Abstract
Purpose
We aimed to assess the clinical value of endorectal magnetic resonance imaging (MRI) in predicting extraprostatic extension and seminal vesicle invasion in patients with clinically localized prostate cancer.
Materials and Methods
A total of 54 patients who underwent radical prostatectomy for clinically localized prostate cancer were retrospectively analyzed. The findings of endorectal MRI, performed at least 3 weeks after biopsy, were compared with the pathological results of radical prostatectomy specimens. The sensitivity, specificity, and accuracy of the detection of extraprostatic extension and seminal vesicle invasion were calculated.
Results
The sensitivity, specificity, and accuracy of the endorectal MRI findings were 50.0%, 82.6%, and 77.8% for the detection of extraprostatic extension, respectively, and 75.0%, 92.0%, and 90.7% for the detection of seminal vesicle invasion, respectively. The sensitivity of endorectal MRI in the detection of extraprostatic extension improved as the Gleason score increased.
Conclusions
Endorectal MRI findings demonstrated modest sensitivity for predicting extraprostatic extension, whereas specificity was relatively high. In addition, endorectal MRI showed better sensitivity for detecting high-grade tumors.
Keywords: Magnetic resonance imaging, Neoplasm staging, Prostatic neoplasms
INTRODUCTION
Treatment of prostate cancer is greatly dependent on local staging, particularly the presence or absence of extraprostatic extension. As a general rule, patients with disease localized to the prostate are candidates for radical prostatectomy, whereas those with tumor extension beyond the prostatic capsule are probably more appropriately managed with radiation therapy. Hence, we have to find a way to perform accurate staging at diagnosis. A variety of imaging modalities have been evaluated for staging prostate cancer. However, none of these techniques is sensitive enough to reliably detect extraprostatic extension and seminal vesicle invasion of prostatic cancer [1,2]. For over two decades, magnetic resonance imaging (MRI) has improved our ability to delineate localized versus locally advanced prostate cancer [3]. Although it seems to have limitations with respect to the diagnosis of microscopic extraprostatic cancer [4], MRI using an endorectal coil combined with phased-array coils remains the most promising technique for the detection and staging of prostate cancer [5].
The present study was undertaken to evaluate the ability of endorectal coil MRI to predict extraprostatic extension and seminal vesicle invasion in patients with clinically localized prostate cancer. In addition, we investigated the changes in sensitivity, specificity, and accuracy when patients were subdivided into groups according to their Gleason scores and serum prostate-specific antigen (PSA) values.
MATERIALS AND METHODS
A total of 54 patients who underwent radical prostatectomy for clinically localized prostate cancer were retrospectively analyzed. By use of the same protocol, endorectal MRI (1.5 T) was performed before surgery in all patients. Patients who received neoadjuvant treatment after the endorectal MRI examination and patients who had undergone a prostate biopsy and endorectal MRI within 3 weeks of each other were excluded from the present study [6,7]. The criteria for diagnosis of extraprostatic extension on endorectal MRI included the following: a localized bulge of the prostatic contour, a thickening or disruption of the prostatic capsule, an infiltrative strand in the periprostatic fat, or asymmetry of the neurovascular bundle [8-10]. Seminal vesicle invasion was defined by abnormal tissue with low signal intensity within the seminal vesicle or dilatation of the seminal vesicle with asymmetry [8-10]. Radiologic interpretations were made by the consensus of two radiologists. The endorectal MRI findings were compared with the histopathologic findings of the radical prostatectomy specimen in each patient.
1. Protocol for endorectal MRI
The endorectal coil was inserted and the patient was positioned supine between the phased-array coils. All patients underwent MRI with a superconducting MRI scanner operating at 1.5 Tesla (Signa Horizon LX, GE Yokogawa Medical Systems, Hino, Japan). Axial T2-weighted fast spin echo (TR/TE 3,500 ms/102 ms, 16 echo train lengths, 256×192 matrix, 4-mm slice thickness, 0.5-mm interslice gap, number of excitations: 4), T1-weighted spin echo (TR/TE 400 ms/9 ms), and coronal T2-weighted fast spin echo (TR/TE 3,200 ms/92 ms) images were obtained. After precontrast image acquisition, 0.1 mmol/kg of gadopentetate dimeglumine was administered intravenously and axial T1-weighted spin echo images with the same imaging parameters or axial T1-weighted fast spoiled gradient recalled acquisition in steady state (GRASS) with fat suppression (TR/TE/flip angle 200 ms/3.1 ms/90°) images were obtained. The field of view was 14×14 cm. A four-channel phased-array coil was used that consisted of four surface coils (Pelvic Array, GE Yokogawa Medical Systems, Hino, Japan) and one endorectal coil (MRInnervu BPX-15, Nihon Medrad KK, Osaka, Japan) that were used together. One channel of the phased-array coil was replaced with an endorectal coil so that the detection system could encompass the entire pelvis and prostate.
2. Statistical analysis
Because the number of positive cases was low, we performed the chi-square trend test instead of the traditional chi-square test to determine a statistically significant linear trend in sensitivity for detecting extraprostatic extension based on the Gleason score. Differences were considered statistically significant at p<0.05.
RESULTS
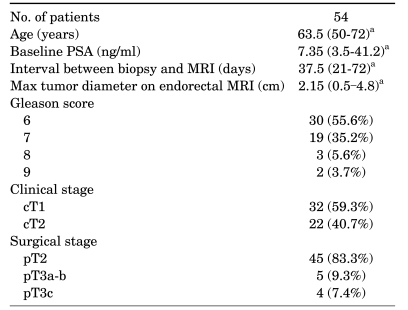
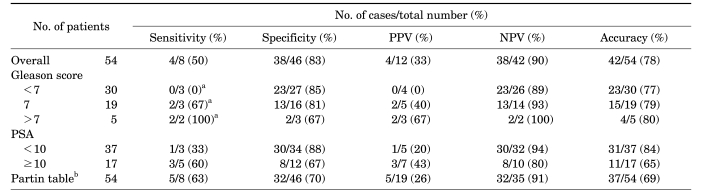
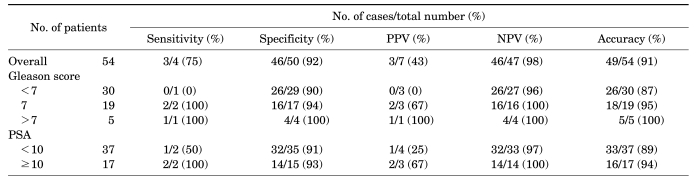
Clinicopathologic parameters are summarized in Table 1. Surgical-pathologic analysis revealed eight (14.8%) patients with extraprostatic extension, including four (7.4%) patients with seminal vesicle invasion. Endorectal MRI demonstrated suspected extraprostatic extension in 12 patients and suspected seminal vesicle invasion in 7 patients. The sensitivity, specificity, and accuracy of endorectal MRI for the detection of extraprostatic extension were 50.0%, 82.6%, and 77.8%, respectively, and the sensitivity, specificity, and accuracy for the detection of seminal vesicle invasion were 75.0%, 92.0%, and 90.7%, respectively (Table 2, 3). The sensitivity of endorectal MRI for the detection of extraprostatic extension and seminal vesicle invasion tended to increase as the Gleason score or PSA increased. In particular, chi-square trend analysis showed a significant linear trend in groups with different Gleason scores (p=0.034). When the Gleason score was 8 or greater, endorectal MRI predicted seminal vesicle invasion perfectly.
TABLE 1.
Clinicopathologic characteristics of patients included in the study sample
PSA: prostate-specific antigen, MRI: magnetic resonance imaging, a: data are presented as the median value with the range in parentheses
Table 2.
Diagnostic performance of endorectal MRI for detecting extraprostatic extension of prostate cancer
MRI: magnetic resonance imaging, PPV: positive predictive value, NPV: negative predictive value, PSA: prostate-specific antigen, a: chi-square test: linear by linear association, p=0.034, b: data was presumed on the basis of the Partin tables [28]
Table 3.
Diagnostic performance of endorectal MRI for detecting seminal vesicle invasion of prostate cancer
MRI: magnetic resonance imaging, PPV: positive predictive value, NPV: negative predictive value, PSA: prostate-specific antigen
DISCUSSION
The typical presentation of prostate cancer has changed greatly over the past two decades. Currently, more men are presenting with well-differentiated tumors, low PSA levels, and nonpalpable disease. This dramatic shift in presentation, which may be due to PSA use, has caused a major stage migration for prostate cancer, with nearly 80% of newly diagnosed cases revealing only localized disease [11,12]. In patients with localized prostate cancer, radical prostatectomy is the most effective treatment tool and offers the best chance of a cure [13]. However, over 30% of men with clinically localized prostate cancer are found to have extraprostatic extension by surgical pathologic analysis [14,15]. Moreover, many of the long-term complications of radical prostatectomy, such as urinary incontinence and impotence, have a large impact on patients' quality of life and, in some patients, may offset the clinical benefits [16]. Therefore, accurate cancer staging at diagnosis is crucial.
The use of computed tomography and MRI to evaluate the local extent of disease is not routinely recommended because of the low sensitivity and accompanying low cost-effectiveness of these modalities [1,10,17]. Nevertheless, MRI using an endorectal coil combined with phased-array coils remains the most promising technique for the detection and staging of prostate cancer [5].
The sensitivity of the detection and correct localization of peripheral zone disease by use of T2 sequences was reported to vary significantly between 37% and 96% [18]. For the detection of extraprostatic extension, the sensitivity, specificity, and accuracy of endorectal MRI has been reported to range from 13-71%, 47-97%, and 58-91%, respectively, whereas for the detection of seminal vesicle invasion, the sensitivity, specificity, and accuracy of endorectal MRI has been reported to range from 33-71%, 83-99%, and 80-95%, respectively [19-21]. In the present study, the sensitivity, specificity, and accuracy for the detection of extraprostatic invasion was 50%, 83%, and 78%, respectively, and that for the detection of seminal vesicle invasion was 75%, 92%, and 91%, respectively. As a result of the inability of MRI to visualize microscopic disease, its sensitivity in detecting extraprostatic extension is uniformly less than 75%. However, in clinical practice, a staging tool must have a high sensitivity to prevent unnecessary surgeries that could negatively impact quality of life. Therefore, it is imperative that we develop a method with an appropriately high sensitivity. Ellis et al reported that high-grade tumors are more likely to be detected on T2 sequences [22]. Later, Ikonen et al confirmed that endorectal MRI detects poorly differentiated prostate cancer lesions more accurately than well-differentiated tumors, although there was no statistically significant difference between PSA groups in the detection of tumors [4]. In our study, chi-square trend analysis also showed a significant linear trend in groups with different Gleason scores. This observation seems to be the result of greater variation in the tumor's microstructure [23]. Therefore, endorectal MRI could be advocated in patients with high Gleason scores.
There are several limitations to our study. First, the number of enrolled cases was small and just eight (14.8%) patients had extraprostatic extension. Therefore, the statistical power is unavoidably weak. Second, we could not compare the sensitivity of endorectal MRI for detecting seminal vesicle invasion according to Gleason scores because the number of positive cases was too small. Third, only prostate cancer in the peripheral zone was assessed in the present study; if prostate cancer in the central gland had been included, the overall accuracy and sensitivity might have been lower. Finally, the use of MRI in prostate cancer is evolving. Recently, several studies have convincingly shown that dynamic contrast enhancement sequencing and spectroscopy each improve the detection rate and sensitivity of MRI [24,25]. In addition, diffusion-weighted imaging of standard T2-weighted sequences and 3T MRI may improve cancer identification [26,27]. The use of these tools will likely yield even better results.
CONCLUSIONS
Endorectal MRI demonstrated modest sensitivity and a relatively high specificity for predicting extraprostatic extension of clinically localized prostate cancer in a small sample of patients. The sensitivity was higher in poorly differentiated prostatic cancer than in well-differentiated prostate cancer. Using endorectal MRI, we were able to more accurately predict seminal vesicle invasion than extraprostatic extension.
Footnotes
This study was supported by the IN-SUNG Foundation for Medical Research.
The authors have nothing to disclose.
References
- 1.Rifkin MD, Zerhouni EA, Gatsonis CA, Quint LE, Paushter DM, Epstein JI, et al. Comparison of magnetic resonance imaging and ultrasonography in staging early prostate cancer. Results of a multi-institutional cooperative trial. N Engl J Med. 1990;323:621–626. doi: 10.1056/NEJM199009063231001. [DOI] [PubMed] [Google Scholar]
- 2.Manyak MJ, Javitt MC. The role of computerized tomography, magnetic resonance imaging, bone scan, and monoclonal antibody nuclear scan for prognosis prediction in prostate cancer. Semin Urol Oncol. 1998;16:145–152. [PubMed] [Google Scholar]
- 3.Nishimoto K, Nakashima J, Hashiguchi A, Kikuchi E, Miyajima A, Nakagawa K, et al. Prediction of extraprostatic extension by prostate specific antigen velocity, endorectal MRI, and biopsy Gleason score in clinically localized prostate cancer. Int J Urol. 2008;15:520–523. doi: 10.1111/j.1442-2042.2008.02042.x. [DOI] [PubMed] [Google Scholar]
- 4.Ikonen S, Kärkkäinen P, Kivisaari L, Salo JO, Taari K, Vehmas T, et al. Magnetic resonance imaging of prostatic cancer: does detection vary between high and low gleason score tumors? Prostate. 2000;43:43–48. doi: 10.1002/(sici)1097-0045(20000401)43:1<43::aid-pros6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Engelbrecht MR, Jager GJ, Laheij RJ, Verbeek AL, van Lier HJ, Barentsz JO. Local staging of prostate cancer using magnetic resonance imaging: a meta-analysis. Eur Radiol. 2002;12:2294–2302. doi: 10.1007/s00330-002-1389-z. [DOI] [PubMed] [Google Scholar]
- 6.White S, Hricak H, Forstner R, Kurhanewicz J, Vigneron DB, Zaloudek CJ, et al. Prostate cancer: effect of postbiopsy hemorrhage on interpretation of MR images. Radiology. 1995;195:385–390. doi: 10.1148/radiology.195.2.7724756. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Hricak H, Kalbhen CL, Kurhanewicz J, Vigneron DB, Weiss JM, et al. Hormonal ablation of prostatic cancer: effects on prostate morphology, tumor detection, and staging by endorectal coil MR imaging. AJR Am J Roentgenol. 1996;166:1157–1163. doi: 10.2214/ajr.166.5.8615261. [DOI] [PubMed] [Google Scholar]
- 8.Outwater EK, Petersen RO, Siegelman ES, Gomella LG, Chernesky CE, Mitchell DG. Prostate carcinoma: assessment of diagnostic criteria for capsular penetration on endorectal coil MR images. Radiology. 1994;193:333–339. doi: 10.1148/radiology.193.2.7972739. [DOI] [PubMed] [Google Scholar]
- 9.Schiebler ML, Schnall MD, Pollack HM, Lenkinski RE, Tomaszewski JE, Wein AJ, et al. Current role of MR imaging in the staging of adenocarcinoma of the prostate. Radiology. 1993;189:339–352. doi: 10.1148/radiology.189.2.8210358. [DOI] [PubMed] [Google Scholar]
- 10.Tempany CM, Zhou X, Zerhouni EA, Rifkin MD, Quint LE, Piccoli CW, et al. Staging of prostate cancer: results of Radiology Diagnostic Oncology Group project comparison of three MR imaging techniques. Radiology. 1994;192:47–54. doi: 10.1148/radiology.192.1.8208963. [DOI] [PubMed] [Google Scholar]
- 11.Augustin H, Auprich M, Stummvoll P, Lipsky K, Pummer K, Petritsch P. Shift of tumor features in patients with clinically localized prostate cancer undergoing radical prostatectomy since the beginning of the PSA era. Wien Klin Wochenschr. 2006;118:348–354. doi: 10.1007/s00508-006-0608-z. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 13.Schostak M, Miller K, Schrader M. Radical prostatectomy in the 21st century - the gold standard for localized and locally advanced prostate cancer. Front Radiat Ther Oncol. 2008;41:7–14. doi: 10.1159/000139873. [DOI] [PubMed] [Google Scholar]
- 14.Makarov DV, Trock BJ, Humphreys EB, Mangold LA, Walsh PC, Epstein JI, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095–1101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barocas DA, Han M, Epstein JI, Chan DY, Trock BJ, Walsh PC, et al. Does capsular incision at radical retropubic prostatectomy affect disease-free survival in otherwise organ-confined prostate cancer? Urology. 2001;58:746–751. doi: 10.1016/s0090-4295(01)01336-x. [DOI] [PubMed] [Google Scholar]
- 16.Turini M, Redaelli A, Gramegna P, Radice D. Quality of life and economic considerations in the management of prostate cancer. Pharmacoeconomics. 2003;21:527–541. doi: 10.2165/00019053-200321080-00001. [DOI] [PubMed] [Google Scholar]
- 17.Levran Z, Gonzalez JA, Diokno AC, Jafri SZ, Steinert BW. Are pelvic computed tomography, bone scan and pelvic lymphadenectomy necessary in the staging of prostatic cancer? Br J Urol. 1995;75:778–781. doi: 10.1111/j.1464-410x.1995.tb07390.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirkham AP, Emberton M, Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol. 2006;50:1163–1174. doi: 10.1016/j.eururo.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima J, Tanimoto A, Imai Y, Mukai M, Horiguchi Y, Nakagawa K, et al. Endorectal MRI for prediction of tumor site, tumor size, and local extension of prostate cancer. Urology. 2004;64:101–105. doi: 10.1016/j.urology.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Ikonen S, Kärkkäinen P, Kivisaari L, Salo JO, Taari K, Vehmas T, et al. Magnetic resonance imaging of clinically localized prostatic cancer. J Urol. 1998;159:915–919. [PubMed] [Google Scholar]
- 21.Rorvik J, Halvorsen OJ, Albrektsen G, Ersland L, Daehlin L, Haukaas S. MRI with an endorectal coil for staging of clinically localised prostate cancer prior to radical prostatectomy. Eur Radiol. 1999;9:29–34. doi: 10.1007/s003300050622. [DOI] [PubMed] [Google Scholar]
- 22.Ellis JH, Tempany C, Sarin MS, Gatsonis C, Rifkin MD, McNeil BJ. MR imaging and sonography of early prostatic cancer: pathologic and imaging features that influence identification and diagnosis. AJR Am J Roentgenol. 1994;162:865–872. doi: 10.2214/ajr.162.4.8141009. [DOI] [PubMed] [Google Scholar]
- 23.Padhani AR. MRI for assessing antivascular cancer treatments. Br J Radiol. 2003;76:S60–S80. doi: 10.1259/bjr/15334380. [DOI] [PubMed] [Google Scholar]
- 24.Ogura K, Maekawa S, Okubo K, Aoki Y, Okada T, Oda K, et al. Dynamic endorectal magnetic resonance imaging for local staging and detection of neurovascular bundle involvement of prostate cancer: correlation with histopathologic results. Urology. 2001;57:721–726. doi: 10.1016/s0090-4295(00)01072-4. [DOI] [PubMed] [Google Scholar]
- 25.Younes P, Chemla N, Hamze B, Mani J, Naouri JF. Prostate MRI spectroscopy. Ann Urol (Paris) 2007;41:145–157. doi: 10.1016/j.anuro.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 26.deSouza NM, Riches SF, Vanas NJ, Morgan VA, Ashley SA, Fisher C, et al. Diffusion-weighted magnetic resonance imaging: a potential non-invasive marker of tumour aggressiveness in localized prostate cancer. Clin Radiol. 2008;63:774–782. doi: 10.1016/j.crad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Kim CK, Park BK. Update of prostate magnetic resonance imaging at 3 T. J Comput Assist Tomogr. 2008;32:163–172. doi: 10.1097/RCT.0b013e3180683b99. [DOI] [PubMed] [Google Scholar]
- 28.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–848. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]