Abstract
Background Dementia is considered widely under‐detected in primary care, and general practitioners (GPs) frequently ask for easy to use tools to assist in its early detection.
Aim To determine the degree of correlation between the Mini‐Cog Assessment (Mini‐Cog) as performed by GPs and the Mini‐Mental State Examination (MMSE).
Design of study This was a prospective study (2005, 2006) comparing two cognitive screening instruments.
Setting Ten general practices in Austria, with patients with a hitherto undiagnosed suspicion of dementia seen consecutively.
Method Sensitivity, specificity and positive and negative predictive values (PPVs and NPVs) of the Mini‐Cog (applying both a colour‐coded and the original rating method) were assessed for degree of correlation with the MMSE. In phase one GPs examined patients suspected of having dementia using the Mini‐Cog; in phase two a neurologist retested them applying the MMSE, a clock‐drawing test (CDT) and a routine clinical examination. A questionnaire on the practicability of the Mini‐Cog was answered by GPs.
Results Of the 107 patients who participated 86 completed the whole study protocol. The Mini‐Cog, as performed by the ten GPs, displayed a sensitivity of 0.85 (95% CI: 0.71, 0.98), a specificity of 0.58 (95% CI: 0.46, 0.71), a PPV of 0.47 (95% CI: 0.33, 0.61) and an NPV of 0.90 (95% CI: 0.80, 0.99) as against the MMSE carried out by neurologists. The GPs judged the Mini‐Cog useful and time saving.
Conclusion The Mini‐Cog has a high sensitivity and acceptable specificity in the general practice setting and has proved to be a practicable tool for the diagnosis of dementia in primary care.
Keywords: dementia, early detection, family practice
Introduction
There is ample evidence that the treatment of mental disorders in primary care has many advantages for patients including, in particular, easier access to medical care and the avoidance of stigmatisation, an aspect which is especially important when alarming symptoms point towards incipient dementia. 1 Despite these obvious advantages GPs and specialists are often undecided as to which patients should be referred to and treated in secondary care. 2
Disorders of the central nervous system, in particular dementia, are a main cause of chronic disability and the need for permanent home care in the elderly.3,4 GPs are ideally placed to recognise and treat dementia. 5 In most cases they have been familiar with their patients for a number of years, so that the potential decline in intellectual capacities, cognitive abilities and ways of coping with everyday tasks can be directly observed. 6 Nevertheless, dementia remains widely under‐detected in general practice and recommendations to screen for it in primary care are controversial. 7–10 It has been suggested that, in addition to clinical judgement, GPs should make use of a cognitive test for the early detection or reliable exclusion of this disorder. 11
There are many tests for the early detection of dementia, but not all are appropriate for use by GPs. 12–14 The ideal test, or tool, should be very brief, simple, sensitive, acceptable to older patients and uninfluenced by poor education and/or language barriers. 15 The Mini‐Cog fulfils these conditions in a near‐perfect manner.16, 17 It makes use of the strongest component of the MMSE, 18 namely the three‐item recall (remembering three words after roughly one minute), 19 and a CDT, which plays an essential role worldwide in the early detection of dementia. 20
In using the Mini‐Cog, Strotzka et al observed that a number of their patients passed the three‐word recall without any trouble, but that some of these scored badly on the clock‐drawing part of the test. 21 From their clinical experience they felt that these patients were at a higher risk of developing dementia in the near future. Consequently, they suggested a slightly different colour‐coded evaluation method for the Mini‐Cog that gives equal weight to both parts of the test. In addition, it was felt that the use of colour‐coding would make the test easier to use and thus encourage more GPs to take advantage of it.
Our aim was to determine whether the Mini‐Cog proved adequate in helping GPs to detect dementia during a routine visit and, in particular, to assess to what extent the results of the Mini‐Cog – as performed in the GP's surgery – agreed with or differed from those of the MMSE subsequently performed by a neurologist, since there is evidence that the MMSE is still the cognitive screening instrument preferred by psychogeriatricians worldwide. 22 We also assessed whether there is any meaningful difference between the results of the test's original rating method and the colour‐coded method.
The authors of this study declare explicitly that they applied and used the Mini‐Cog published by Borson and Scanlan 15 and are not suggesting or wishing to create a new name for a well‐established test with the intention of bypassing copyright.
Methods
Study design
This was a two‐phase comparison study of two screening tools, carried out ‘blind’. In phase one, patients were tested by the GP using the colour‐coded Mini‐Cog; in phase two by a neurologist using the MMSE, including a CDT and a routine clinical evaluation. Careful attention was paid by both the GP and the neurologist to accurate documentation of the patient's medical history, including any relevant information supplied by family members. This is the standard approach of GPs and neurologists in Austria and one designed to meet State health insurance requirements for MMSE results.
Participating GPs were asked to reply to the questionnaire using an anonymous data sheet, including information on patients' socio‐economic status, level of education and co‐morbidities. The results of the Mini‐Cog were entered on a data sheet. Patients were not told their test results at this stage but were referred to a neurologist for retesting within six weeks (the neurologist also remained uninformed as to the result of the GP's test). As part of his/her routine clinical examination the neurologist performed an MMSE and a CDT using the Sunderland method. 20 All the findings were then sent to the evaluating study centre, where the results of the GPs' clock‐drawing part of the Mini‐Cog were subjected to a ‘control check’ by an experienced psychiatrist. After being informed about the study's aim and procedures patients were asked to sign an informed consent form. The study period was from June 2005 to September 2006.
Test description
With the original form of the Mini‐Cog a score of 0 to 3 marks is given for the recall test, one point being given for each word remembered after the CDT. A score of 0 or 2 is awarded for the CDT part of the test – 2 points for a correct drawing, none for a wrong one. To obtain the full Mini‐Cog score the recall and CDT scores were combined. A score of 3 and below indicates a suspicion of dementia. In most cases the Mini‐Cog takes no more than three minutes to perform.
In contrast, with the modified colour‐coded evaluation method we gave both parts of the Mini‐Cog the same weight, three colours (red, yellow, green) being used as ratings. Patients were given three words (for example ‘book’, ‘house’, ‘flower’), which they had to repeat. They were then asked to draw a clock face showing all twelve numbers and with the hands pointing to ten past eleven. When this was done, they were asked to recall the three words.
For their clock‐drawing performance patients were awarded one of three colours – green for a perfect clock face with all 12 numbers shown, the number 12 correctly positioned and the hour and minute hands pointing exactly to 11 and 10; yellow for a good clock face with minor errors but wrongly positioned hands; and red for a clock face with major errors, that is, with hands missing or numbers wrongly ordered. Figure 1 shows examples of three different test results.
Figure 1.
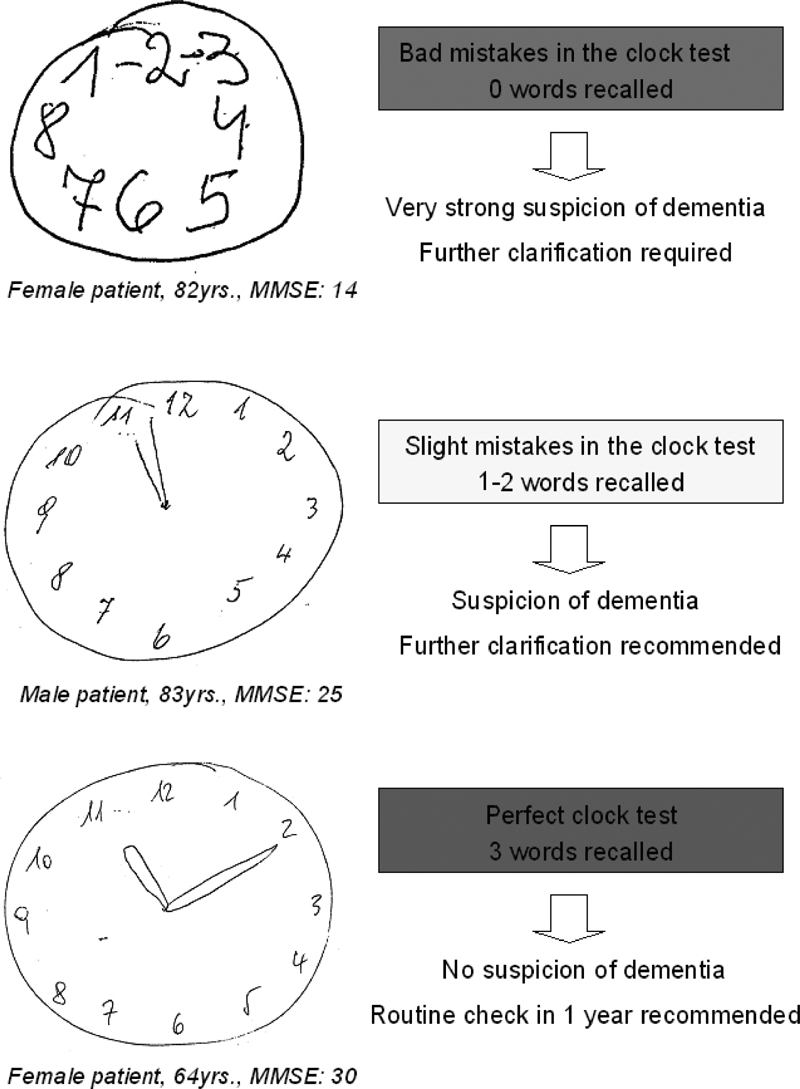
Examples of three different CDT results
The same colour system was used for the word recall part of the Mini‐Cog. Green was awarded for correct recall of all three words, yellow for one or two words, and red for no words remembered. The results of the two parts of the Mini‐Cog were then combined to give the ratings red/red, red/yellow, yellow/red, red/green, green/red, yellow/yellow, yellow/green, green/yellow, and green/green, the first colour being the assessment of the clock drawing, the second that of the word recall. Patients with a test result of red/red, red/yellow, yellow/red, red/ green, green/red or yellow/yellow were considered to be under serious suspicion of dementia. The results yellow/green, green/yellow and green/green indicated no suspicion of the disorder.
The GPs were asked to give their opinion of the colour‐coded Mini‐Cog by answering four questions put to them at the end of the study, applying a rating system ranging from one (top score) to five (no score).
Participants
Patients in primary care suspected of having impaired memory or cognitive decline, selected consecutively by GPs in the provinces of Lower Austria and Vienna.
Recruitment strategies
Participants were recruited by ten different GPs from their own practices. Inclusion criteria were a minimum age of 65 and the suspicion by the GP, the patient him/herself, or a close relative of impaired memory or cognitive decline. The patient's visit to the doctor was not necessarily in connection with that particular complaint. Exclusion criteria were: 1) patients dependent on the care of others and unable to give their consent and 2) patients already diagnosed with dementia or mild cognitive impairment (MCI).
Analysis
We assessed the sensitivity and specificity of both the original and the colour‐coded Mini‐Cog along with the positive and negative predictive values and 95% confidence intervals. Cohen's Kappa coefficient was used for measuring the agreement between the two evaluation methods. We used Fisher's Exact test and the Chi‐square test to determine any significant correlation between the two test parts of the modified Mini‐Cog and between these and the equivalent tests carried out by the neurologist. We also determined the correlation between the CDT as evaluated by the physician and the quality control check performed by a psychiatrist. The degree of correlation was measured applying the contingency coefficient C and Spearman's rank correlation coefficient. For statistical analyses and graphs we used the R Language and Environment for Statistical Computing and Graphics, Version 2.4.1, and Epi Info, Version 3.2.2, CDC, Atlanta.
Results
In all, 107 patients (m: 32; f: 75) were selected, and 86 (m: 24; f: 62) completed the study. Ages ranged from 65 to 98 years. Participants' data are listed in Table 1.
Table 1.
Baseline data of whole study population and of those who were neuropsychologically tested
|
| ||||
|---|---|---|---|---|
| Baseline personal data | Study population (n = 107) | Neuropsychologically tested (n = 86) | ||
|
| ||||
| Mean (SD) age | 77.5 | (6.8) | 77.3 | (6.9) |
| No. (%) of women | 75 | (70) | 63 | (72) |
| No. (%) with higher education* | 50 | (47) | 43 | (50) |
| Regular physical activity (at least one hour a week) N (%) | 16 | (15) | 15 | (17.4%) |
| No. (%) with chronic illnesses† | ||||
| Coronary heart disease | 30 | (28) | 23 | (26.7) |
| Diabetes (NIDDM) | 22 | (20.6) | 13 | (15.1) |
| Hypertension | 20 | (18.7) | 13 | (15.1) |
| Cerebral occlusive disease (TIA, PRIND, not stroke) | 17 | (15.9) | 12 | (13.9) |
| Chronic heart failure | 13 | (12.1) | 11 | (12.8) |
|
| ||||
*Further schooling following the eight years of basic education
†Frequency in study population > 10%
As Table 2 shows, the Mini‐Cog with the colour‐coded (positive) results ‘red/red’, ‘red/yellow’, ‘yellow/red’, ‘red/green’, ‘green/red’ or ‘yellow/yellow’ has a sensitivity of 0.846 (CI 0.71–0.98) and a specificity of 0.58 (CI 0.46–0.71). Positive and negative predictive values were 0.47 (CI 0.33–0.61) and 0.90 (CI 0.80–0.99) respectively.
Table 2.
Diagnostic effectiveness of the modified and the original evaluation of the Mini‐Cog for predicting an MMSE <27*
|
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | |||||
|
| ||||||||
| Modified scheme† | 0.846 | (0.71–0.98) | 0.58 | (0.46–0.71) | 0.47 | (0.33–0.61) | 0.90 | (0.80–0.99) |
| Original scheme‡ | 0.77 | (0.56–0.91) | 0.60 | (0.47–0.72) | 0.45 | (0.31–0.58) | 0.86 | (0.71–0.95) |
|
| ||||||||
*As the traditional cut‐off point of 23/24 was regarded to be too low for our study population, a more sensitive cutoff point of <27 was felt to be more appropriate
†Colour‐coding of the clock drawing part: green – perfect drawings; yellow – small errors; red – serious errors Colour‐coding of the three‐word recall: green – all words remembered; yellow – one or two words remembered; red – no words remembered. The colour combinations red/red, red/yellow, yellow/red, red/green, green/red or yellow/yellow indicate a positive test result
‡A Mini‐Cog result of less than three points indicates a positive test result
With the original scoring method sensitivity (0.77; CI 0.56–0.91), positive (0.45; CI 0.31–0.58) and negative (0.86; CI 0.71–0.95) predictive values were slightly lower and specificity (0.6; CI 0.47–0.72) was slightly higher. So there are no relevant differences between the two with regard to these values.
Table 3 shows a detailed comparison between the original and the colour‐coded evaluation method of the Mini‐Cog. Only three cases generated different results from the two methods, resulting in a Kappa coefficient of 0.93, which means a near‐perfect agreement between both scoring methods.
Table 3.
Original and colour‐coded evaluation scheme of the Mini‐Cog in detail
|
| ||||
|---|---|---|---|---|
| Mini‐Cog original evaluation | Mini‐Cog colour‐coded evaluation* | MMSE< 27 points | MMSE≥ 27 points | Total |
|
| ||||
| Not passed | red/red | 4 | 0 | 4 |
| Not passed | red/yellow | 5 | 1 | 6 |
| Not passed | yellow/red | 0 | 2 | 2 |
| Not passed | green/red | 0 | 1 | 1 |
| Not passed | yellow/yellow | 11 | 20 | 31 |
| Passed | yellow/green | 2 | 6 | 8 |
| Passed | green/yellow | 2 | 12 | 14 |
| Passed | green/green | 0 | 17 | 17 |
| Passed† | red/green† | 2 | 1 | 3 |
| Total | 26 | 60 | 86 | |
|
| ||||
*The colour combinations red/red, red/yellow, yellow/red, red/green, green/red or yellow/yellow indicate that the test has not been passed
†In only three cases a discrepancy between the original and the colour‐coded evaluation was observed. Kappa index of both scoring systems: 0.93
A statistically significant correlation was found between the results of the clock‐drawing part of the Mini‐Cog as evaluated by the GP and those of the CDT (Sunderland method) carried out by the neurologist (P‐value<0.001; Spearman's Rho = 0.56). There was also significant agreement between the two parts of the Mini‐Cog (P<0.001; Contingency Coefficient C = 0.46).
For the majority of clock drawings independent evaluation by a psychiatrist showed exactly the same ratings as recorded by the GP. Only 11.2% were evaluated differently. It is worth noting that none of the drawings was rated ‘red’ by the GP and later (by the psychiatrist) ‘green’ or vice versa. The agreement was statistically highly significant (P‐value <0.001), and the contingency coefficient revealed a strong correlation (C = 0.77) (see Table 4).
Table 4.
Number of colour‐coded clock drawings evaluated by the general practitioner and by a second evaluation from a psychiatrist
|
| ||||
|---|---|---|---|---|
| Second evaluation by psychiatrist (colour‐coded results)* | ||||
| Evaluation of general practitioner (colour‐coded results)* | Red | Yellow | Green | Total |
|
| ||||
| Red | 20 | 1 | 0 | 21 |
| Yellow | 3 | 41 | 6 | 50 |
| Green | 0 | 2 | 34 | 36 |
| Total | 23 | 44 | 40 | 107 |
|
| ||||
*Colour‐coding of the clock drawing part of the test: green – perfect drawings; yellow – small errors; red – serious errors
The proportion of those with a positive result in the Mini‐Cog was higher (though not to a statistically significant degree) among patients who did not see a neurologist than among those who completed the whole study protocol (76% vs. 55%, P = 0.087). There was no significant difference in the mean age (78.05 vs. 77.35 years, P = 0.67). Also, the proportion of those who did not consult a neurologist was higher for male patients (38.5%) than female (21%) (see Table 5).
Table 5.
Results of the Mini‐Cog with regard to the probability of a further complete testing by the neurologist (n = 107)
|
| ||
|---|---|---|
| Mini‐Cog at GP's | Number of patients fully tested by the neurologist | |
| No | Yes | |
|
| ||
| Positive* | 16 | 47 |
| Negative† | 5 | 39 |
|
| ||
*Positive results: red/red, red/yellow, yellow/red, red/green, green/red, yellow/yellow
†Negative results: yellow/green, green/yellow, green/green
Fisher's Exact test, P‐value = 0.08677. Odds ratio = 2.63 (95% CI 0.825, 10.03)
Those with a positive clock drawing/three‐word recall are 2.6 times more likely to reject detailed neuropsychological testing than those with a negative test result
The majority of the ten participating GPs regarded the modified Mini‐Cog as easy to use (ten out of ten), time saving (eight out of ten) and causing no negative reaction on the part of the patients (nine out of ten). The referral to the neurologist was sometimes regarded as difficult (seven out of ten) (table not shown).
Discussion
The Mini‐Cog is an easy to use tool and has a high sensitivity and acceptable specificity in the general practice setting. Comparison of the Mini‐Cog performed by the GP with the MMSE carried out by the neurologist showed close agreement between the results of the two, in particular with regard to sensitivity and negative predictive value, thus making the Mini‐Cog a viable diagnostic tool for use in general practice.
The results were almost identical to those of the original non‐modified Mini‐Cog, showing only slightly lower specificity and a slightly higher sensitivity. The high Kappa coefficient indicated a near‐perfect agreement. There exists some evidence that patients with dementia of the Lewy body kind show a lower performance in the CDT. 23 The initiators of this study therefore proposed that patients who score badly in the clock‐drawing part of the Mini‐Cog should be failed, even if they remember all three items of the word recall. Our study found only three patients with a discrepancy between the colour‐coded and the original rating method, a number clearly too small to support our hypothesis.To arrive at any statistically meaningful results it would be necessary to cover a much larger group of patients.
As a rule GPs only check patients for incipient cognitive impairment or dementia when suspicion of these disorders arises. It is typical of the general practice setting to exclude the possible existence of dementia rather than detect it. If, however, there is a strong enough suspicion of the disorder the GP will, of course, advise immediate referral to a neurologist. But in many cases the situation is not so clear cut and a quick and easy test to support the clinical impression could prove helpful. On the one hand, the GP wishes to avoid unnecessary referrals, which would in many cases impair the doctor–patient relationship, the suspicion of dementia being considered stigmatising by a large number of patients. On the other hand, the GP does not want to miss the chance of an early intervention and a possible consequent benefit for the patient. Consequently, a quick test like the Mini‐Cog, well correlated with the MMSE, could in this case be helpful for the GP. Since lack of time is always a key issue in general practice, this time saving test could prove a good substitute for the MMSE and would do away with the need for longer and more complicated tests. Since the MMSE is one of the essential brief cognitive screening instruments used by neurologists, 22 and since there is a good correlation between the two tests, the GP can be confident that the result of the Mini‐Cog will predict to some degree the result of the MMSE and the clinical judgement of the neurologist, making it easier for the GP to justify a referral. Standardised brief assessment instruments have been shown to be helpful in estimating the severity of a condition and hence justifying the necessity of a referral from primary to secondary mental health care, with the goal of improved primary–secondary care communication. 24
Another important aspect made clear by our study was the reluctance of patients to consult a neurologist, an observation which was also reported in other studies. 25 Only 80% of the 107 patients underwent testing by the neurologist. Of the 20% who were not tested by the neurologist the majority (though not a statistically significant number) scored positive ratings on the GPs' Mini‐Cog, in contrast to those patients who were tested by both GP and neurologist. This 20% loss stresses the importance of an accurate, quick diagnostic test, for it gives GP the opportunity to gain valuable objective information, even when no further testing by a specialist takes place. This information could be used as a baseline from which to monitor patients' future care.
The study was focused on simulating the real‐world situation in daily general practice with its all too well known restraints of time and resources. Our intention was not to compare the efficacy of the Mini‐Cog or the MMSE in detecting dementia against a ‘gold standard’ which would require a battery of neuropsychological tests. In a post hoc analysis of a population based sample Borson et al (2003) demonstrated that the Mini‐Cog has the same sensitivity and specificity as a conventional neuropsychological battery when using an MMSE cut‐off of 25 points. 26
Cohort studies have shown memory related disorders to be a sensitive indicator in predicting future dementia. The use of a simple diagnostic tool such as the Mini‐Cog in the GP's surgery, where patients', their relatives' or the GP's own suspicions of memory failure are first expressed, is therefore justified, for it ensures a timely decision by the GP to refer his/her patient to a neurologist. 27 The GP's close and often long‐term relationship with his/her patients proves a big advantage in addressing this problem. With the aid of the two‐stage Mini‐Cog GPs can offer timely advice and reassurance to both patients and relatives.
A key merit of our study was its design. The predictive nature of the test, performed ‘blind’ and in a primary care setting and administered to patients not previously diagnosed or tested, followed by clarification by a neurologist and ‘quality control’ by a psychiatrist, not only ensures a higher measure of validity, but also emulates real‐life conditions and reveals problems that occur only during the actual diagnostic procedure.
Limitations
One of the limitations of our study was that the number of patients who complained to the GP for the first time of symptoms suggestive of dementia or MCI was not as high as it could have been. A further weakness of our study is the fact that neurologists did not use a complete battery of neurological instruments to establish a diagnosis of dementia or mild cognitive impairment. One intention of our study was to describe the use of the Mini‐Cog in general practice and its correlation with the judgment of the neurologist based on his/her use of the MMSE and clinical investigation. Since MMSE findings are still a cornerstone of the neurologist's diagnosis of dementia, resulting as a rule in full payment for specific drugs by health insurance companies (at least in Austria), we consider our study design justified.
A further limitation is that the coexistence of depression was not definitely excluded or verified by means of a standardised and validated test instrument, which means that the results could have been influenced by the presence of an underlying depressive condition.
Conclusions
The quick and easy to use Mini‐Cog with combined colour‐coded rating found a high degree of acceptance among both practitioners and patients. In view of its close correlation with the MMSE we consider it an appropriate tool for the early detection by GPs of dementia in patients suspected, for the first time, of suffering from that disorder.
ACKNOWLEDGEMENTS
The authors wish to thank the Austrian primary care physicians and their staff who helped with this study and also Professor Philip Lupton, MA cantab, PhD Vienna, for reviewing the English version of this article.
Contributor Information
Gustav Kamenski, Department of General Practice, Centre for Public Health, Medical University, Vienna, Austria.
Thomas Dorner, Institute of Social Medicine, Centre for Public Health, Medical University, Vienna, Austria.
Kitty Lawrence, General Secretary, Association Altern mit Zukunft, Vienna, Austria.
Georg Psota, Psychiatrist, Centre for Psychogeriatrics, Vienna, Austria.
Anita Rieder, Professor for Social Medicine, Institute of Social Medicine, Centre for Public Health, Medical University, Vienna, Austria.
Franz Schwarz, Vienna Institute of Demography, Austrian Academy of Sciences, Vienna, Austria.
Asita Sepandj, Psychiatrist, Centre for Psychogeriatrics, Vienna, Austria.
Wolfgang Spiegel, Department of General Practice, Centre for Public Health, Medical University, Vienna, Austria.
Stefan Strotzka, Psychologist, Centre for Psychogeriatrics, Vienna, Austria.
REFERENCES
- 1.Ivbijaro G, Kolkiewicz L, Lionis C, et al. Primary care mental health and Alma‐Ata: from evidence to action. Mental Health in Family Medicine 2008;5:67–9 [PMC free article] [PubMed] [Google Scholar]
- 2.Nolan P, Orford J, White A, et al. Professional views on managing common mental health problems in primary care. Primary Care Mental Health 2003;1:27–36 [Google Scholar]
- 3.Ferri C, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. The Lancet 2005;366:2112–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamenski G, Fink W, Maier M, et al. Characteristics and trends in required home care by GPs in Austria: diseases and functional status of patients. BMC Family Practice 2006;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.England E. Improving the management of dementia. BMJ 2006;332:681–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramakers I, Visser P, Aalten P, et al. Symptoms of preclinical dementia in general practice up to five years before dementia diagnosis. Dementia and Geriatric Cognitive Disorders 2007;24:300–6 [DOI] [PubMed] [Google Scholar]
- 7.Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Archives of Internal Medicine 2000;160:2964–8 [DOI] [PubMed] [Google Scholar]
- 8.Boustani M, Peterson B, Hanson L, et al. Screening for dementia in primary care: a summary of the evidence for the US Preventive Services Task Force. Annals of Internal Medicine 2003;138:927–42 [DOI] [PubMed] [Google Scholar]
- 9.Stoppe G, Maeck L, Haak S, et al. Early diagnosis of dementia in primary care: a representative eight‐year follow‐up study in Lower Saxony, Germany. International Journal of Geriatric Psychiatry 2006;22:23–31 [DOI] [PubMed] [Google Scholar]
- 10.Spiegel W. Should family physicians screen for cognitive impairment and/or dementia (Commentary). American Journal of Geriatric Psychiatry 2007;15:726–7 [DOI] [PubMed] [Google Scholar]
- 11.Palmer K, Bäckman L, Wingblad B, et al. Detection of Alzheimer's disease and dementia in the preclinical phase: population based cohort study. BMJ 2003;326:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodaty H, Low LF, Gibson L, et al. What is the best dementia screening instrument for general practitioners to use? American Journal of Geriatric Psychiatry 2006;14:391–400 [DOI] [PubMed] [Google Scholar]
- 13.Holsinger T, Deveau J, Boustani M, et al. Does this patient have dementia? Journal of the American Medical Association 2007;297:2391–404 [DOI] [PubMed] [Google Scholar]
- 14.Lorentz WJ, Scanlan J, Borson S. Brief screening tests for dementia. Canadian Journal of Psychiatry 2002;47:723–33 [DOI] [PubMed] [Google Scholar]
- 15.Borson S, Scanlan J, Brush M, et al. The Mini‐Cog: a cognitive ‘vital signs’ measure for dementia screening in multi‐lingual elderly. International Journal of Geriatric Psychiatry 2000;15:1021–7 [DOI] [PubMed] [Google Scholar]
- 16.Scanlan J, Borson S. The Mini‐Cog: receiver operating characteristics with expert and naive raters. International Journal of Geriatric Psychiatry 2001;16:216–22 [DOI] [PubMed] [Google Scholar]
- 17.Borson S, Scanlan JM, Watanabe J, et al. Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample. Journal of the American Geriatric Society 2005;53:871–4 [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. Mini‐mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 19.Braekhus A, Laake K, Engedal K. The Mini‐Mental State Examination: identifying the most efficient variables for detecting cognitive impairment in the elderly. Journal of the American Geriatric Society 1992;40:1139–45 [DOI] [PubMed] [Google Scholar]
- 20.Sunderland T, Hill JL, Mellow AM, et al. Clock drawing in Alzheimer's disease: a novel measure of dementia severity. Journal of the American Geriatric Society 1989;37:725–9 [DOI] [PubMed] [Google Scholar]
- 21.Strotzka S, Psota G, Sepandj A. Uhrentests in der Demenzdiagnostik – Auf der Suche nach der verlorenen Zeit [in German]. Psychopraxis 2003;4:16–24 [Google Scholar]
- 22.Shulman KI, Herrmann N, Brodaty H, et al. IPA survey of brief cognitive screening instruments. International Psychogeriatrics 2006;18:281–94 [DOI] [PubMed] [Google Scholar]
- 23.Nagahama Y, Okina T, Suzuki N, et al. Cerebral substrates related to impaired performance in the clock‐drawing test in dementia with Lewy bodies. Dementia and Geriatric Cognitive Disorders 2008;25:524–30 [DOI] [PubMed] [Google Scholar]
- 24.Slade M, Cahill S, Kelsey W, et al. Threshold 4: an evaluation of the Threshold Assessment Grid as an aid to mental health referrals. Primary Care Mental Health 2003;1:45–54 [Google Scholar]
- 25.Boustani M, Perkins AJ, Fox C, et al. Who refuses the diagnostic assessment for dementia in primary care? International Journal of Geriatric Psychiatry 2006;21:556–63 [DOI] [PubMed] [Google Scholar]
- 26.Borson S, Scanlan M, Chen P, et al. The Mini‐Cog as a screen for dementia: validation in a population‐based sample. Journal of the American Geriatric Society 2003;51:1451–4 [DOI] [PubMed] [Google Scholar]
- 27.Reid L, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dementia and Geriatric Cognitive Disorders 2006;22:471–85 [DOI] [PubMed] [Google Scholar]
FUNDING
This work was supported by an independent research grant awarded by Pfizer Corporation Austria Ges.mbH. Pfizer had no role in the design and execution of the study or the writing of the paper.
All researchers of the study declare that they have no financial or any other obligations towards or dependency on Pfizer.
ETHICS
Approved by the ethics committees of Vienna, Lower Austria, Upper Austria, Vorarlberg. The test procedures followed in this study were in accordance with the ethical standards on human experimentation of the regional Austrian ethics committees and in accordance with the Helsinki Declaration of 1975, revised in 1983. Patients tested in this study have given their informed consent.
CONFLICTS OF INTEREST
All authors declare that there are no personal or financial relationships between themselves or with others that might bias their work. They further explicitly state that there exist no potential conflicts that might bias their work.
References
We certify that all the authors have made a substantial written contribution to the paper and have participated in the work to such an extent as to take public responsibility for the whole contents and have seen and approved the final version. The submitting author certifies that all the authors have read the papers and have agreed to be listed as authors.


