Abstract
Background
Neural-glial signaling in the spinal cord may underlie pain and sensitization after peripheral injury. Here we test the role of a glial activator, the chemokine CCL2 on mechanical hypersensitivity following plantar incision in a rat model of postoperative pain.
Methods
Twenty four hours after hindpaw incision rats were intrathecally administered an anti-CCL2 neutralizing antibody (3 μg and 10 μg) or control IgG (10 μg). Mechanical hypersensitivity was assessed acutely and for several days following administration of anti-CCL2 antibody using von Frey filaments. Immunohistochemical analysis was conducted on spinal cord sections to examine the effects of treatment on measures of microglial activation including levels of ionized calcium binding adaptor molecule 1 (IBA1) and phosphorylated p38 mitogen activated protein kinase.
Results
Neutralization of spinal CCL2 acutely reversed mechanical hypersensitivity within 30 minutes in a dose dependent manner. A single administration also produced a sustained decrease in mechanical hypersensitivity 48 and 72 hours following incision. Anti-CCL2 antibody reduced microglial activation as measured by the levels of IBA1 immunoreactivity and the number of microglia containing phosphorylated p38 mitogen activated protein kinase 48 hours following incision but not within 30 minutes of administration.
Conclusions
These results provide evidence that CCL2 contributes to the maintenance of mechanical hypersensitivity following plantar incision and establishes a role for neural glial signaling in postoperative pain. The long term effects of anti-CCL2 treatment correlate with reduced microglial activation. Spinal blockade of CCL2 may serve as a useful therapy for the treatment of certain aspects of postoperative pain.
Introduction
An estimated 23 million people in the United States and 234 million worldwide undergo surgical procedures each year 1. Despite increased preclinical and clinical research on pathological mechanisms of postoperative pain and recent advances in analgesic therapies, many patients do not receive adequate analgesic support during the postoperative setting. Clinical reports indicate that 50-70% of patients experience moderate to severe pain after surgery 2-4.
Preclinical models of acute postoperative pain have been developed involving surgical incision of the skin, muscle and fascia which leads to evoked and non-evoked pain-related behaviors that mirror the symptoms observed in patients undergoing surgery 5-7. It is now widely recognized that activation of spinal glial cells including microglia and astrocytes are involved in central sensitization and mechanical hypersensitivity in acute and persistent pain states8-11. However, the contribution of glial cells to postoperative pain states has just begun to be investigated. Following surgical incision, spinal microglia upregulate the cell surface molecules CD11b (recognized by OX42 antibody) and the ionized calcium binding adaptor molecule 1 (IBA1) in lumbar dermatomes ipsilateral to incision 9,12,13. Further, phosphorylated p38 mitogen activated protein kinase (p-p38 MAPK)14 and cyclooxygenase 1 15,16 are upregulated in microglia within hours of surgical incision. These data suggest that microglia may serve as sources of proinflammatory cytokines and prostaglandins which could maintain central sensitization after incision. In support of this idea, intrathecal injection of the glial metabolic inhibitor fluorocitrate dose dependently reversed mechanical hypersensitivity 24 hours after incision 9 and spinal administration of agents that target microglial mediated signaling including p38 MAPK inhibitors 14 and the COX1 preferring inhibitor ketorolac 16,17 also partially reverse or prevent the development of mechanical hypersensitivity post incision. Recently, it has been shown that the cannabinoid receptor type 2 agonist JWH015 reduced microglial activation and mechanical hypersensitivity following surgical incision in rats 18.
What has not been determined is what factors activate glial cells in the postoperative setting. Several factors that activate glial cells in the spinal cord have been identified in neuropathic pain states including adenosine triphosphate, tumor necrosis factor-α, CCL2, fractalkine (CX3CL1), and toll like receptor agonists 19. As part of the current study, we investigated the effects of spinal blockade of the chemokine CCL2 on mechanical hypersensitivity and spinal markers of microglial activation including IBA1 and p-p38 MAPK following surgical incision.
Materials and Methods
Animal preparation and surgery
Male Sprague–Dawley rats (Harlan Industries, Indianapolis, IN), weighing 250–275 g, were used for experiments. All studies conformed to the Wake Forest University Guidelines on the ethical use of animals, and studies were performed under Animal Care and Use Committee (Winston-Salem, NC) approval. Animals were housed under a 12-h light–dark cycle, with food and water ad libitum.
Plantar incision was performed as previously described 5. In brief, animals were anesthetized with inhalational isoflurane (2%) in oxygen, and the plantar aspect of the left hindpaw was prepared in a sterile manner with a 10% povidone-iodine solution. A midline incision (1 cm) was made using a No. 11 blade on the left hindpaw starting 0.5 cm from the heel. The plantaris muscle was elevated and incised longitudinally. The wound was closed with two 5.0 nylon mattress sutures. For sham procedures, animals were anesthetized with inhalational isoflurane (2%) in oxygen and the plantar aspects of the left hindpaw were prepared in a sterile manner with 10% povidone-iodine solution.
Drug administration
Rats that received plantar incision were intrathecally administered CCL2 neutralizing antibody (goat anti-mouse CCL2/MCP-1/LE 15 μl at 200ng/ml or 15 μl at 667 ng/ml for 3 and 10 ng total, respectively) or the same amount of goat anti-mouse IgG (Both antibodies from R&D Systems, Minneapolis, MN). Anti-CCL2 antibody and control IgG were prepared in 0.9% saline solution as the vehicle. The goat anti-mouse CCL2/MCP-1/LE neutralizing antibody used for this study also recognizes rat CCL2 20. Percutaneous intrathecal injections were performed between the L5 and L6 vertebrae of the spine using a 30 gauge ½ inch needle under 2% isoflurane/O2 anesthesia. Successful puncture of the dura mater was assumed by the presence of a tail flick.
Behavioral analysis
Paw withdrawal thresholds to mechanical stimuli were determined using von Frey filaments as previously described 21. Briefly, rats were placed in individual Plexiglas chambers with a plastic mesh floor and allowed to acclimate to the test apparatus at least 30 minutes prior to testing. Filaments were applied to the bending point for 6 s, and a brisk paw withdrawal was considered a positive response. Withdrawal threshold was determined using an up–down statistical method 21. Individuals conducting behavioral assays were blinded to the treatment.
Tissue preparation for Immunohistochemistry
Rats were anesthetized with sodium pentobarbital (intraperitoneal injection; 100 mg/kg), the thorax was opened, and 0.1 M phosphate buffered saline (PBS, pH 7.4) followed by fixative (4% paraformaldehyde in 0.1 M PBS, pH 7.4) was perfused through the left ventricle with a peristaltic pump (20 ml/min). The spinal cord was removed, immersed in fixative for 12 hours at 4° C followed by immersion in 30% sucrose at 4°C for cryoprotection until ready to be sectioned. Spinal cord cross sections (40 μm) were cut on a cryostat and every fourth section was processed for immunohistochemistry for a given marker. For single labeling of microglia an antibody against IBA1 (rabbit anti-rat IBA1, Wako Chemicals, Richmond, VA) was used. Spinal cord sections were processed free floating and incubated over night at 4°C with primary antibody. Antibodies were diluted in a solution consisting of PBS containing 1% normal donkey serum and 0.1% Triton X-100. Sections were washed in 0.1 M PBS solution and incubated in rabbit biotinylated secondary antiserum (1:500, Jackson Immunoresearch, West Grove, PA) for 2 hours at room temperature. The Elite Vectastain ABC kit (Vector Laboratories, Burlingame, CA) was used to link the antigen-antibody complex to horseradish peroxidase which was then visualized with 3, 3-diaminobenzidinetetrahydrochloride histochemistry. Finally, the sections were washed thoroughly in PBS, mounted on plus-slides, air-dried, dehydrated in ethanol, cleared in xylene, and cover slipped with DPX mounting media (Sigma-Aldrich, St. Louis, MO)
For double immunofluorescense labeling, sections were incubated with a mixture of primary antibodies rabbit anti p-p38 MAPK (1:500, Cell Signaling Technologies, Danvers, MA) antibody and mouse monoclonal antibody against the microglial specific cell surface receptor CD11b (clone OX-42,1:250, Serotec Ltd, Raleigh, NC) overnight at 4 ° C. The sections were washed in 0.1 M PBS and incubated in a mixture of CY3-conjugated donkey anti-rabbit IgG (1:600, Jackson Immunoresearch) and CY2-conjugated donkey anti-mouse IgG (1:200, Jackson Immunoresearch) for 2 hours at room temperature. Then sections were washed in PBS mounted on plus –slides air-dried, dehydrated in ethanol, cleared in xylene, and cover slipped with DPX.
Image analysis and quantification
Sections were examined with brightfield illumination and images were captured with a CCD digital camera attached to the microscope using a 10× objective at a resolution of 1,600 × 1,200 pixels. Images of ipsilateral and contralateral L4-5 dorsal horn of sham operated and incision rats were captured. A square with a fixed area (250 × 250 μm2) covering the region of laminae I-II was randomly positioned in the middle one third of the mediolateral extent of the spinal cord dorsal horn. The number of pixels occupied by IBA1 immunoreactive cells within a defined threshold was measured using image analysis software (Image J; NIH Image, National Institutes of Health, Bethesda, MD). Immunodensity measurements were obtained from a minimum of 5 spinal cord sections/rat and averaged.
For assessment of spinal levels of p-p38 MAPK, images of p-p38 MAPK immunoreactivity and OX42 immunoreactivity within ipsilateral and contralateral L4-5 dorsal horn of sham operated and incision rats were sequentially captured using a 20× objective for each section by alternating between filter sets to capture in separate overlapping images CY3 and CY2 labeling, respectively. A square with a fixed area (250 × 250 μm2) covering the dorsal medial aspects of the spinal cord was randomly positioned in the dorsal horn of the spinal cord images. The number of p-p38 MAPK immunoreactive cells was counted on six randomly chosen sections from each animal and averaged for each animal. The microglial phenotype of p-p38 MAPK immunoreactive cells in each section was confirmed by overlaying the p-p38 MAPK images with the OX42 image. The observer quantifying the sections was blind to the condition of the sample being quantified.
Statistical analysis
Statistical analysis was conducted using Sigma Plot (Version 11.0, Systat software Inc, San Jose, CA) software. The effects of treatment on withdrawal thresholds were examined at each time point using the Kruskal-Wallis test. Significant effects were followed by pairwise comparisons of the mean ranks of the treatment groups at each time point using Student Newman Keuls post hoc test. The effects of treatment over time on withdrawal thresholds were examined using Friedman repeated-measures analysis of variance on ranks test. If significant effects were found pairwise comparisons of the mean ranks at each time point within a treatment group were conducted using Student Newman Keuls post hoc test. If group sizes were not equal Dunn's test was conducted for post hoc analysis. Immunocytochemical data was analyzed using a two way ANOVA assessing for effects of treatment and side followed by Bonferroni post hoc tests for pairwise comparisons. To examine whether markers of microglial activation (p-p38 MAPK immunoreactivity and IBA1 immunoreactivity) were associated with measures of mechanical hypersensitivity following sham procedure or plantar incision linear regression analysis was conducted using a nonparametric Spearman's rho test. The criterion for statistical significance for all analysis was P< 0.05.
Results
Acute intrathecal administration of CCL2 sequestering antibody24 hours following incision partially reverses mechanical hypersensitivity
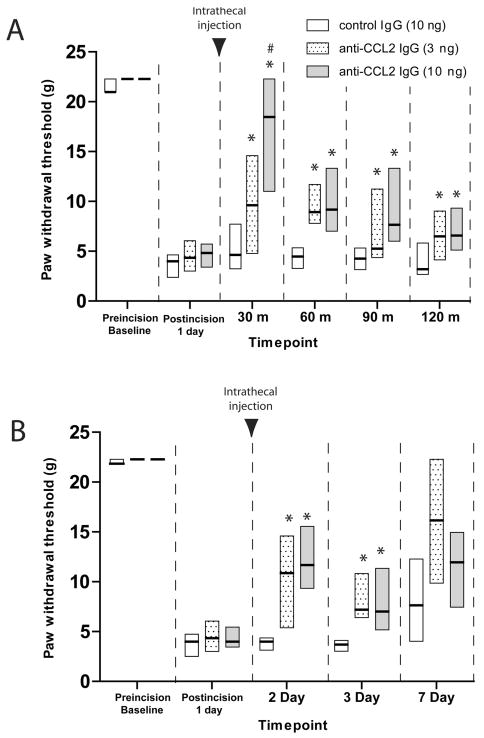
To examine the contribution of endogenous CCL2 to mechanical hypersensitivity following surgical incision, we measured the acute behavioral effect of intrathecal anti-CCL2 neutralizing antibody (10 ng, 3 ng) on mechanical withdrawal thresholds when administered 24 hours following plantar incision. Withdrawal thresholds were significantly reduced 24 hours following incision compared to baseline values (P<0.05) in all groups examined (Figure 1A.) Both doses of anti-CCL2 antibody significantly attenuated mechanical hypersensitivity compared to control IgG treated rats at 30, 60, 90 and 120 minutes post administration. At 30 minutes post administration the higher dose of anti-CCL2 antibody (10 ng) increased paw withdrawal thresholds significantly greater than the 3 ng dose indicating a dose dependent attenuation of mechanical hypersensitivity at this time point (Figure 1A, P<0.05).
Figure 1.
Time course of mechanical hypersensitivity induced by paw incision following intrathecal treatment with anti-CCL2 IgG (3 ng, dotted bars; 10 ng, gray bars) or control IgG (10 ng, open bars) was examined acutely for several minutes following treatment beginning one day post surgical incision (A) and for several days following single treatment beginning one day post surgical incision (B). Withdrawal thresholds to von Frey filaments values are expressed as the median (solid line) and 25th and 75th percentiles of 8-14 rats per group. * P< 0.05 for comparisons to control IgG values at each timepoint or # P<0.05 for comparison between 3 ng anti-CCL2 and 10 ng ant-CCL2 treated values at each timepoint by Kruskal-Wallis analysis of variance on ranks followed by Dunn's post hoc test. Black arrowhead indicates timepoint of intrathecal injection.
Acute intrathecal administration of anti-CCL2 IgG following plantar incision reduces mechanical hypersensitivity for several days
Because single spinal administration of exogenous CCL2 has been shown to induce long term persistent changes in microglial activation and pain facilitation 20,22, we examined the ability of anti-CCL2 neutralizing antibody to reduce mechanical hypersensitivity at later time points post administration. In a separate group of rats, a single administration of 3 ng or 10 ng anti-CCL2 IgG or control IgG was administered intrathecally one day following incision and rats were assessed for mechanical hypersensitivity on day 2, 3 and 7 post incision (Figure 1B). Both doses of anti-CCL2 IgG significantly reduced mechanical hypersensitivity day 2 and 3 post incision (1 and 2 days post administration) compared to control IgG (P<0.05, Kruskal-Wallis followed by Dunn's test). The difference in the median values of the treatment groups 7 days post-incision was not significantly different compared to values from control IgG treated rats (P=0.264, Kruskal-Wallis).
Effects of spinal blockade of CCL2 on microglial activation following plantar incision
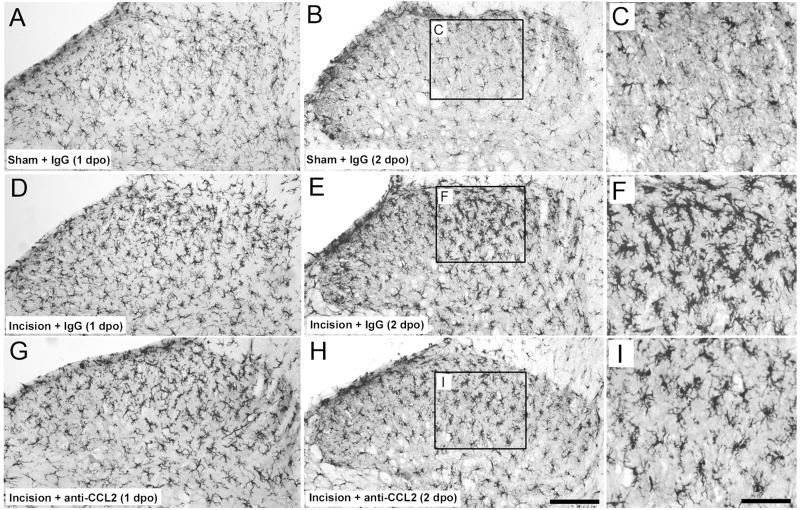
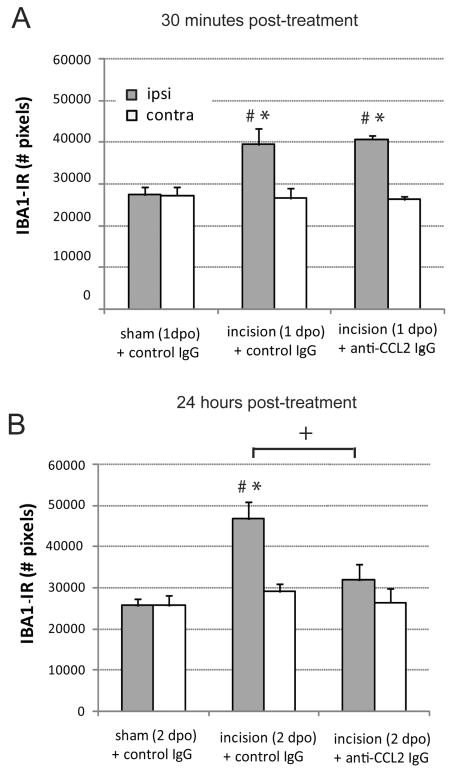
Spinal microglia are activated following plantar incision as indicated by increases in the cell surface protein CD11b (OX42), upregulation of the microglial specific protein IBA1 and increased levels of phosphorylated p38 MAPK. Immunohistochemical staining of IBA1 was significantly greater in the spinal cord dorsal horn ipsilateral to plantar incision compared to the ipsilateral dorsal horn of rats with sham surgery 24 and 48 hours following surgery (Figures 2 and 3, P<0.05). Rats with plantar incision had significantly greater number of IBA1 immunoreactive pixels in the ipsilateral spinal cord dorsal horn 1 day and 2 days postoperatively compared to contralateral values (Figure 3, P<0.05). Microglia in the spinal cord of incised rats exhibited characteristic morphology of activation including larger cell bodies and thicker processes compared to the thin and highly ramified processes of microglia within the spinal cord of rats that underwent sham procedure (Figure 2). We examined the short and long term effects of blocking spinal CCL2 on the number of IBA1 immunoreactive pixels beginning 24 hours postoperatively following plantar incision. Rats treated with anti-CCL2 IgG via percutaneous lumbar intrathecal injections had similar number of IBA1 immunoreactive pixels in the ipsilateral dorsal horn as in control IgG treated incision rats 30 minutes following administration (Figure 3A). Conversely, when IBA1 was examined 24 hours following spinal anti-CCL2 treatment the number of pixels was significantly reduced in the ipsilateral dorsal horn compared to incision vehicle treated rats (Figure 3B). At this later time point the IBA1 immunoreactive pixel values from ipsilateral dorsal horn of CCL2 treated rats were not significantly different compared to contralateral CCL2 treated values (P=0.832) or values from rats that underwent sham procedures (P=0.226)
Figure 2.
Representative images of microglial staining with an antibody against ionized calcium-binding adapter molecule 1 (IBA1). Ipsilateral lumbar dorsal spinal cord of rats that underwent sham procedures administered control IgG (10ng; A, B, C) or ipsilateral spinal cord of rats with plantar incision administered 10 ng anti-CCL2 IgG (G, H, I) or 10 ng control IgG (D, E, F). Microglial IBA1 immunostaining was increased in the ipsilateral dorsal spinal cord of rats 1 and 2 days postoperatively (1dpo and 2dpo) following plantar incision compared to levels in rats that underwent sham procedure. A single intrathecal injection of anti-CCL2 IgG (10 ng) administered 1 day following plantar incision reduced the levels of IBA1-IR in the spinal cord of incision rats 24 hours following administration (H compared to E) but not 30 minutes following administration (G compared to D)compared to incision rats that received intrathecal control IgG. Insets depict the morphological differences in microglia 48 hours following incision. Not increased size of microglia in control IgG treated rats versus anti-CCL2 treated rats (F compared to I). Scale bar in H = 150 micron, Scale bar in I = 120 microns.
Figure 3.
Quantification of ionized calcium-binding adapter molecule 1 (IBA1) immunostaining in ipsilateral (gray bar) and contralateral (open bar) dorsal spinal cord of sham operated and incision rats 30 minutes following intrathecal administration of anti-CCL2 (10 ng) or control IgG (10 ng) on day 1 post operatively (1dpo; A) or 24 hours following administration of anti-CCL2 (10 ng) or control IgG (10 ng) on day 2 postoperatively (2 dpo; B). * P<0.05 compared with sham + control IgG value within side. # P<0.05 compared to contralateral side within treatment group. +P<0.05 compared to incision control IgG treated values by two way ANOVA with Bonferroni post hoc test. n=8 rats per group.
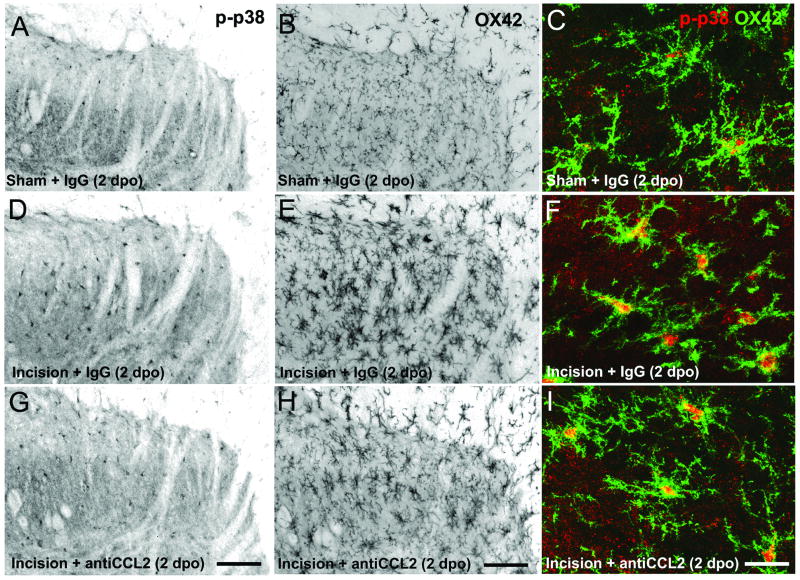
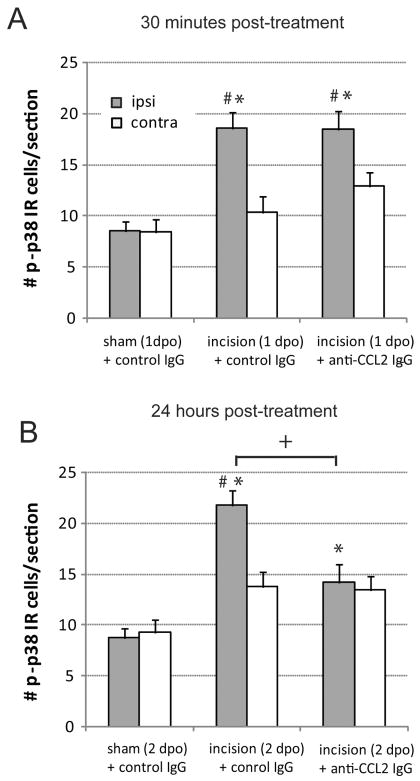
We also examined the number of p-p38 MAPK immunoreactive microglia. Spinal cord sections were labeled with antibodies against p-p38 MAPK and the microglial cell surface antigen CD11b (OX42, Figure 4). Immunohistochemistry for both markers was performed in the same sections to confirm co-labeling of p-p38 MAPK in microglia (Figure 4C, F, I) and allow for cell type specific quantification. Incision increased the number of p-p38 MAPK microglia ipsilateral to surgery compared to sham operated rats and compared to the contralateral side 24 and 48 hours following incision (Figure 5; P<0.05). Anti-CCL2 IgG did not alter the number of p-p38 MAPK microglia in the ipsilateral dorsal horn 30 minutes following administration on the first postoperative day compared to vehicle (Figure 5A). Likewise the number of p-p38 MAPK microglia were significantly greater in ipsilateral versus contralateral spinal cord sections of anti-CCL2 treated rats 30 minutes post treatment (Figure 5A). Twenty four hours following treatment the number of p-p38 MAPK immunoreactive microglia were significantly reduced in the ipsilateral dorsal horn of anti-CCL2 treated incision rats compared to control IgG treated incision rats, although not to the levels in rats that underwent sham procedures treated with control IgG (Figure 4 D,G; Figure 5B).
Figure 4.
Representative images of phosphorylated p38 MAP kinase (p-p38 MAPK) labeling (A, D, G) and Cd11b (OX42; B, E, H) in the ipsilateral dorsal spinal cord of sham operated (A, B, C) and incision rats treated with 10 ng control IgG (D, E, F) or 10 ng anti-CCL2 IgG (G, H, I) rats 2 days postoperatively (2 dpo) following plantar incision. Rats received a single acute administration of anti-CCL2 IgG (10 ng) on day 1 following plantar incision. High power confocal images show colocalization of p-p38 MAPK (red) and OX42 (green). Note increased levels of cytoplasmic p-p38 MAPK in OX42 immunoreactive microglia of incision rats treated with control IgG compared to sham operated rats (F compared to C) and incision rats treated with anti-CCL2 IgG (F compared to I). Fluorescent images in panels A, B, D, E, G, H were inverted in Photoshop (Adobe Systems Incorporated, San Jose, CA) and converted to grayscale to enhance contrast. Scale bars in G and H = 75 µm and scale bar in I = 5 µm
Figure 5.
Quantification of microglial phosphorylated p38 mitogen activated protein kinase immunostaining in ipsilateral (gray bar) and contralateral (open bar) dorsal spinal cord of sham operated and incision rats 30 minutes following intrathecal administration of anti-CCL2 (10 ng) or control IgG (10 ng) on day 1 post operatively (1dpo; A) or 24 hours following administration of anti-CCL2 (10 ng) or control IgG (10 ng) on day 2 postoperatively (2 dpo; B). * P<0.05 compared with sham + control IgG value within side. # P<0.05 compared to contralateral side within treatment group. +P<0.05 compared to incision control IgG treated values by two way ANOVA with Bonferroni post hoc test. n=8 rats per group.
Correlation of markers of microglial activation to mechanical hypersensitivity following plantar incision
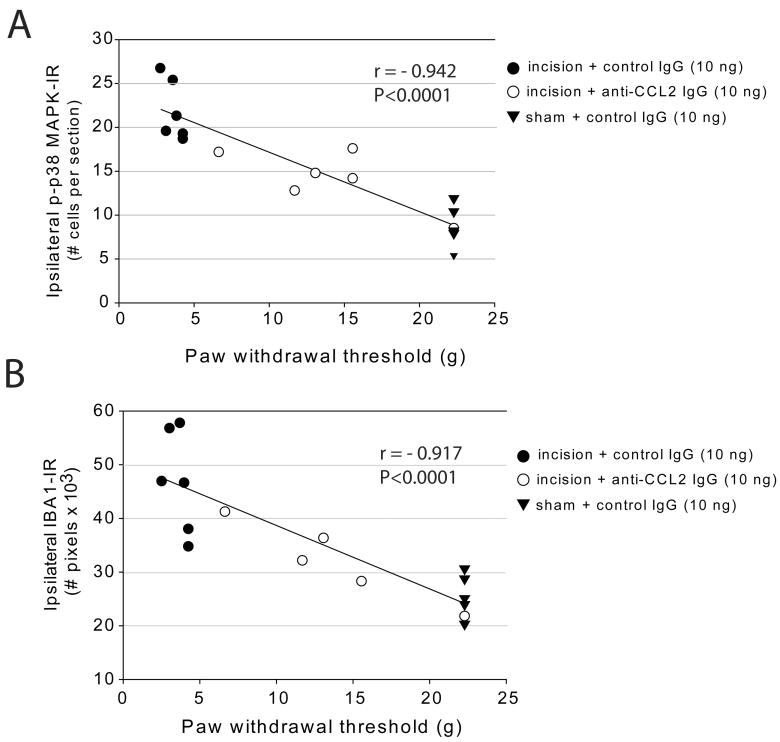
In a separate group of rats, we conducted behavioral analysis and collected spinal cord tissue 48 hour following plantar incision or sham operation and 24 hours following spinal treatment with anti-CCL2 antibodies or control IgG. We observed a strong correlation between the number of p-p38 MAPK immunoreactive microglia in the ipsilateral spinal cord and the degree of mechanical hypersensitivity in rats with plantar incision or sham procedure based on linear regression analysis (Figure 6A). There was also a strong correlation between number of IBA1 immunoreactive pixels in the spinal cord and mechanical hypersensitivity at this time point (Figure 6B).
Figure 6.
Correlation between spinal markers of microglial activation and behavioral measures of mechanical hypersensitivity 2 days following surgical incision or sham procedure. The association between number of microglial IBA1-IR pixels (A) or number of microglia positive for phosphorylated p38 mitogen activated protein kinase (B) and mechanical paw withdrawal thresholds (g) were examined using Spearman rho non-parametric linear regression analysis.
Discussion
In the current study, we provide behavioral evidence that endogenous spinal CCL2 contributes to mechanical hypersensitivity following surgical incision. First, we demonstrate that acute blockade of spinal CCL2 with a neutralizing antibody partially reverses mechanical hypersensitivity within minutes of administration independent of effects on microglial activation. Second, we demonstrate that a single spinal administration of anti-CCL2 antibody attenuates mechanical hypersensitivity for days following incision by inhibiting microglial activation and p38 MAPK signaling. These results carry implications for the mechanisms of neural glial signaling in pain facilitation after incision and for postoperative pain treatment.
CCL2 also known as monocyte chemotactic protein 1 is a small 14 kDa protein which signals through the G-protein coupled receptor CCR2. CCL2, similar to other chemokines, was initially identified as an immunomodulatory factor that regulates activation and migration of peripheral immune cells. Recently, it has been shown to have a neuromodulatory role in spinal nociceptive processing (For review see 23). CCL2 messenger RNA and protein are constitutively expressed in a population of predominantly small and medium sized neurons within the dorsal root ganglia (DRG) 22. CCL2 immunoreactivity colocalizes with markers of peptidergic 20,22 and non-peptidergic 20,24 primary afferents in the DRG and their central terminals within the dorsal horn of the spinal cord 25. Under normal conditions, CCL2 is not present at detectable levels within non-neuronal cells including microglia and astrocytes of the rat spinal cord22,26,27. However, CCL2 may be upregulated in sensory neurons of rats following peripheral nerve injury and inflammation 20,22,25-29 and has also been shown to be upregulated within spinal cord astrocytes following chronic constriction injury of the sciatic nerve in mice 30.
CCR2 receptors have been localized in the spinal cord based on autoradiographical binding studies that reported dense binding of radiolabeled CCL2 in the dorsal horn (laminae I-IV)29. There is some debate regarding the cell type expression of CCR2 within the spinal cord and immunohistochemical and transgenic approaches have provided evidence of CCR2 on predominantly microglia and macrophage 31,32 while other studies report it predominantly in second order neurons and terminals of primary afferents30,33. Therefore, in the context of postoperative pain state primary afferent derived CCL2 may have actions on both neuronal as well as non-neuronal cell populations within the spinal cord.
In the current study, we provide evidence that endogenous spinal CCL2 contributes in part to mechanical hypersensitivity following surgical incision in the rats. In support of our findings, there is substantial evidence that endogenous CCL2 and CCR2 are involved in the development of mechanical hypersensitivity in acute and persistent pain states. CCR2 antagonists attenuate mechanical hypersensitivity in neuropathic pain models 20,28,30 and transgenic mice lacking CCR2 do not develop mechanical hypersensitivity following partial sciatic nerve ligation 32. In two recent studies, intrathecal administration of a CCL2 neutralizing antibody attenuated ipsilateral mechanical allodynia for several days following chronic constriction injury in rats 20 and transiently reversed mechanical hypersensitivity following spinal nerve ligation in mice 30. In acute inflammatory pain states, CCR2 knockout mice exhibited decreased phase II formalin induced nociceptive behavior and a modest attenuation of mechanical allodynia induced by hindpaw injection of complete Freund's adjuvant. Spinal administration of exogenous CCL2 in naïve rats induces mechanical allodynia which is sustained for several days following single injection 20,22 suggesting long term effects of CCL2 on spinal pain processing.
In the current study, we provide evidence that microglial mechanisms may be relevant to CCL2 induced central sensitization in the postoperative pain setting. We observed a reduction in IBA1 immunoreactivity and a reduced number of p-p38 MAPK immunoreactive microglia in the ipsilateral spinal cord of rats with plantar incision 24 hours following administration of neutralizing anti-CCL2 antibody. Consistent with our findings, there is substantial evidence in support of a microglial mediated mechanism contributing to CCL2s pro hypersensitivity effects. A causal relationship between microglial activation and CCR2 mediated signaling was initially suggested based on observations that upregulation of CCL2 in primary afferent nerve terminals correlates with the spatial and temporal profile of activated microglia within the spinal cord following peripheral nerve injury 27 and exogenous administration of CCL2 in the spinal cord of naïve rats induces extensive spinal microglial activation at the site of injection 20 as indicated by increased OX42 immunoreactivity. Additionally, spinal CCR2 antagonists attenuate microglial activation (reduce OX42 immunoreactivity) following peripheral nerve injury 20 and CCR2 knockout mice have a reduced number of p-p38 MAPK positive microglia following partial sciatic nerve injury compared to wild type controls 32. Similar to our results, Wen et al. observed a significant increase in the number of p-p38 MAPK immunoreactive microglia one and two days post hindpaw incision in rats. In their study, a single intrathecal injection of the p38 inhibitor ZR167653 prior to incision attenuated mechanical hypersensitivity and reduced the number of p-p38 MAPK microglia following incision. These results and our findings demonstrating a strong correlation between measures of mechanical hypersensitivity and the number of p-p38 MAPK microglia in the ipsilateral spinal cord of treated rats suggest that microglial signaling via p38 MAPK is important for the persistence of mechanical hypersensitivity following surgical incision. In contrast to our findings, Ito et al. reported that single systemic administration of the microglial inhibitor minocycline only slightly attenuated mechanical hypersensitivity when administered acutely one day after plantar incision 38. In the same study, intrathecal administration of the p38 inhibitor SB203580 one and three days after incision was without effect. Chronic administration of minocycline twice daily for three days after surgical incision failed to attenuate mechanical hypersensitivity despite producing a reduction in OX42 immunoreactive microglia. While these data suggest microglia may have a minor role in postoperative pain, it is also possible that the systemic doses of minocycline and p38 inhibitor used in this former study were insufficient to block phosphorylation of p38 MAPK which appears to be essential for the development and maintenance of mechanical hypersensitivity in this postoperative pain model 14. The effect of preemptive systemic minocycline on mechanical hypersensitivity and levels of p-p38 MAPK were not examined in the former study. This may be an important distinction as OX42 immunoreactivity does not parallel the development of mechanical hypersensitivity following incision unlike the upregulation of p-p38 MAPK and IBA1 in microglia which has been observed as early as one to 24 hours following plantar incision, respectively. Several studies suggest that microglial changes in IBA1 immunoreactivity or levels of p-p38 MAPK in microglia may be a more functionally relevant marker of microglial activation particularly in acute pain states 10,34,35.
Interestingly in the current study, spinal neutralization of CCL2 rapidly attenuated mechanical hypersensitivity within 30 minutes of administration but failed to reduce the number of p-p38 MAPK immunoreactive microglia or IBA1 immunoreactivity at this acute time point. There are several explanations for the lack of acute effect on microglial activation. First, it is possible that the immunohistochemical approaches used in the current study are not sensitive enough to detect acute effects of treatment. However, several studies have observed acute effects of pharmacological agents on p-p38 MAPK immunoreactivity in the peripheral and central nervous system 36. Another possibility is that CCL2 contributes to mechanical hypersensitivity at early time points by direct actions on spinal cord neurons in addition to the delayed effects on microglia that we observed at 48 hours following incision. There is increasing in vitro and in vivo evidence that CCL2 directly excites or sensitizes neurons in the superficial laminae of the spinal cord and DRG. Spinal administration of exogenous CCL2 in vivo induces phosphorylation of the mitogen activated protein kinase pERK in neurons within the superficial dorsal horn and in vitro following application to spinal cord slices 30. Patch clamp recordings of lamina II spinal cord neurons have been conducted in slice preparations to examine direct functional effects of CCL2. Bath application of CCL2 increased excitatory synaptic transmission as indicated by an increased frequency and amplitude of spontaneous excitatory postsynaptic currents 30. Whole cell patch clamp recordings of cultured spinal neurons demonstrated that CCL2 inhibits gamma amino butyric acid (GABA) induced currents suggesting a neuromodulatory role for this chemokine 29. The presence of CCL2 in primary afferents fibers and localization of CCR2 on sensory neurons suggest that CCL2 may act in an autocrine or paracrine manner on the central terminals of primary afferent fibers to promote the release of neurotransmitters into the spinal cord. In support of this, CCL2 has been shown to trigger the release of calcitonin gene related peptide from cultured DRG neurons 37 It is not known if similar mechanisms occur following surgical incision. Clearly, additional research is warranted to more precisely define the mechanisms by which CCL2 modulates nociceptive processing in the context of postoperative pain.
In summary, we administered a CCL2 neutralizing antibody beginning one day following plantar incision in the rat after the postoperative pain state was established. Using this post-treatment paradigm, we observed that spinal blockade of CCL2 attenuated mechanical hypersensitivity within minutes of administration an effect that was sustained for several days. These findings establish a role for spinal CCL2 mediated signaling in the maintenance of mechanical hypersensitivity following surgical incision and suggest that spinal CCL2 antagonists may have utility for treating postoperative pain.
Acknowledgments
Source of funding: This work was supported from grant GM48085 from the National Institute of General Medical Sciences (Bethesda, Maryland) and an Individual Postdoctoral Ruth L. Kirchstein National Research Service Award 1F32GM089076-01 from the National Institute of General Medical Sciences and the National Institutes of Health (Bethesda, Maryland).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: A modelling strategy based on available data. Lancet. 2008;372:139–44. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 3.Pavlin DJ, Chen C, Penaloza DA, Buckley FP. A survey of pain and other symptoms that affect the recovery process after discharge from an ambulatory surgery unit. J Clin Anesth. 2004;16:200–6. doi: 10.1016/j.jclinane.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Pavlin DJ, Chen C, Penaloza DA, Polissar NL, Buckley FP. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg. 2002;95:627–34. doi: 10.1097/00000539-200209000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 6.Buvanendran A, Kroin JS, Kerns JM, Nagalla SN, Tuman KJ. Characterization of a new animal model for evaluation of persistent postthoracotomy pain. Anesth Analg. 2004;99:1453–60. doi: 10.1213/01.ANE.0000134806.61887.0D. [DOI] [PubMed] [Google Scholar]
- 7.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: A model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M. Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J Pain. 2006;7:816–22. doi: 10.1016/j.jpain.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL. Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J Neurochem. 2005;92:1508–20. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- 11.Svensson CI, Hua XY, Protter AA, Powell HC, Yaksh TL. Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. Neuroreport. 2003;14:1153–7. doi: 10.1097/00001756-200306110-00010. [DOI] [PubMed] [Google Scholar]
- 12.Romero-Sandoval A, Chai N, Nutile-McMenemy N, Deleo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–26. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroin JS, Takatori M, Li J, Chen EY, Buvanendran A, Tuman KJ. Upregulation of dorsal horn microglial cyclooxygenase-1 and neuronal cyclooxygenase-2 after thoracic deep muscle incisions in the rat. Anesth Analg. 2008;106:1288–95. doi: 10.1213/ane.0b013e318163faa6. [DOI] [PubMed] [Google Scholar]
- 14.Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, Kohno T, Sun WZ, Wang CC. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009;110:155–65. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- 15.Ririe DG, Prout HD, Barclay D, Tong C, Lin M, Eisenach JC. Developmental differences in spinal cyclooxygenase 1 expression after surgical incision. Anesthesiology. 2006;104:426–31. doi: 10.1097/00000542-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X, Conklin D, Eisenach JC. Cyclooxygenase-1 in the spinal cord plays an important role in postoperative pain. Pain. 2003;104:15–23. doi: 10.1016/s0304-3959(02)00465-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhu X, Conklin DR, Eisenach JC. Preoperative inhibition of cyclooxygenase-1 in the spinal cord reduces postoperative pain. Anesth Analg. 2005;100:1390–3. doi: 10.1213/01.ANE.0000148127.53832.8E. [DOI] [PubMed] [Google Scholar]
- 18.Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106:787–94. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- 19.Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 20.Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13:263–72. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 22.Dansereau MA, Gosselin RD, Pohl M, Pommier B, Mechighel P, Mauborgne A, Rostene W, Kitabgi P, Beaudet N, Sarret P, Melik-Parsadaniantz S. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J Neurochem. 2008;106:757–69. doi: 10.1111/j.1471-4159.2008.05429.x. [DOI] [PubMed] [Google Scholar]
- 23.Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–34. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeon SM, Lee KM, Park ES, Jeon YH, Cho HJ. Monocyte chemoattractant protein-1 immunoreactivity in sensory ganglia and hindpaw after adjuvant injection. Neuroreport. 2008;19:183–6. doi: 10.1097/WNR.0b013e3282f3c781. [DOI] [PubMed] [Google Scholar]
- 25.White FA, Wilson NM. Chemokines as pain mediators and modulators. Curr Opin Anaesthesiol. 2008;21:580–5. doi: 10.1097/ACO.0b013e32830eb69d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: Possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48:463–9. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–83. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- 28.Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: A mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, Melik-Parsadaniantz S. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem. 2005;95:1023–34. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- 30.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–52. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, Miller RJ. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J Neurosci. 2009;29:8051–62. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–40. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- 35.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–44. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 36.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005;82:51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- 38.Ito N, Obata H, Saito S. Spinal microglial expression and mechanical hypersensitivity in a postoperative pain model: comparison with a neuropathic pain model. Anesthesiology. 2009;111:640–8. doi: 10.1097/ALN.0b013e3181b05f42. [DOI] [PubMed] [Google Scholar]