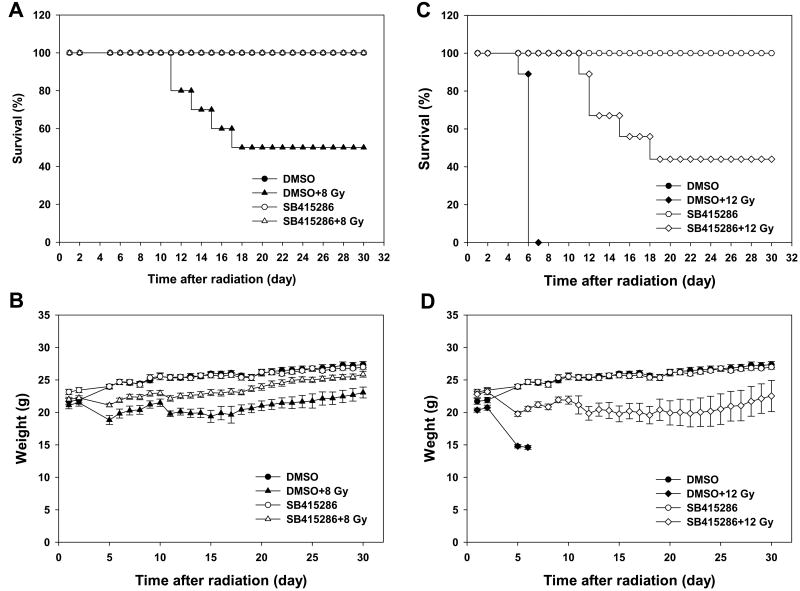
To determine whether GSK-3β is a molecular target for radiation protecting drugs, we performed animal survival analysis. Ten week-old mice were treated with 1.0 mg/kg of SB415286 followed by a single dose of 8 or 12 Gy whole body radiation. Treatment with SB415286 protected mice from the 50% lethal dose of 8 Gy (100% and 50%, survival, DMSO+8 Gy and SB415286+8 Gy, respectively, Fig. 1A) and the 100% lethal dose of 12 Gy (44% and 0%, survival, DMSO+12 Gy and SB415286+12 Gy, respectively, Fig. 1C). At both doses, radiation-induced animal death correlated closely with weight loss (5 to 10% per day, Fig. 1B and D).
Fig. 1. GSK-3β inhibition improves survival of irradiated mice.
Ten week-old C57/BL6 mice were treated with daily IP injections of SB415286 (1.0 mg/kg) or DMSO for 2 days followed by 8 Gy or 12 Gy whole body irradiation. Mice were weighed daily and observed for the signs of pre-morbid state. Shown are Kaplan-Meyer survival curves for 8 Gy (A) and 12 Gy (C) and graphs of the average animal weight for 8 Gy (B) and 12 Gy (D) with SD for each treatment group (9-10 mice).
GSK-3β inhibition protects intestinal crypt cells from radiation-induced apoptosis
Studies of whole body-irradiated mice demonstrated that radiation-induced apoptosis in small intestine cells results in animal death (4, 6, 30, 31). To determine whether GSK-3β inhibition could affect radiation-induced apoptosis in intestinal crypt cells, we treated 10 week-old mice with 0.6 mg/kg of SB216763 or 1.0 mg/kg of SB415286 followed by a single dose of 4 or 8 Gy radiation. Four and 12 h later the proximal jejunum was analyzed using TUNEL staining. At 12 h after irradiation, mice pretreated with SB216763 or SB415286 showed significantly less TPC per crypt at both 4 Gy (1.5 and 1.7 TPC, Fig. 2A, B) and 8 Gy (2.7 and 2.4 TPC, Fig. 2A, B) as compared to radiation alone (5 and 6.7 TPC, 4 and 8 Gy, respectively, Fig. 2A, B). Similar results were observed at the earlier time after irradiation, 4 h (0.5, 1.2 and 4.2 TPC, SB216763+8 Gy, SB415286+8 Gy and 8 Gy alone, respectively, Fig. 2C, D).
Fig. 2. GSK-3β inhibitors protect small intestine epithelium in vivo from radiation-induced apoptosis.
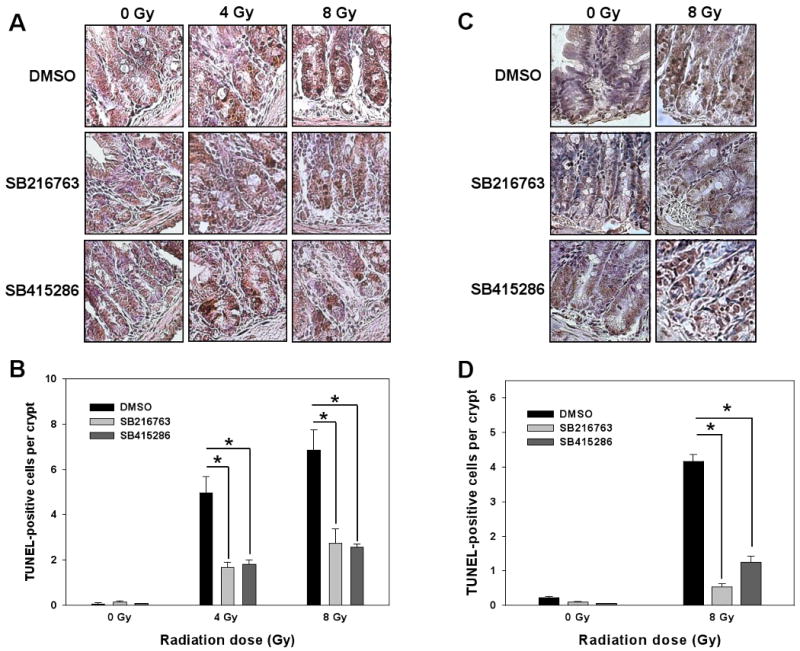
Ten week-old C57/BL6 mice were treated with daily IP injections of SB216763 (0.6 mg/kg), SB415286 (1.0 mg/kg) or DMSO for 2 days followed by 4 Gy or 8 Gy of whole body irradiation. Twelve (A, B) or 4 h (C, D) later, proximal jejunum was fixed, sectioned and stained using TUNEL kit. Shown are representative micrographs of TUNEL staining (A, C) and bar graphs of the average numbers of TUNEL-positive cells per crypt in each treatment group with SEM from three experiments; *, P < 0.05 (B, D).
GSK-3β inhibition increases survival of irradiated IEC-6 cells due to attenuated apoptosis
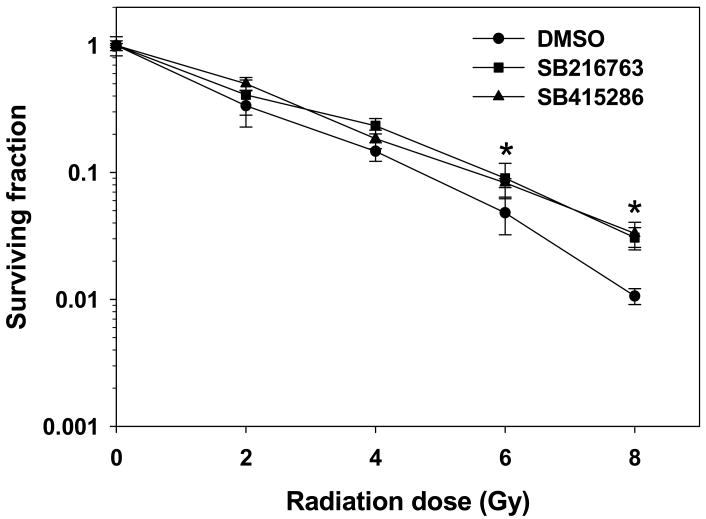
To determine the role of GSK-3β in cell viability, we examined the effects of GSK-3β inhibitors on clonogenic survival of irradiated IEC-6 cells, a non-transformed crypt cell line derived from rat small intestine (32). Pretreatment of IEC-6 cells with 10 μM SB216763 or 25 μM SB415286 for 16 h before irradiation significantly increased cell survival as compared to cells treated with radiation alone (Fig. 3).
Fig. 3. GSK-3β inhibition increases clonogenic survival of irradiated IEC-6 cells.
IEC-6 cells were treated with DMSO (●), 10 μM SB216763 (■) or 25 μM SB415286 (▲) for 16 h followed by irradiation with 0, 2, 4, 6 and 8 Gy and plated for clonogenic survival assay. Shown are the surviving fractions and SEM from three experiments; *, P<0.05.
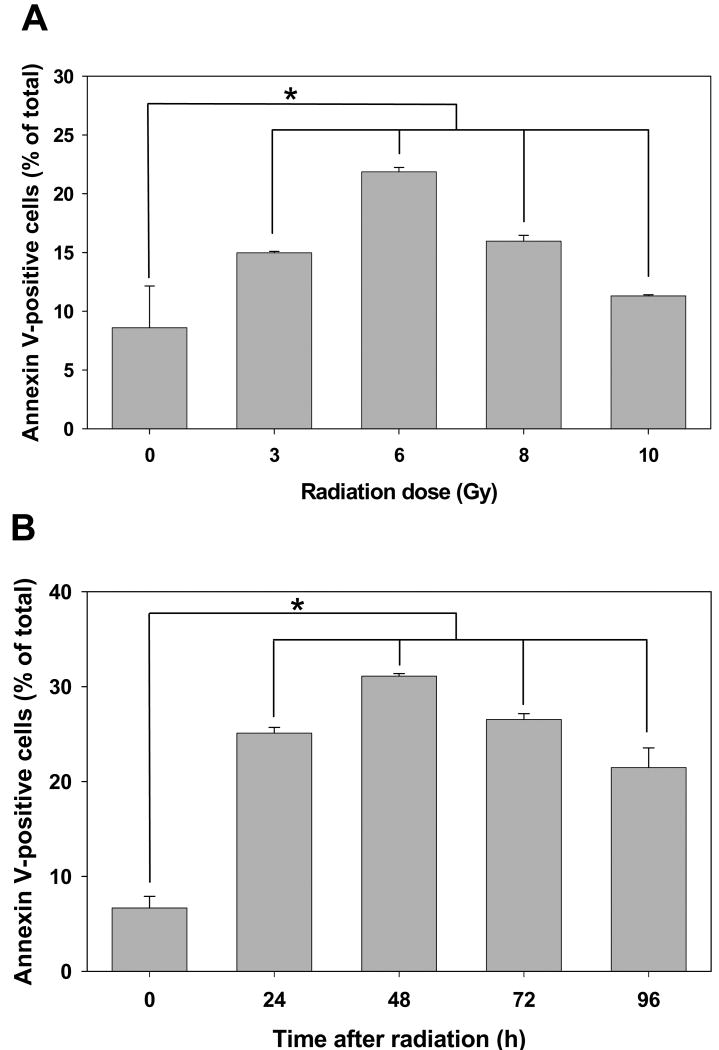
To study the mechanism of increased survival in small intestine epithelial cells, we analyzed apoptosis by Annexin V-APC and propidium iodide staining in flow cytometry assay. We determined that effect of radiation on the level of apoptosis in IEC-6 cells is dose- and time-dependent (Fig. 4). We determined that maximal apoptosis occurred in cells 48 h after treatment with a radiation dose of 6 Gy (Fig. 4). Since we did not observe dramatic changes in apoptosis from 24 to 72 h (Fig. 4B), the optimal dose and time to evaluate effect of GSK-3β inhibitors on radiation-induced apoptosis in IEC-6 cells were 6 Gy and 24 h, respectively (Fig. 4).
Fig. 4. Radiation induces apoptosis in IEC-6 cells in a time- and dose-dependent manner.
IEC6 cells were treated with various doses of radiation (A) or with 6 Gy (B). Cells were collected at 24 h (A) or at various time points (B) after irradiation, stained with Annexin V-APC/propidium iodide and analyzed by flow cytometry. Shown are bar graphs of the average percent of Annexin V-positive cells vs. total cell number for each treatment with SEM from three experiments; *, P<0.05.
To determine whether GSK-3β inhibitors regulate apoptosis in irradiated IEC-6 cells, cells were treated with 25 μM SB415286 for 16 h followed by radiation with 6 Gy. The cells were analyzed for apoptosis by Annexin V-APC assay 24 h post irradiation. This assay was applied only for the cells treated with SB415286 because SB216763 showed the artifact of fluorescence similar to that of APC. IEC-6 cells showed significantly less radiation-induced apoptosis when treated with SB415286 (8.23%) as compared to cells treated with vehicle DMSO (19.93%) (Fig. 5A, B). We further evaluated the nuclear morphology of dying cells using DAPI staining (Fig. 5C). Irradiated IEC-6 cells pretreated with GSK-3β inhibitors demonstrated a protective effect with reduced number of apoptotic cells to 4.6% (SB216763) and 3.62% (SB415286) as compared to 28.53 % in DMSO-pretreated irradiated cells (Fig. 5D).
Fig. 5. GSK-3β inhibitors attenuate radiation-induced apoptosis in IEC-6 cells.
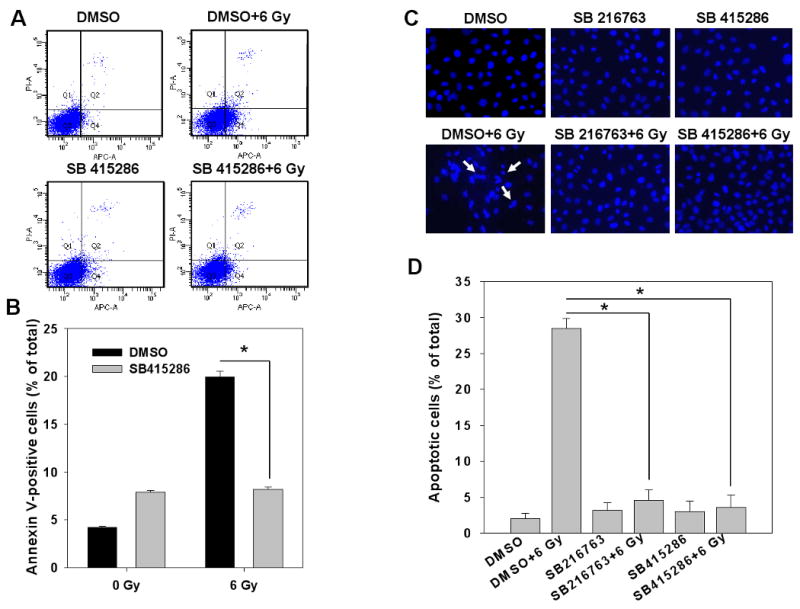
IEC6 cells were treated with DMSO, 10 μM SB216763 or 25 μM SB415286 16 h prior to irradiation with 6 Gy. At 24 h after irradiation, cells were stained with Annexin V-APC/propidium iodide and analyzed by flow cytometry (A, B). Alternatively, cells were fixed and stained with DAPI, and apoptotic cells indicated by arrows were counted in multiple randomly selected fields (C, D). Shown are representative diagrams of distribution of stained cells (A), representative micrographs (C) and bar graphs of the average percent of apoptotic cells vs. total cell number for each treatment with SEM from three experiments; *, P<0.05 (B, D).
GSK-3β inhibitors prevent radiation-induced increase in Bax and caspase-3 cleavage, while up-regulating Bcl-2
To analyze effects of radiation on the GSK-3β-dependent apoptotic pathway, IEC-6 cells were treated with 10 μM SB216763 and 25 μM SB415286 for 16 h followed by irradiation with 6 Gy. The cells were lysed 6 h after irradiation and the lysates were studied by Western immunoblot analysis. Level of expression of β-catenin was used as a marker of GSK-3β activity. Since GSK-3β phosphorylates β-catenin and promotes its degradation (33), the increased accumulation of β-catenin correlates with decreased activity of GSK-3β. Treatment of IEC-6 cells with both GSK-3β inhibitors SB216763 and SB415286 showed significant accumulation of β-catenin protein as compared to controls cells (1.8- to 2.2-fold increase, Fig. 6A).
Fig. 6. GSK-3β inhibitors prevent radiation-induced changes in pro-apoptotic proteins Bax and caspase-3 and activate anti-apoptotic protein Bcl-2.

A. IEC-6 cells were pretreated with DMSO, 10 μM SB216763 or 25 μM SB415286 for 16 h, then irradiated with 6 Gy and harvested 6 h later. Cellular proteins were immunoblotted using antibodies to β-catenin, Bcl-2, Bax, and Caspase-3. Actin was used to evaluate protein loading in each lane. Relative protein levels were determined by densitometry, normalized to actin and calculated as the ratio of treated samples to sham-irradiated DMSO-pretreated controls. B. Ten week-old C57/BL6 mice were treated with daily IP injections of SB216763 (0.6 mg/kg), SB415286 (1.0 mg/kg) or DMSO for 2 days followed by 8 Gy irradiation. Four h later, proximal jejunum was fixed, sectioned and stained using antibodies to Bax and Bcl-2. Shown are representative micrographs.
Upon examination of expression of Bcl-2 family proteins, IEC-6 cells treated with 6 Gy showed moderate increase of 1.5-fold in pro-apoptotic protein Bax as compared to control (Fig. 6A). In addition, radiation-induced cleavage of caspase-3 (17 kD and 12 kD) was observed (Fig. 6A). These changes were abrogated in irradiated IEC-6 cells when pretreated with SB216763 and SB415286 (Fig. 6A). Moreover, GSK-3β inhibition in irradiated IEC-6 cells led to an increased level of anti-apoptotic protein Bcl-2 (2.67- and 4.04-fold increase over control, SB216763 and SB415286, respectively, Fig. 6A) that was also sustained after combined treatment with radiation (2.15- and 3.13-fold increase, SB216763 and SB415286, respectively, Fig. 6A).
We extended analysis of the expression of Bcl-2 family proteins to in vivo study and observed similar results. Immunohistochemical staining of mouse small intestinal epithelium revealed increased positivity for expression of pro-apoptotic protein Bax in irradiated animals compared to sham-irradiated DMSO-pretreated controls (brown staining, top panel, Fig. 6B). Pretreatment with GSK-3β inhibitors prevented this increase (brown staining, top panel, Fig. 6B). On the contrary, radiation did not affect expression of anti-apoptotic protein Bcl-2, while GSK-3β inhibition led to an increase in Bcl-2 positive staining in intestinal crypt cells (brown staining, bottom panel, Fig. 6B).
Discussion
Intestinal injury is a limiting factor in abdominal and pelvic radiotherapy. In the present study, we explore the possibility of developing small molecule inhibitors of GSK-3β as radioprotectors against intestinal injury. In the survival study, we have demonstrated a radioprotective effect of SB415286 at both the 100% lethal (12 Gy) and 50% lethal (8 Gy) doses. The dose-modifying factor (DMF, the fold change in irradiation dose lethal for 50% of animals) of SB415286 in C57Bl6 mice for 30-day survival was 1.5. Upon whole body radiation with 12 Gy, the animal deaths occurred within 5-8 days after exposure (less than 10 days) indicating involvement of mixed hematopoietic/gastrointestinal injury (34, 35); while mortality caused by 8 Gy (deaths happened within 11-18 after exposure, greater than 10 days) is primarily due to the bone marrow failure (34). Interestingly, mice treated with SB415286 before radiation with 12 Gy showed a delayed death (greater than 10 days) suggesting that this mortality may be, in part, due to complications from hematopoietic insufficiency after irradiation.
In the small intestine, proliferation occurs in continuously renewing cells near the base of the crypt (9, 10). Cells migrate up the crypts and give rise to terminally differentiated cells that populate the villi (36). The crypt cells proliferation is associated with sensitivity to radiation (7). Normal and healthy small intestinal epithelium undergo spontaneous apoptosis; however, the number of apoptotic cells is very low and relatively constant over time. Even small doses of radiation can induce elevated apoptosis in the continuously renewing cells in the crypts. The number of apoptotic cells increases with increasing doses of radiation (10). In the present study, we also observed a dose-dependent increase in apoptosis with increasing doses of radiation. Importantly, GSK-3β small molecule inhibitors tested in our study, SB415286 and SB216763, protected stem cells in intestinal crypts from radiation-induced apoptosis at late (12 h after radiation) and at early (4 h after radiation) time points. It was demonstrated that p53-deficiency prevents early apoptosis in the crypt cells, occurring 3–6 h following irradiation, but leads to accelerated animal death (11, 12). This suggests that radioprotective effect of GSK-3β inhibitors could utilize the p53 pathway but is not limited to this pathway. In addition to protection from early apoptosis, GSK-3β inhibitors decreased apoptosis at 12 h after irradiation and, most importantly, increased survival of irradiated mice.
GSK-3β has been shown to induce apoptosis in a wide variety of conditions including DNA damage (37), hypoxia (38) and stress of the endoplasmic reticulum (39). Direct over-expression of wild-type GSK-3β is known to induce apoptosis in various cell types in culture, and specific inhibitors of GSK-3β are able to ameliorate this apoptotic process (24-26). In our earlier study, over-expression of a kinase inactive mutant of GSK-3β attenuated apoptosis in hippocampal neurons (21). In the present study, irradiated small intestine epithelial IEC-6 cells pretreated with GSK-3β inhibitors SB216763 or SB415286 showed an increased survival as compared to cells treated with radiation alone. Moreover, one of the mechanisms of this radioprotection was an abrogation of radiation-induced apoptosis.
GSK-3β was shown to promote apoptosis by inhibiting pro-survival transcription factors, such as CREB and heat shock factor-1 (23), and by facilitating pro-apoptotic transcription factors, such as p53 (37) and Bcl-2 family proteins (15-17). In regulation of apoptotic response, mammalian cells employ multiple pro-survival proteins from Bcl-2 family (Bcl-2, Bcl-XL, Bcl-w, Mcl1 and A1) that antagonize the pro-apoptotic function of Bax and Bak (40). Bax and Bak localize on the mitochondrial outer membrane and trigger death signals leading to cytochrome c release to the cytosol (40, 41). Apoptosis requires a group of effector caspases to dismantle the cells. Cytochrome c activates caspase-9 which subsequently activates caspase-3 (42). The activation of caspase-3 is an essential step leading to cleavage of the DNA repair enzyme, poly (ADP-ribose) polymerase (PARP), resulting in genomic DNA fragmentation. We observed that radiation increased both Bax protein level and cleavage (activation) of caspase-3 in IEC-6 cells and that this effect was abrogated by GSK-3β inhibitors. This observation suggests that radiation-induced apoptosis in intestinal epithelial cells involves the Bax/caspase-3 pathway and that GSK-3β regulates the activation of this pathway.
The anti-apoptotic protein Bcl-2 blocks caspase-3 activation by preventing cytochrome c release. A definitive role of Bcl-2 in regulating intestinal apoptosis has been demonstrated by increased epithelial apoptotic index in the small intestine of Bcl-2-null mice (17). In the present study, we found that, in addition to inhibition of radiation-induced increase of Bax in intestinal epithelial cells, GSK-3β inhibitors also increased the levels of anti-apoptotic protein Bcl-2. These data suggest that the anti-apoptotic effect of GSK-3β inhibitors is mediated, in part, by Bcl-2 and Bax levels.
Alternatively or additively, the radioprotective effect of GSK-3β inhibitors could be transduced through Wnt signaling that plays one of the central roles in the renewal of the damaged intestinal epithelium (27). Inhibition of GSK-3β could prevent phosphorylation of β-catenin that targets it for proteasomal degradation. This allows for the activation of β-catenin-dependent transcription factors that promote renewal, division and progression of stem cells within intestinal crypts.
Conclusion
In previous studies, we found that GSK-3β signaling is a key regulator of radiation-induced damage in hippocampal neurons and that small molecule inhibitors of GSK-3β effectively protect irradiated hippocampal neurons from apoptosis and improve cognitive performance in irradiated mice (21, 26). In the present study, we show that the small molecule inhibitors of GSK-3β prevent radiation-induced death in mouse intestine by reducing apoptosis of the epithelial cells of the crypts and dramatically increase animal survival. Cell culture experiments demonstrated that protective effect of GSK-3β inhibitors is associated with elevation of the anti-apoptotic protein Bcl-2 and abrogation of radiation-induced increase of Bax and cleavage of caspase-3. Therefore, small molecule inhibitors of GSK-3β may serve as a new class of radioprotectors and, in addition to the earlier reported radioprotection of normal hippocampal neurons, they could have a therapeutic role in protecting the intestine from radiation injury.
Acknowledgments
Financial support: NIH Grants R01-CA125757, R01-CA89674, R01-CA125656 and R01-CA115556
Footnotes
Meeting presentation: Poster presented at the 50th Annual Meeting of American Society for Therapeutic Radiology and Oncology (ASTRO), Boston, MA, September 21-25, 2008
Conflict of interest notification: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol. 2007;8(11):1007–1017. doi: 10.1016/S1470-2045(07)70341-8. [DOI] [PubMed] [Google Scholar]
- 2.Arlow FL, Dekovich AA, Priest RJ, et al. Bile acids in radiation-induced diarrhea. South Med J. 1987;80(10):1259–1261. doi: 10.1097/00007611-198710000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Weiss RG, Stryker JA. 14C-lactose breath tests during pelvic radiotherapy: the effect of the amount of small bowel irradiated. Radiology. 1982;142(2):507–510. doi: 10.1148/radiology.142.2.7054844. [DOI] [PubMed] [Google Scholar]
- 4.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 5.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269(5628):518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 6.Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320(5873):226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol. 1990;58(6):925–973. doi: 10.1080/09553009014552281. [DOI] [PubMed] [Google Scholar]
- 8.Anno GH, Baum SJ, Withers HR, et al. Symptomatology of acute radiation effects in humans after exposure to doses of 0.5-30 Gy. Health Phys. 1989;56(6):821–838. doi: 10.1097/00004032-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161(2):123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- 10.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997;78(4):219–243. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merritt AJ, Potten CS, Kemp CJ, et al. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994;54:614–617. [PubMed] [Google Scholar]
- 12.Komarova EA, Kondratov RV, Wang K, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23:3265–3271. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]
- 13.Qiu W, Carson-Walter EB, Liu H, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ch'ang HJ, Maj JG, Paris F, et al. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med. 2005;11(5):484–490. doi: 10.1038/nm1237. [DOI] [PubMed] [Google Scholar]
- 15.Przemeck SM, Duckworth CA, Pritchard DM. Radiation-induced gastric epithelial apoptosis occurs in the proliferative zone and is regulated by p53, bak, bax, and bcl-2. Am J Physiol Gastrointest Liver Physiol. 2007;292(2):G620–627. doi: 10.1152/ajpgi.00391.2006. [DOI] [PubMed] [Google Scholar]
- 16.Rotolo JA, Maj JG, Feldman R, et al. Bax and Bak do not exhibit functional redundancy in mediating radiation-induced endothelial apoptosis in the intestinal mucosa. Int J Radiat Oncol Biol Phys. 2008;70(3):804–815. doi: 10.1016/j.ijrobp.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 17.Pritchard DM, Potten CS, Korsmeyer SJ, et al. Damage-induced apoptosis in intestinal epithelia from bcl-2-null and bax-null mice: investigations of the mechanistic determinants of epithelial apoptosis in vivo. Oncogene. 1999;18(51):7287–7293. doi: 10.1038/sj.onc.1203150. [DOI] [PubMed] [Google Scholar]
- 18.Maisin JR. Bacq and Alexander Award lecture--chemical radioprotection: past, present, and future prospects. Int J Radiat Biol. 1998;73:443–450. doi: 10.1080/095530098142284. [DOI] [PubMed] [Google Scholar]
- 19.Thotala D, Chetyrkin S, Hudson B, et al. Pyridoxamine protects intestinal epithelium from ionizing radiation-induced apoptosis. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.06.020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann N Y Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 21.Thotala DK, Hallahan DE, Yazlovitskaya EM. Inhibition of glycogen synthase kinase 3 beta attenuates neurocognitive dysfunction resulting from cranial irradiation. Cancer Res. 2008;68(14):5859–5868. doi: 10.1158/0008-5472.CAN-07-6327. [DOI] [PubMed] [Google Scholar]
- 22.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65(4):391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 24.Bijur GN, De Sarno P, Jope RS. Glycogen synthase kinase-3beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. The Journal of biological chemistry. 2000;275(11):7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez JF, Sniderhan LF, Williamson ALF, et al. Glycogen synthase kinase 3beta-mediated apoptosis of primary cortical astrocytes involves inhibition of nuclear factor kappaB signaling. Mol Cell Biol. 2003;23(13):4649–4662. doi: 10.1128/MCB.23.13.4649-4662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yazlovitskaya EM, Edwards E, Thotala D, et al. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66(23):11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 27.Scoville DH, Sato T, He XC, et al. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 28.Coghlan MP, Culbert AA, Cross DAE, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chemistry & Biology. 2000;7(10):793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 29.Potten CS, Hendry JH. Cell clones: manual of mammalian cell techniques. Edinburgh (NY): Churchill Livingstone; 1985. [Google Scholar]
- 30.Potten CS, Wilson JW, Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells. 1997;15:82–93. doi: 10.1002/stem.150082. [DOI] [PubMed] [Google Scholar]
- 31.Potten CS. Protection of the small intestinal clonogenic stem cells from radiation-induced damage by pretreatment with interleukin 11 also increases murine survival time. Stem Cells. 1996;14:452–459. doi: 10.1002/stem.140452. [DOI] [PubMed] [Google Scholar]
- 32.Quaroni A, Wands J, Trelstad RL, et al. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979;80(2):248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JR, Moon RT. Signal transduction through beta-catenin and specification of cell fate during embryogenesis. Genes & development. 1996;10(20):2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- 34.Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 35.Mason KA, Withers HR, McBride WH, Davis, et al. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat Res. 1989;117:480–488. [PubMed] [Google Scholar]
- 36.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974;141(4):461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- 37.Watcharasit P, Bijur GN, Zmijewski JW, et al. Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage. Proc Natl Acad Sci U S A. 2002;99(12):7951–7955. doi: 10.1073/pnas.122062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loberg RD, Vesely E, Brosius FC., 3rd Enhanced glycogen synthase kinase-3beta activity mediates hypoxia-induced apoptosis of vascular smooth muscle cells and is prevented by glucose transport and metabolism. The Journal of biological chemistry. 2002;277(44):41667–41673. doi: 10.1074/jbc.M206405200. [DOI] [PubMed] [Google Scholar]
- 39.Song L, De Sarno P, Jope RS. Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. The Journal of biological chemistry. 2002;277(47):44701–44708. doi: 10.1074/jbc.M206047200. [DOI] [PubMed] [Google Scholar]
- 40.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 41.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7(12):989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 42.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]