Abstract
Sepsis is a complex, incompletely understood and often fatal disorder,1 typically accompanied by hypotension,2 that is considered to represent a dysregulated host response to infection.3,4,5 Neurotensin (NT) is a 13-amino-acid peptide that, among its multiple effects, induces hypotension.6 We find that intraperitoneal and plasma concentrations of NT are increased in mice after severe caecal ligation and puncture (CLP), a model of sepsis, and that mice treated with a pharmacological antagonist of NT, or NT-deficient mice, exhibit reduced mortality during severe CLP. In mice, mast cells can degrade NT and reduce NT-induced hypotension and CLP-associated mortality, and optimal expression of these effects requires mast cell expression of neurotensin receptor 1 and neurolysin. These findings show that NT contributes to sepsis-related mortality in mice during severe CLP and that mast cells can lower NT concentrations, and suggest that mast cell-dependent reduction in NT levels contributes to the ability of mast cells to enhance survival after CLP.
INTRODUCTION
Sepsis is the most common cause of death in intensive care units in the United States.4 The host response to sepsis is complex, yet the factors responsible for the pathology and death associated with sepsis are not fully understood.3–5,7 Moreover, there is significant interest in identifying additional biomarkers and therapeutic targets in this disorder.8,9
NT is a tridecapeptide that exerts potent effects in the central nervous system and the periphery.6 There are two G protein-coupled receptors for NT, the high affinity receptor, Ntsr1, and the low affinity receptor, Ntsr2.10,11 Ntsr3, originally identified as the intracellular sorting protein sortilin12, is a non-G protein coupled receptor that binds NT with high affinity but is of unknown function.6 Although NT can induce hypotension,13 a common problem in sepsis,2 the potential contributions of NT to the pathology of sepsis have not previously been investigated.
We provide pharmacological and genetic evidence that NT can contribute to mortality in mice subjected to severe caecal ligation and puncture (CLP), a model of acute septic peritonitis.14 Mast cells (MCs) can be activated by several mechanisms in CLP and can promote survival in this setting,15,16,17 and both TNF,18,19,20 and mouse MC protease-6 (mMCP-6)21 have been reported to reduce mortality in CLP19,20 and/or other models of infection.18,21 Because certain MC-derived proteases can degrade NT,22,23 we hypothesized that an additional mechanism by which MCs can promote survival in CLP is by reducing levels of NT. We now report evidence that strongly supports that hypothesis.
RESULTS
NT contributes to mortality in sepsis
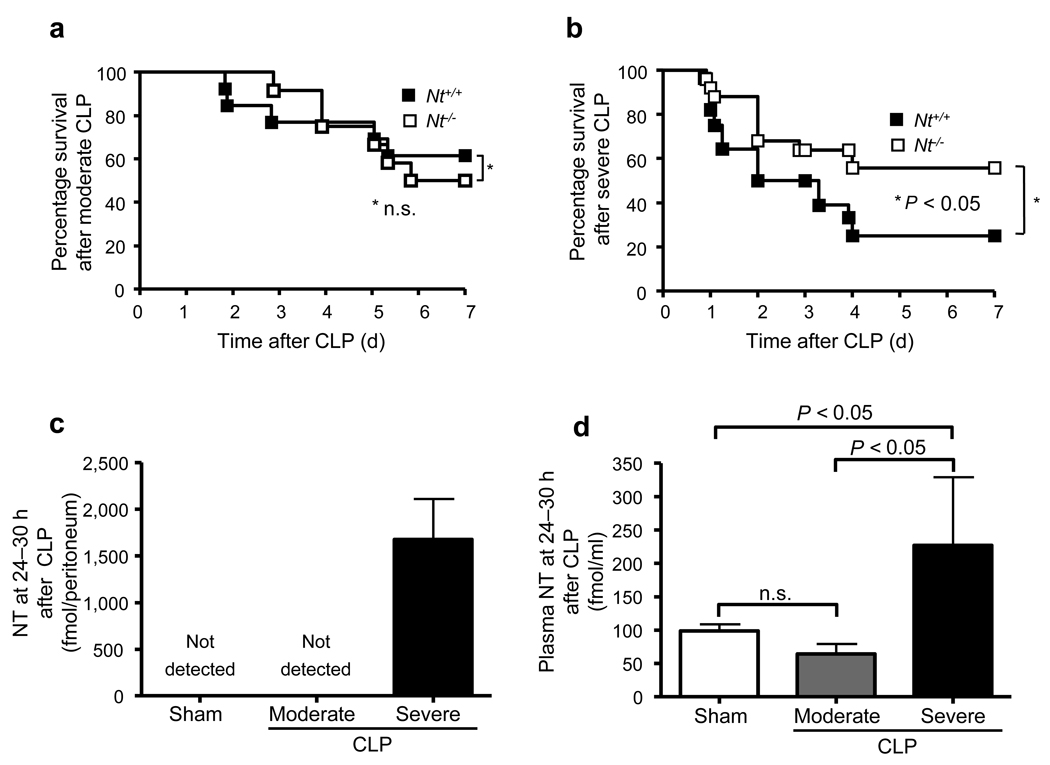
Nt−/− mice resemble wild type (WT) mice in general appearance, gross anatomy, body weight, reproduction and overt behavior.24 Nt+/+ and Nt−/− mice were similar in % of MCs in the peritoneal cavity (2.1 ± 0.1% or 1.7 ± 0.2% of cells in peritoneal lavage fluid), distribution of MCs in the mesentery (Supplementary Fig. 1), and survival after moderate CLP (P = 0.74) (Fig. 1a). However, after severe CLP, Nt−/− mice exhibited significantly higher systemic blood pressure (Supplementary Fig. 2a) and enhanced survival (56% vs. 25% survival, at 1 week after CLP, P < 0.05) (Fig. 1b) compared to Nt+/+ mice.
Figure 1. NT levels are increased and contribute to mortality after CLP in mice.
(a) Survival after moderate CLP (ligation of distal half of caecum; one puncture with a 22G needle) in 8–12-week old female or male wild type (Nt+/+) (n = 13) or NT-deficient (Nt−/−) (n = 12) mice. (b) Survival after severe CLP (ligation of distal half of caecum; one puncture with a 20G needle) in 8–12-week old female or male wild type (Nt+/+) (n = 28) or NT-deficient (Nt−/−) (n = 25) mice. Data in a and b were pooled from the two or five experiments performed, respectively, each of which gave similar results. (c, d) Amounts of NT in the peritoneal lavage fluid (c) and plasma concentrations of NT (d) at 24–30 h after induction of moderate or severe CLP in 8–12-week old female or male C57BL/6 wild type (Nt+/+) (n = 3–9/group).
Higher levels of NT were detected in the peritoneal cavity and plasma of C57BL/6 mice in severe vs. moderate CLP (Fig. 1c and 1d). As previously reported,17 endothelin-1 (ET-1) was also increased in the peritoneal fluid of C57BL/6 mice after CLP (87.2 ± 13.2 [n = 10] vs. 18.0 ± 1.0 [n = 4] 18 h after severe CLP vs. sham operation, respectively, P < 0.002). Compared to WT mice, survival after severe CLP was significantly enhanced in Ntsr1−/− but not Ntsr2−/− mice (Supplementary Fig. 2b), indicating that Ntsr1 is more important than Ntsr2 in the pathways by which NT promotes pathology in this setting.
Mast cells regulate NT levels during sepsis
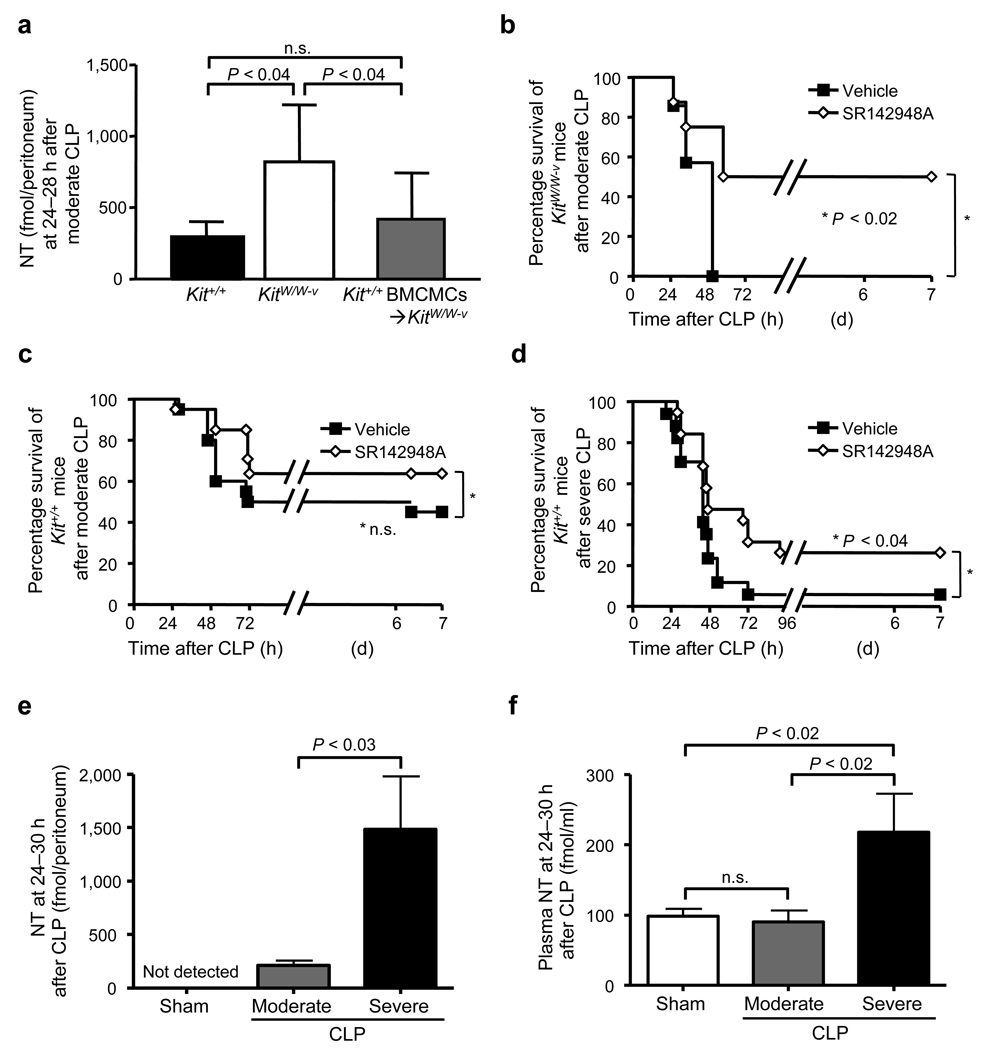
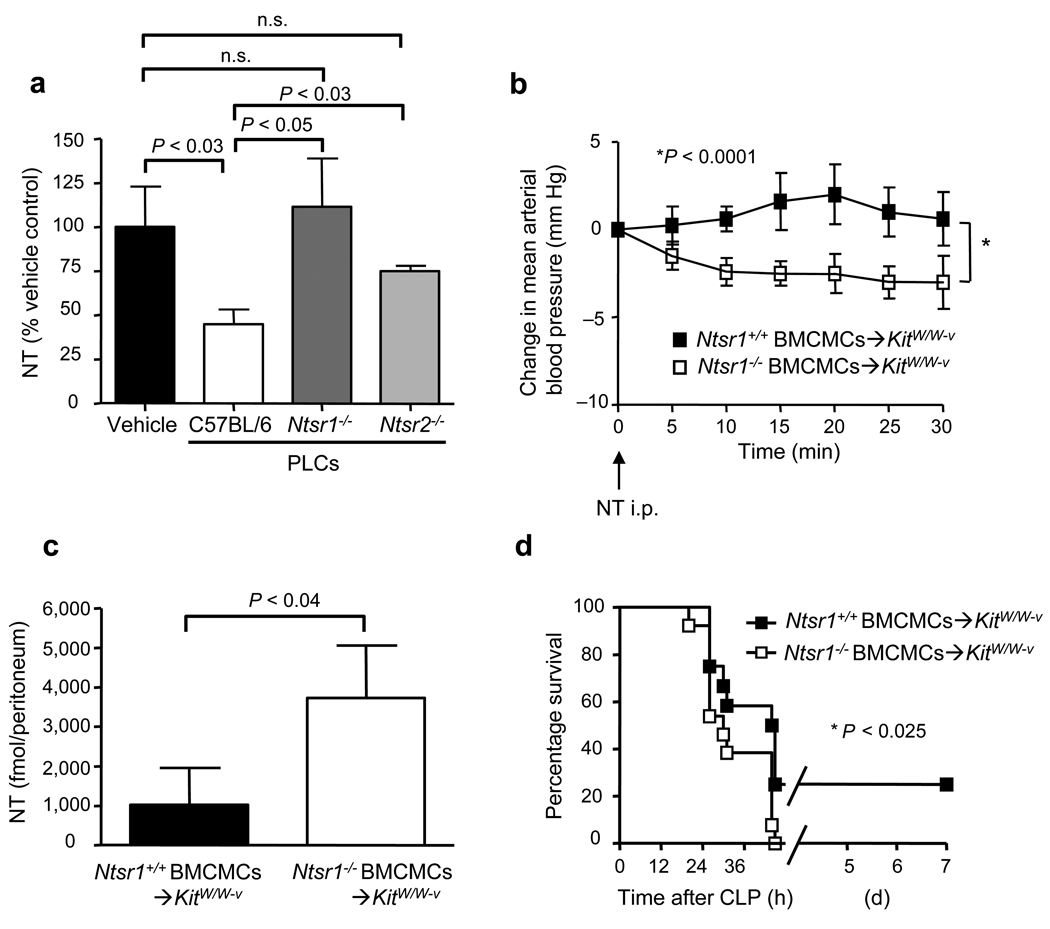
MCs can promote survival in mice subjected to CLP,19 and there is evidence that MC activation by ET-1 can contribute to both MC-dependent reduction in ET-1 in the peritoneal cavity and enhanced survival in CLP.17 Certain MC populations can also be activated by NT in vitro.25,26 Amounts of NT in the peritoneal cavity were significantly higher in genetically MC-deficient KitW/W-v mice vs. congenic normal (Kit+/+) mice 24–28 h after moderate CLP (Fig. 2a).
Figure 2. Evidence that NT can contribute to mortality after CLP in WT or KitW/W-v MC-deficient mice.
(a) Amounts of NT in the peritoneal lavage fluid at 24–28 h after moderate CLP (ligation of distal half of caecum; one puncture with a 22G needle) in wild type (Kit+/+) (n = 18), KitW/W-v mast cell [MC]-deficient (n = 15) or Kit+/+ MC-engrafted KitW/W-v (Kit+/+ BMCMCs➔KitW/W-v) (n = 6) mice. (b, c) Survival in 12-week old female KitW/W-v mast cell deficient (n = 7–8) (b) or wild type (Kit+/+) mice (n = 20) (c) after moderate CLP. (d) Survival in 12-week old female wild type (Kit+/+) mice (n = 20) after severe CLP (ligation of distal 2/3rds of caecum; one puncture with a 20G needle). In b–d, mice received two i.p. injections of SR142948A (a non-selective antagonist of Ntsr1 and Ntsr2; 100 µg/kg in 200 µl of 0.01% Tween 80 in saline [Vehicle]), or 200 µl of Vehicle, 1 h before and 8 h after CLP. Data in b–d were pooled from the three experiments performed, each of which gave similar results. (e, f) Amounts of NT in the peritoneal lavage fluid (e) and plasma concentrations of NT (f) at 24–30 h after induction of moderate CLP (ligation of the distal half of the caecum; one puncture with a 22G needle) or severe CLP (ligation of the distal 2/3 of the caecum; one puncture with a 20G needle) in 12-week old female Kit+/+ mice) (n = 3–9/group).
To assess the extent to which this difference reflected the MC deficiency of KitW/W-v mice, as opposed to other consequences of their c-kit mutations, we analyzed KitW/W-v mice selectively engrafted i.p. with Kit+/+ bone marrow-derived cultured MCs (BMCMCs) (Kit+/+ BMCMCs➔KitW/W-v mice).27 Amounts of NT in the peritoneal cavity after moderate CLP in Kit+/+ BMCMCs➔KitW/W-v mice were very similar to those in Kit+/+ mice (Fig. 2a).
Treatment with SR142948A, a non-selective antagonist of Ntsr1 and Ntsr2,28 significantly improved survival in KitW/W-v mice subjected to moderate CLP (50% survival 1 week after CLP vs. all vehicle-treated mice dead by 48 h, P < 0.02) (Fig. 2b). Survival of Kit+/+ mice subjected to moderate CLP was not significantly influenced by treatment with SR142948A (P = 0.15) (Fig. 2c). However, SR142948A treatment significantly enhanced survival of Kit+/+ mice after severe CLP (26% at 1 week vs. 6% in vehicle-treated mice, P < 0.04) (Fig. 2d). As in C57BL/6 (Nt+/+) mice (Fig. 1c and 1d), NT levels were significantly higher in the peritoneal cavity and plasma of WBB6F1-Kit+/+ mice that were subjected to severe as opposed to moderate CLP (Fig. 2e and 2f).
Taken together with the enhanced survival of Nt−/− (Fig. 1b) or Ntsr1−/− (Supplementary Fig. 2b) vs. WT mice in severe CLP, our pharmacological data and the results obtained with MC-deficient mice indicate that NT can contribute significantly to the mortality associated with severe CLP and that MCs can regulate NT concentrations in the peritoneal cavity after CLP.
Mast cells degrade NT and prevent NT-induced hypotension
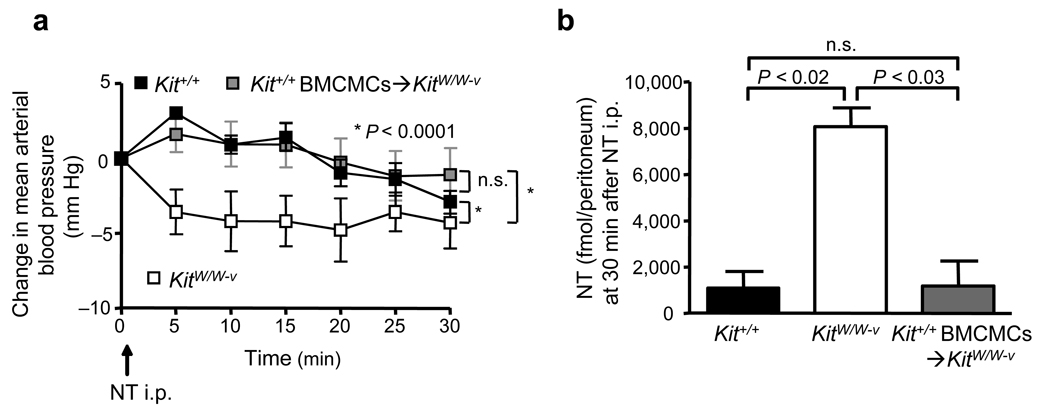
Rat peritoneal MCs (PMCs) can degrade NT in vitro.22 In accord with that result, peritoneal lavage cells (PLCs) of Kit+/+, but not MC-deficient KitW/W-v, mice reduced concentrations of NT in vitro (Supplementary Fig. 3a). To assess whether MCs can reduce levels of NT in vivo in mice not subjected to CLP, we administered NT i.p (6 nmol in 300 µl saline), and then measured mean arterial blood pressure (MAP). NT induced more significant reductions in MAP in KitW/W-v mice than in Kit+/+ or Kit+/+ BMCMCs➔KitW/W-v mice (Fig. 3a). Moreover, 30 min after NT injection, amounts of NT were significantly higher in the peritoneal cavities of KitW/W-v mice than in Kit+/+ or Kit+/+ BMCMCs➔KitW/W-v mice (Fig. 3b). Thus, MCs can reduce levels of NT in vitro or in vivo, and can also limit the extent of NT-induced hypotension in vivo.
Figure 3. MCs reduce peritoneal NT concentrations and NT-induced hypotension.
(a, b) Changes in mean arterial pressure (MAP) versus baseline levels (= “0”, measured 3 min after injection of 300 µl saline, i.p.) at various times after injection of NT (6 nmol in 300 µl saline, i.p.) (a) and amounts of NT in the peritoneal fluids of these mice at 30 min after injection of NT (b) in wild type (Kit+/+) (n = 5), KitW/W-v MC-deficient (n = 4) or Kit+/+ BMCMCs➔KitW/W-v (n = 6) mice.
Mast cells express neurolysin
Using inhibitors of various proteases that can degrade NT,22,23,29 we found that NT degradation by PLCs was significantly inhibited by the mouse MC carboxypeptidase A (mMC-CPA) inhibitor, potato carboxypeptidase inhibitor (PCI),26 and by the neurolysin (NLN) inhibitor, phosphodiepryl 03 (p03)30 (Supplementary Fig. 3b). Neither the chymase inhibitor chymostatin (Chym)31 nor the inhibitor of thimet oligopeptidase, CFp-Ala-Ala-Phe-pAB (CFp),32 significantly influenced NT degradation by PLCs (Supplementary Fig. 3b). Human skin chymase can degrade NT.23 However, NT was degraded to the same extent by PLCs of WT control mice and mouse MC protease-4 (mMCP-4)-deficient (Mcpt4−/−) mice, which lack mMCP-4, the major protease of mouse PMCs with chymotryptic activity33 (Supplementary Fig. 3c).
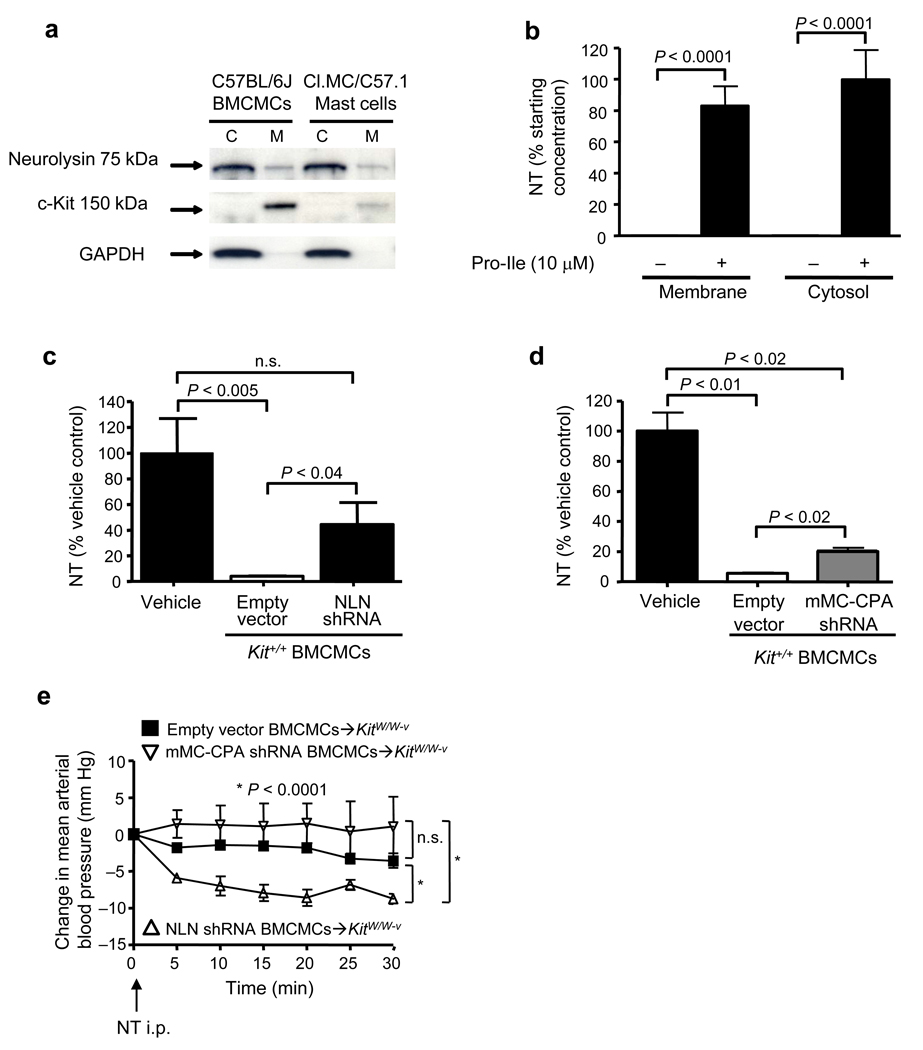
NLN, a zinc metalloendopeptidase expressed in tissues such as kidney, ileum and testis,34 has not been previously described in MCs. PLCs which contained MCs exhibited significantly higher amounts of NLN activity than did PLCs which virtually lacked MCs (Supplementary Fig. 3d). Mouse MCs contained NLN mRNA and protein (Supplementary Fig. 4a and 4b), and active NLN was present in both the membrane and cytosol fractions of such cells (Fig. 4a and Supplementary Fig. 4c). Moreover, either membrane or cytosol preparations of mouse BMCMCs degraded NT, an ability that was almost completely inhibited by the NLN inhibitor, Pro-Ile (10 µM) (Fig. 4b).35
Figure 4. MC-associated neurolysin (NLN) contributes to the ability of MCs to reduce NT levels and NT-induced hypotension.
(a) Identification of NLN in membrane and cytosol fractions of C1.MC/C57.1 MCs and C57BL/6J BMCMCs. (b) Degradation of NT (10 µM) by membrane or cytosol preparations from Kit+/+ BMCMCs (2 × 106 cells/preparation) that were pre-treated with either vehicle (“−”) or with Pro-Ile (10 µM, 15 min at 37 °C). Results are expressed as the percentage of NT remaining after incubation with membrane or cytosol preparations as compared to samples of NT incubated in vehicle alone (n = 6/group, data were pooled from two independent experiments that gave similar results). (c, d) Degradation of NT (10 µM) by A23187 (5 µM)-activated Kit+/+ BMCMCs (2 × 105) that were pre-treated with either empty vector, NLN-shRNA or mMC-CPA-shRNA. Cells were incubated with NT for 30 min at 37 °C. Results are expressed as the percentage of NT remaining in the samples compared to that in samples of NT incubated in vehicle alone at 37 °C (n = 9/group, data were pooled from three independent experiments that gave similar results). (e) Changes in MAP versus baseline levels (= “0”, measured 3 min after injection of 300 µl saline, i.p.) at various times after injection of NT (6 nmol in 300 µl saline, i.p.) in KitW/W-v mice which had been engrafted i.p. with Kit+/+ BMCMCs treated with either empty vector (empty vector BMCMCs➔KitW/W-v), NLN-shRNA (NLN-shRNA BMCMCs➔KitW/W-v) or mMC-CPA-shRNA (mMC-CPA-shRNA BMCMCs➔KitW/W-v) (n = 4/group).
PCI may inhibit NT degradation by rat MCs in part by interfering with the binding of NT to MCs, thus reducing MC degranulation.26 We therefore also used a non-pharmacological approach to assess the extent to which MC-derived mMC-CPA or NLN can degrade NT in vitro. Kit+/+ BMCMCs infected with a lentivirus that delivered short hairpin RNAs (shRNAs) to stably silence expression of NLN or mMC-CPA exhibited reductions of 80% or 90% in levels of NLN or mMC-CPA protein, respectively (Supplementary Fig. 5a and 5b). By contrast, infection of BMCMCs with mMC-CPA or NLN shRNA did not detectably reduce expression of NLN or mMC-CPA, respectively (Supplementary Fig. 5c and 5d). In agreement with results reported for PMCs from mMC-CPA-deficient mice,36 the reduced levels of mMC-CPA were associated with reduced expression of mMCP-5, but not mMCP-4, protein, whereas no effects on these proteases were observed in MCs with reduced levels of NLN (Supplementary Fig. 5e).
MC degranulation results in secretion of mMC-CPA and other MC mediators, and either mMC-CPA- or NLN-shRNA-transduced BMCMCs exhibited significantly reduced ability to degrade NT after induction of BMCMC degranulation with A23187 (5 µM) (Fig. 4c and 4d). Notably, BMCMCs transduced with shRNAs for NLN or mMC-CPA degranulated to the same extent as empty vector-treated cells upon FcεRI cross-linking (Supplementary Fig. 5f), indicating that these treatments didn’t globally reduce MC secretory function. These results indicate that either mMC-CPA or NLN can contribute to the ability of MCs to degrade NT when MCs are induced to degranulate in vitro.
Role of mast cell NLN in reducing NT-induced hypotension
To assess the extent to which NLN or mMC-CPA can contribute to MC-dependent reduction of NT-induced hypotension, KitW/W-v mice were engrafted with BMCMCs transduced with shRNA to silence either NLN or mMC-CPA. Compared to results in control mice, i.p. injection of NT significantly reduced MAP in NLN-shRNA BMCMCs➔KitW/W-v mice but not in mMC-CPA-shRNA BMCMCs➔KitW/W-v mice (Fig. 4e). The percentage of PMCs among total cells in the peritoneal cavity were similar in KitW/W-v mice engrafted with MCs transduced with either empty vector, NLN-shRNA or mMC-CPA-shRNA (2.8 ± 0.1%, 3.5 ± 0.8% or 4.0 ± 0.9%, respectively), as was the distribution of MCs in the mesentery (Supplementary Fig. 6). These results indicate that MC derived-NLN is more important than mMC-CPA in protecting mice from hypotension induced by i.p. injection of NT in vivo.
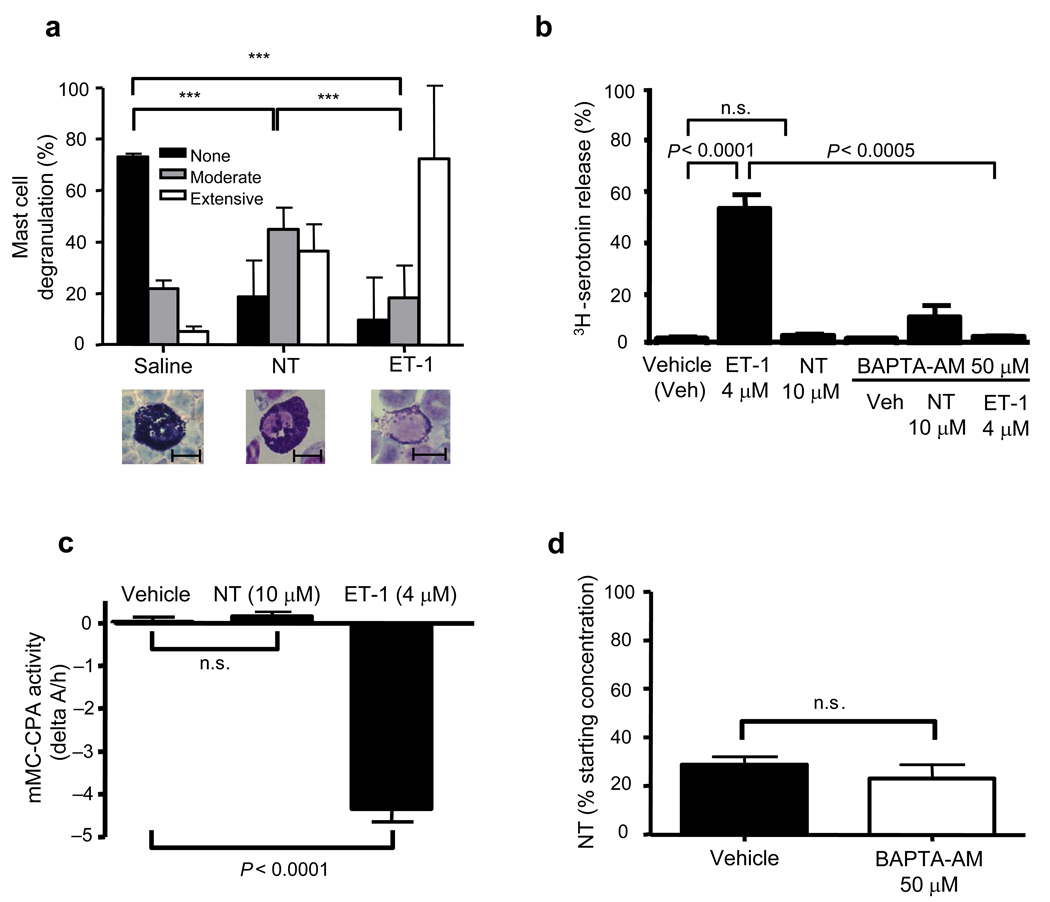
Mast cells can degrade NT without extensive degranulation
Notably, PMCs in Kit+/+ mice exhibited only moderate degranulation at 30 min after i.p. injection of NT, especially when compared with PMCs in mice injected i.p. with ET-1 (Fig. 5a). Moreover, NT had little or no ability to induce PMCs to release serotonin or mMC-CPA in vitro (Fig. 5b and 5c). Also, inhibition of PMC degranulation with the membrane-permeable Ca2+ chelator BAPTA-AM (50 mM, 30 min),17 that markedly inhibited ET-1-induced PMC serotonin release (Fig. 5b), did not reduce the ability of PMCs to degrade NT in vitro (Fig. 5d). These results indicate that PMCs can degrade NT even when such PMCs have not been induced to undergo extensive degranulation.
Figure 5. MCs can degrade NT in the absence of extensive degranulation.
(a) Percentage of PMCs obtained from Kit+/+ mice exhibiting > 50% (“Extensive”), 10–50% (“Moderate”) or < 10% (“None”) degranulation at 30 min after injection of NT (6 nmol/300 µl saline), ET-1 (1.2 nmol/300 µl saline) or saline. *** P < 0.0001 for comparisons shown by brackets. Photomicrographs are of May Grunwald-modified Giemsa-stained PLC preparations illustrating PMCs that exhibit no (left), moderate (middle) or extensive (right) degranulation (scale bars = 10 mm). (b) PLCs containing 1 × 104 PMCs obtained from C57BL/6 mice were incubated for 2 h with 3H-serotonin and stimulated for 15 min at 37 °C with either ET-1 (4 µM), NT (10 µM), or vehicle. In a separate set of experiments, cells were pre-incubated with BAPTA-AM (50 mM, 15 min, 37 °C) before the addition of the stimuli (n = 6/group). (c) mMC-CPA activity was measured in supernatants obtained from Kit+/+ PLCs containing 5 × 104 PMCs that were stimulated for 30 min at 37 °C with either ET-1 (4 µM), NT (10 µM), or vehicle (n = 3–4/group). (d) PLCs containing 5 × 104 PMCs from C57BL/6J mice were pre-treated for 15 min with BAPTA-AM (50 mM) and then incubated with NT (10 µM) for 30 min at 37 °C. Results are expressed as the percentage NT remaining after incubation with cells compared to that in samples of NT incubated in vehicle alone at 37 °C (n = 4/group). Data in b–d were pooled from the two independent experiments performed in each case, each of which gave similar results.
Role of mast cell Ntsr1
To assess the potential involvement of NT receptors in MC degradation of NT, we assessed the NT degradation ability of PLCs isolated from Ntsr1−/− and Ntsr2−/− mice. NT degradation was significantly impaired in Ntsr1−/− PLCs and, to a lesser extent, in Ntsr2−/− PLCs (Fig. 6a). However, upon stimulation with ET-1 (4 µM), both Ntsr1−/− -and Ntsr2−/− -derived PMCs released serotonin to the same extent as did PMCs from congenic wild type mice, indicating that a NT receptor-deficiency does not impair PMC degranulation in response to an agonist other than NT (Supplementary Fig. 7a). These results suggest that Ntsr1 and, to a lesser extent, Ntsr2 can contribute to NT degradation by PLCs. However, we found that mouse MCs expressed Ntsr1, as was reported for rat PMCs,26,37 but not Ntsr2 (Supplementary Fig. 7b). This result suggests that Ntsr2 expression by PLCs other than MCs can influence NT degradation, either directly and/or through effects on additional cell types.
Figure 6. MC expression of Ntsr1 is required for optimal MC-dependent reduction of NT-induced hypotension and enhancement of survival after CLP.
(a) PLCs containing 5 × 104 PMCs from C57BL/6, Ntsr1−/− or Ntsr2−/− mice were incubated with NT (10 µM) for 30 min at 37 °C. Results are expressed as the percentage of NT remaining after incubation with cells compared to that in samples of NT incubated in vehicle alone at 37 °C (data were pooled from triplicate determinations in the two independent experiments performed, each of which gave similar results). (b, c) Changes in MAP vs. baseline levels (= “0”, measured 3 min after injection of 300 µl saline, i.p.) at various times after injection of NT (6 nmol in 300 µl saline, i.p.) (b), and amounts of NT in the peritoneal fluids of these mice at 30 min after NT injection (c), in KitW/W-v mice that had been engrafted i.p. with BMCMCs of C57BL/6-Ntsr1+/+ (n = 7) or -Ntsr1−/− (n = 6) origin. (d) Survival after moderate CLP (ligation of distal half of caecum; one puncture with a 22G needle) in KitW/W-v mice that had been engrafted i.p. with BMCMCs of C57BL/6-Ntsr1+/+ (n = 14) or -Ntsr1−/− (n = 13) origin.
Intraperitoneal injection of NT resulted in significantly lower MAP in Ntsr1−/− BMCMCs➔KitW/W-v mice than in Ntsr1+/+ BMCMCs➔KitW/W-v mice (P < 0.0001) (Fig. 6b). However, MAP was not as low in NT-injected Ntsr1−/− BMCMCs➔KitW/W-v mice as in NT-injected KitW/W-v mice (P < 0.0001) (Fig. 3a). Intraperitoneal NT levels were also significantly higher in Ntsr1−/− BMCMCs➔KitW/W-v mice than in Ntsr1+/+ BMCMCs➔KitW/W-v mice (P < 0.04) (Fig. 6c), but levels in NT-injected Ntsr1−/− BMCMCs➔KitW/W-v mice were not as high as those in NT-injected KitW/W-v mice (P < 0.05) (Fig. 3b). Finally, survival after CLP was significantly higher in Ntsr1+/+ BMCMCs➔KitW/W-v mice than in Ntsr1−/− BMCMCs➔KitW/W-v mice (23% vs. 0%, respectively, P < 0.025) (Fig. 6d). The % of PMCs in the peritoneal cavity of Ntsr1+/+ BMCMCs➔KitW/W-v or Ntsr1−/− BMCMCs➔KitW/W-v mice were very similar (3.2 ±0.4% or 3.8 ± 0.8% of total cells), as was the distribution of MCs in the mesentery of these mice (Supplementary Fig. 8).
DISCUSSION
Given the large number of molecules that are thought to influence the pathogenesis of sepsis, it is remarkable that mouse survival after severe CLP was significantly enhanced by either the genetic deletion of NT or the pharmacological inhibition of this peptide. Our findings thus reveal a heretofore unknown contribution of NT to the mortality associated with this model of sepsis.
Our findings also identify a previously unknown and important role for MCs, and MC-associated NLN, in regulating NT levels, and in turn the extent of NT-associated pathology, in vivo. While it has been reported that certain MC populations can degranulate in response to NT25 and that some MC-derived proteases can degrade NT in vitro,22,23 the effects of MCs and their products on the degradation of NT in vivo has not previously been investigated. We found that significantly higher amounts of NT occur during CLP in the peritoneal cavity of MC-deficient KitW/W-v mice than in Kit+/+ wild type mice or MC-engrafted KitW/W-v mice, indicating that MCs can regulate levels of endogenously generated NT in this sepsis model.
MCs also can significantly reduce NT-induced hypotension upon i.p. injection of the peptide in vivo. In the past, NT-induced changes in blood pressure have been attributed to NT-induced release of serotonin or histamine from MCs, suggesting that the effect of NT on MCs actually contributes to the hypotension induced by this agent.38 However, these assumptions had not been tested using MC-deficient mice. High-affinity NT receptors are expressed on vascular endothelial cells as well as on MCs, suggesting that endothelium-derived vasodilators such as prostacyclin, rather than MC-derived products, may contribute to the hypotensive effects of NT.39,40
We found that MCs can express the NT-degrading enzyme, NLN34, and that the active enzyme was present in both cytosol and membrane fractions of these cells. In vitro studies have shown that NLN also can degrade bradykinin and angiotensin-I, implicating NLN in the regulation of blood pressure.41 Mouse MCs are known to express CPA (i.e., mMC-CPA),42 an exopeptidase that preferentially cleaves C-terminal aliphatic amino acids.43 Pharmacological evidence indicated that either mMC-CPA or NLN can contribute to NT degradation when MCs are activated to degranulate extensively in vitro. However, in vivo studies with KitW/W-v mice containing adoptively-transferred MC populations in which either mMC-CPA or NLN was knocked down using an shRNA-based approach indicated that MC-associated NLN is more important than MC-associated mMC-CPA in reducing NT-induced hypotension in this setting.
Evidence derived from two different in vivo models supports the conclusion that the expression of Ntsr1 by MCs contributes to their ability to enhance resistance to the adverse effects of NT. Ntsr1 expression by MCs was required for optimal MC-dependent reduction in NT levels in the peritoneal cavity after i.p. injection of the peptide (Fig. 6c) and for optimal reduction of NT-induced hypotension (Fig. 6b). However, the hypotensive effects of NT, and the levels of peritoneal NT, were greater in MC-deficient KitW/W-v mice than in KitW/W-v mice engrafted with Ntsr1−/− BMCMCs. Taken together, these findings suggest that MCs can confer protection against the hypotensive effects of NT by both Ntsr1-dependent and Ntsr1-independent mechanisms.
In our second in vivo model, CLP, KitW/W-v mice engrafted with Ntsr1−/− BMCMCs exhibited significantly poorer survival than did Ntsr1+/+ BMCMC-engrafted KitW/W-v mice (Fig. 6d). This finding further strengthens the conclusion that optimal MC function in diminishing adverse effects of NT, either after NT injection or in CLP, requires MC expression of Ntsr1. In preliminary in vitro studies, we found that rhodamine-labeled NT binds to and is internalized by PMCs to a greater extent than by other PLCs, and that PMCs lacking either Ntsr1 or both Ntsr1 and Ntsr2 exhibit impaired ability to bind and internalize rhodamine-NT (Supplementary Fig. 9 and data not shown). These findings suggest that one mechanism by which Ntsr1 may promote degradation of NT by MC-associated NLN is to facilitate the uptake of NT by MCs.
The origin of the NT produced during CLP in mice remains to be identified. In addition, the relevance of our findings in mice to the roles of NT and MCs in septic shock in humans remains to be assessed. In a pilot study, plasma concentrations of NT were elevated to levels similar to those in mice with severe CLP in patients admitted to the intensive care unit with septic shock (median: 224 fmol/ml [range: 65–1715 fmol/ml, n = 17], versus 30 fmol/ml [range: 13.8–180 fmol/ml, n = 14] in healthy controls, P < 0.0001) (see Supplementary Table 1 for the subjects’ clinical characteristics). Notably, plasma concentrations of NT were similarly elevated in subjects with cardiogenic shock (median: 309 fmol/ml [range: 10–2176 fmol/ml, n = 6], P < 0.04 vs. values for healthy control subjects) (Supplementary Table 1). These data raise the possibility that NT might contribute to hypotension in patients with either septic or cardiogenic shock.
The role of human MCs in regulating levels of NT is not yet clear. It has been reported that plasma levels of the MC protease, tryptase, are not elevated in humans with sepsis.44 Moreover, unlike in murine rodents, few MCs can be recovered in peritoneal lavage fluid in humans.45 However, humans do have MCs within the mesentery and in the mucosa, submucosa and muscularis propria of the gastrointestinal tract.46,47 Moreover, human umbilical cord blood-derived MCs express mRNA for NLN, express Ntsr1 protein, and, upon activation by a calcium ionophore, can degrade NT in vitro (Supplementary Fig. 10). In addition, our evidence in mice suggests that at least certain populations of MCs can reduce levels of NT in vitro or in vivo under conditions in which such MCs do not undergo extensive degranulation. Accordingly, the lack of elevated levels of plasma tryptase during human sepsis, which indicates the probable absence of extensive and ongoing MC degranulation in this setting, does not rule out a role for MCs in regulating levels of NT during sepsis in humans. Establishing the extent to which NT levels are regulated by MCs during septic shock in humans may be difficult, since genetically MC-deficient humans have not been reported. Nevertheless, our studies in mice raise the possibility that inhibiting pathological actions of NT may confer benefit in humans with this highly lethal disorder and may even be beneficial in subjects with cardiogenic shock.
METHODS
Chemicals and reagents
Neurotensin (1–13) (Bachem Peninsula Labs), endothelin-1, A23187 and chymostatin (Sigma), potato carboxypeptidase inhibitor (Calbiochem), and Pro-Ile (Bachem Peninsula Labs) were purchased from the manufacturers.
Animals
C-kit mutant genetically MC-deficient (WB/ReJ-KitW/+ × C57BL/6J–KitW-v/+)F1-KitW/W-v (WBB6F1-KitW/W-v) (KitW/W-v) mice and the congenic normal WBB6F1-+/+ (Kit+/+) mice, and C57BL/6J mice, were purchased from Jackson Laboratories. Mouse MC-protease-4 deficient (Mcpt4−/−), NT deficient (Nt−/−), NT receptor-1 deficient (Ntsr1−/−) and NT receptor-2 deficient (Ntsr2−/−) mice were all on the C57BL/6J background except for the C57BL/6-Ntsr1−/− mice (for which we used littermate C57BL/6-Ntsr1+/+ mice as controls); these mice were bred and maintained at the Stanford University Research Animal Facility. Unless specified otherwise, all mice were 12 week old females when used for experiments. All animal care and experimentation was conducted in accord with current National Institutes of Health guidelines and with the approval of the Stanford University Institutional Animal Care and Use Committee.
Caecal ligation and puncture (CLP)
CLP was performed as described.20 Briefly, mice were deeply anaesthetized by i.m. injection of 100 mg/kg Ketamine and 20 mg/kg Xylazine. The caecum was exposed by a 1–2 cm midline incision on the anterior abdomen and subjected to ligation of its distal portion and a single needle puncture of the ligated segment. For severe CLP, we ligated the distal half or 2/3rds of the caecum (in mice on the C57BL/6 or [WB × C57BL/6]F1 background, respectively) and made a single puncture with a 20G needle; for moderate CLP (in wild type mice on the C57BL/6 or [WB × C57BL/6]F1 background), we ligated the distal half of the caecum and made a single puncture with a 22G needle. Protocols for eliciting severe or moderate CLP were developed so that, on each strain background, > 50% or 20–50% of the wild type mice succumbed within 4 days after CLP. The caecum was then replaced into the abdomen, 1 ml of sterile saline (pyrogen-free 0.9% NaCl) was administrated into the peritoneal cavity, and the incision was closed using 9-mm steel wound clips. Mice were observed for mortality at least four times daily. Mice that were clearly moribund were killed by CO2 inhalation.
Mean arterial blood pressure (MAP) measurements
Mice were anaesthetized with 2% Isoflurane. The carotid artery was exposed via a midline incision in the upper thorax and then was cannulated with a pressure transduction catheter connected to a computerized pressure monitor (Powerlab) to record blood pressure. The transduction system was calibrated using a sphygmomanometer. After the level of isoflurane was reduced to 1%, the blood pressure and respiratory rate of the mouse was allowed to stabilize for several minutes. Then 300 µl of saline was administered i.p. and MAP at baseline was recorded. There were no significant differences among the various groups of mice tested in single experiments in baseline measurements of MAP. 3 min after saline administration, NT was injected i.p. (6 nmol/300 µl saline) and blood pressure was recorded for 30 min. Data are presented as changes in MAP after NT administration in relation to baseline (“time 0”).
MC engraftment of KitW/W-v mice
Some KitW/W-v mice (female, 4–6-week-old) were repaired of their MC deficiency selectively and locally by the i.p. injection of growth factor-dependent congenic Kit+/+ BMCMCs. Briefly, femoral bone marrow cells from Kit+/+ mice were maintained in vitro for ~4 weeks in IL-3-containing medium until MCs represented > 95% of the total cells according to staining with May-Grünwald-Giemsa. 2.0 × 106 BMCMCs in 200 µl of PBS, were injected i.p. (via a 26G needle) and the mice were used for experiments, together with gender-and age-matched MC-deficient KitW/W-v mice, 4–6 weeks after adoptive transfer of BMCMCs. Other KitW/W-v mice received, 4–6 weeks before injection of NT i.p. or CLP, 2.0 × 106 BMCMCs generated from either Ntsr1-deficient mice (Ntsr1−/−) or the congenic normal mice (Ntsr1+/+). Other KitW/W-v mice received, 4 weeks before injection of NT i.p., injections of 1.0 × 106 BMCMCs that had been transduced with NLN- or mMC-CPA-targeting shRNA or with empty vector.
Lentiviral vector production, preparation of NLN shRNA- or mMC-CPA shRNA-containing MCs, RT-PCR, membrane preparations, western blot analysis, CPA enzymatic activity assay, beta-hexosaminidase and serotonin release, NT and ET-1 measurements, evaluation of MCs in the peritoneal cavity and mesenteric windows, and human umbilical cord blood-derived MC generation.
These procedures are described in Supplementary Methods.
Statistical analysis
Analysis of variance (ANOVA) for repeated measures was used to assess differences in the changes in mean arterial blood pressure. We assessed differences in the survival rates after CLP using the Mantel-Haenszel Logrank test, the extent of MC degranulation by the Chi-square test. All other data were analyzed for statistical significance using the unpaired two-tailed Student’s t-test or Mann Whitney U-test. P < 0.05 is considered statistically significant. Unless otherwise specified, all data are presented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank A. Xu and D. Lepard for technical assistance, M. Krupa-Plonowska and G. O’Riordan for their assistance with the collection of human blood samples, A. Patterson and R. Agrawal for the use of equipment to measure blood pressure, and J. Kalesnikoff for critical reading of the manuscript. We thank B. Vincent (Institut de Pharmacologie Moleculaire et Cellulaire, Centre National de la Recherche Scientifique, Valbonne, France) for providing antibodies against NLN, M. Gurish (Brigham and Women’s Hospital and Harvard Medical School, Boston, MA) for providing antibodies against mMCP-4 and mMCP-5, R. Carraway (University of Massachusetts Medical School, Worcester, MA) for providing antibodies against Ntsr1, V. Dive (Commissariat a L’Ennergie Atomique, Saclay, France) for providing phosphodiepryl-03, a NLN inhibitor that can be used with living cells, S. Wilk (Mount Sinai School of Medicine, NY) for providing CFp-Ala-Ala-Phe-pAB, L. Van Parijs for providing pLentiLox 3.7 (pLL3.7), D. Gully (Sanofi-Synthelabo Recherche, Tolouse Cedex, France) for providing SR142948A, Amgen Incorporated for the gifts of rat rSCF, human rSCF164 and human rIL-6. This work was supported by United States Public Health Science Grants (to S.J.G. and C.-C. C.), and by a fellowship from Deutsche Forschungsgemeinschaft (to M. M.).
Footnotes
AUTHOR CONTRIBUTIONS
A.M.P., C.-C.C., T.N., E.J.R., P.R.D., J.D.F., R.P., M.T. and S.J.G. designed research; A.M.P., C.-C.C., T.N., M.M., E.J.R., S.Z. and U.M. performed research; A.M.P., C.-C.C., T.N., E.J.R., P.R.D., E.W., K.W., M.A., G.P., R.P., M.T. and S.J.G. analyzed data; A.M.P. and S.J.G. wrote the manuscript and C.-C.C., T.N., M.M., E.J.R., P.R.D., E.W., K.W., S.Z., U.M., J.D.F., M.A., G.P., R.P. and M.T. contributed to the revision of/approved the manuscript.
References
- 1.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 2.Grocott-Mason RM, Shah AM. Cardiac dysfunction in sepsis: new theories and clinical implications. Intensive Care Med. 1998;24:286–295. doi: 10.1007/s001340050570. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 4.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 5.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyler-McMahon BM, Boules M, Richelson E. Neurotensin: peptide for the next millennium. Regul Pept. 2000;93:125–136. doi: 10.1016/s0167-0115(00)00183-x. [DOI] [PubMed] [Google Scholar]
- 7.Reinhart K, Meisner M, Brunkhorst FM. Markers for sepsis diagnosis: what is useful? Crit Care Clin. 2006;22:503–519. ix–x. doi: 10.1016/j.ccc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Patel GP, Gurka DP, Balk RA. New treatment strategies for severe sepsis and septic shock. Curr Opin Crit Care. 2003;9:390–396. doi: 10.1097/00075198-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, de Carvalho FB, De Backer D. Management of septic shock. Ann Med. 2002;34:606–613. doi: 10.1080/078538902321117832. [DOI] [PubMed] [Google Scholar]
- 10.Remaury A, et al. Targeted inactivation of the neurotensin type 1 receptor reveals its role in body temperature control and feeding behavior but not in analgesia. Brain Res. 2002;953:63–72. doi: 10.1016/s0006-8993(02)03271-7. [DOI] [PubMed] [Google Scholar]
- 11.Maeno H, et al. Comparison of mice deficient in the high- or low-affinity neurotensin receptors, Ntsr1 or Ntsr2, reveals a novel function for Ntsr2 in thermal nociception. Brain Res. 2004;998:122–129. doi: 10.1016/j.brainres.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Mazella J, et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273:26273–26276. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- 13.Rioux F, Kerouac R, Quirion R, St-Pierre S. Mechanisms of the cardiovascular effects of neurotensin. Ann N Y Acad Sci. 1982;400:56–74. doi: 10.1111/j.1749-6632.1982.tb31560.x. [DOI] [PubMed] [Google Scholar]
- 14.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 15.Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 16.Supajatura V, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurer M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 18.Malaviya R, Gao Z, Thankavel K, van der Merwe PA, Abraham SN. The mast cell tumor necrosis factor alpha response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc Natl Acad Sci U S A. 1999;96:8110–8115. doi: 10.1073/pnas.96.14.8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 20.Maurer M, et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med. 1998;188:2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakurdas SM, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 22.Cochrane DE, Carraway RE, Boucher W, Feldberg RS. Rapid degradation of neurotensin by stimulated rat mast cells. Peptides. 1991;12:1187–1194. doi: 10.1016/0196-9781(91)90193-s. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein SM, Leong J, Bunnett NW. Human mast cell proteases hydrolyze neurotensin, kinetensin and Leu5-enkephalin. Peptides. 1991;12:995–1000. doi: 10.1016/0196-9781(91)90049-u. [DOI] [PubMed] [Google Scholar]
- 24.Dobner PR, Fadel J, Deitemeyer N, Carraway RE, Deutch AY. Neurotensin-deficient mice show altered responses to antipsychotic drugs. Proc Natl Acad Sci U S A. 2001;98:8048–8053. doi: 10.1073/pnas.141042198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurose M, Saeki K. Histamine release induced by neurotensin from rat peritoneal mast cells. Eur J Pharmacol. 1981;76:129–136. doi: 10.1016/0014-2999(81)90494-5. [DOI] [PubMed] [Google Scholar]
- 26.Miller LA, Cochrane DE, Feldberg RS, Carraway RE. Inhibition of neurotensin-stimulated mast cell secretion and carboxypeptidase A activity by the peptide inhibitor of carboxypeptidase A and neurotensin-receptor antagonist SR 48692. Int Arch Allergy Immunol. 1998;116:147–153. doi: 10.1159/000023938. [DOI] [PubMed] [Google Scholar]
- 27.Nakano T, et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162:1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gully D, et al. Biochemical and pharmacological activities of SR 142948A, a new potent neurotensin receptor antagonist. J Pharmacol Exp Ther. 1997;280:802–812. [PubMed] [Google Scholar]
- 29.Millican PE, Kenny AJ, Turner AJ. Purification and properties of a neurotensin-degrading endopeptidase from pig brain. Biochem J. 1991;276(Pt 3):583–591. doi: 10.1042/bj2760583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barelli H, Dive V, Yiotakis A, Vincent JP, Checler F. Potent inhibition of endopeptidase 24.16 and endopeptidase 24.15 by the phosphonamide peptide N-(phenylethylphosphonyl)-Gly-L-Pro-L-aminohexanoic acid. Biochem J. 1992;287(Pt 2):621–625. doi: 10.1042/bj2870621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powers JC, et al. Mammalian chymotrypsin-like enzymes. Comparative reactivities of rat mast cell proteases, human and dog skin chymases, and human cathepsin G with peptide 4-nitroanilide substrates and with peptide chloromethyl ketone and sulfonyl fluoride inhibitors. Biochemistry. 1985;24:2048–2058. doi: 10.1021/bi00329a037. [DOI] [PubMed] [Google Scholar]
- 32.Wilk S, Orlowski M. Inhibition of rabbit brain prolyl endopeptidase by n-benzyloxycarbonyl-prolyl-prolinal, a transition state aldehyde inhibitor. J Neurochem. 1983;41:69–75. doi: 10.1111/j.1471-4159.1983.tb11815.x. [DOI] [PubMed] [Google Scholar]
- 33.Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med. 2003;198:423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shrimpton CN, Smith AI, Lew RA. Soluble metalloendopeptidases and neuroendocrine signaling. Endocr Rev. 2002;23:647–664. doi: 10.1210/er.2001-0032. [DOI] [PubMed] [Google Scholar]
- 35.Dauch P, Vincent JP, Checler F. Specific inhibition of endopeptidase 24.16 by dipeptides. Eur J Biochem. 1991;202:269–276. doi: 10.1111/j.1432-1033.1991.tb16372.x. [DOI] [PubMed] [Google Scholar]
- 36.Feyerabend TB, et al. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol Cell Biol. 2005;25:6199–6210. doi: 10.1128/MCB.25.14.6199-6210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldberg RS, et al. Evidence for a neurotensin receptor in rat serosal mast cells. Inflamm Res. 1998;47:245–250. doi: 10.1007/s000110050325. [DOI] [PubMed] [Google Scholar]
- 38.Quirion R, Rioux F, Regoli D, St-Pierre S. Compound 48/80 inhibits neurotensin-induced hypotension in rats. Life Sci. 1980;27:1889–1895. doi: 10.1016/0024-3205(80)90435-x. [DOI] [PubMed] [Google Scholar]
- 39.Schaeffer P, et al. Human umbilical vein endothelial cells express high affinity neurotensin receptors coupled to intracellular calcium release. J Biol Chem. 1995;270:3409–3413. doi: 10.1074/jbc.270.7.3409. [DOI] [PubMed] [Google Scholar]
- 40.Schaeffer P, et al. Neurotensin induces the release of prostacyclin from human umbilical vein endothelial cells in vitro and increases plasma prostacyclin levels in the rat. Eur J Pharmacol. 1997;323:215–221. doi: 10.1016/s0014-2999(97)00041-1. [DOI] [PubMed] [Google Scholar]
- 41.Norman MU, Reeve SB, Dive V, Smith AI, Lew RA. Endopeptidases 3.4.24.15 and 24.16 in endothelial cells: potential role in vasoactive peptide metabolism. Am J Physiol Heart Circ Physiol. 2003;284:H1978–H1984. doi: 10.1152/ajpheart.01116.2002. [DOI] [PubMed] [Google Scholar]
- 42.Serafin WE, Dayton ET, Gravallese PM, Austen KF, Stevens RL. Carboxypeptidase A in mouse mast cells Identification, characterization, and use as a differentiation marker. J Immunol. 1987;139:3771–3776. [PubMed] [Google Scholar]
- 43.Woodbury RG, Everitt MT, Neurath H. Mast cell proteases. Methods Enzymol. 1981;80(Pt C):588–609. doi: 10.1016/s0076-6879(81)80047-x. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–1626. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 45.Fox CC, Dvorak AM, MacGlashan DW, Jr, Lichtenstein LM. Histamine-containing cells in human peritoneal fluid. J Immunol. 1984;132:2177–2179. [PubMed] [Google Scholar]
- 46.Bienenstock J. The mucosal immunologic network. Ann Allergy. 1984;53:535–540. [PubMed] [Google Scholar]
- 47.Metcalfe DD. Mast cell mediators with emphasis on intestinal mast cells. Ann Allergy. 1984;53:563–575. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.