Abstract
Objective
This study examined the NICU Network Neurobehavioral Scale (NNNS) as a predictor of negative medical and behavioral findings one month to 4½ years of age.
Methods
. The sample included 1248 mother-infant dyads (42% born <37 weeks’ gestational age) participating in a longitudinal study of the effects of prenatal substance exposure on child development. Mothers were recruited at 4 urban university-based centers and were mostly African-American and on public assistance. At 1 month of age, infants were tested with the NICU Network Neurobehavioral Scale (NNNS). Latent Profile Analysis (LPA) was carried out on NNNS summary scales to identify discrete behavioral profiles. The validity of the NNNS was examined using logistic regression to predict prenatal drug exposure, medical and developmental outcomes through 4½ years of age including adjustment for gestational age and socioeconomic status (SES).
Results
. Five discrete behavioral profiles were reliably identified with the most extreme negative profile found in 5.8% of the infants. The profiles showed statistically significant associations with prenatal drug exposure, gestational age and birthweight, head ultrasound, neurological and brain disease findings and abnormal scores on measures of behavior problems, school readiness and IQ through 4½ years of age.
Conclusions
The NNNS may be useful to identify infant behavioral needs to be targeted in well-baby pediatric care, as well as for referrals to community based early intervention services.
Keywords: NNNS, neonatal assessment, neurobehavioral, developmental outcomes, in utero drug exposure, latent profile analysis
There has been a long standing interest in the predictive validity of neonatal neurobehavioral assessments. From a scientific point of view, relations between neonatal neurobehavior and later behavioral outcome would support developmental theories that argue for the continuity and biological basis of later child outcome by “ruling out” the effects of the postnatal environment. From a practical point of view, the early detection of infants with poor developmental outcome would invite the study of preventive interventions that could ameliorate or reduce the severity of long term developmental deficits.1
Although the short term predictive validity of neonatal neurobehavioral assessments has been shown, the long term prediction of these assessments has been inconsistent and disappointing.2, 3 The NICU Network Neurobehavioral Scale (NNNS) is a comprehensive evaluation of the neurobehavioral performance of the high-risk infant 4 that includes neurological and behavioral measures and signs of stress. In previous work, infant performance on the NNNS has been related to prenatal drug exposure including cocaine5, 6 methamphetamine,5, 7 marijuana 8 and tobacco,9, 10 prematurity,11,12 MRI findings in preterm infants,11 intrauterine growth retardation,13 fetal behavior,14 infant temperament,15 maternal depression, 16 infants of adolescent mothers,17 treatment of neonatal withdrawal,18 treatment with cocaine using mothers,19 and as a parenting intervention with low birthweight infants.20
In this study we describe a method to classify infants into discrete risk categories or “profiles” based on previously established NNNS summary scores.4, 21 We then examined the ability of the profiles to predict problematic medical and behavioral findings in infancy and early childhood.
Methods
Subjects were enrolled in the Maternal Lifestyle Study (MLS), a multisite longitudinal study of children at risk due to factors such as prenatal exposure to cocaine and other substances and prematurity.5 Details of enrollment and exclusion criteria are described elsewhere.22–25 The study was approved by the institutional review board at each study site and written informed consent was obtained. The exposed group (n=658) was based on mother report of cocaine use during pregnancy and/or a positive meconium assay for cocaine.22 The comparison group (n=730) included children born to mothers who denied cocaine use confirmed by negative meconium results, group matched to the exposed group by gestational age categories (<32 weeks, 33–36 weeks and >36 weeks), child gender, race, and ethnicity within study site. Background substances associated with cocaine use, alcohol, tobacco, and marijuana, were included in both groups. Children were seen at 10 visits from 1 month to 4½ years with an average retention rate of 78%.
Measures
NNNS
The NNNS was administered by certified, blinded psychometrists at the hospital clinic 1-month visit to 1248 (90%) of the original 1388 infants. Items from the NNNS were scored using previously established summary scores4 (Table 1).
Table 1.
NNNS Summary Scale Score Definitions
| 1. |
Attention: Ability to localize and track (follow?) animate and inanimate auditory and visual stimuli |
| 2. |
Asymmetric reflexes: Number of asymmetric responses to elicited reflexes |
| 3. |
Excitability: High levels of motor, state, and physiologic reactivity |
| 4. |
Habituation: Response decrement to repeated auditory and visual stimuli during sleep. |
| 5. |
Handling: Extent to which handling strategies were used during attention sequence to maintain alert state |
| 6. |
Hypertonicity: Hypertonic responses in arms, legs, or trunk or in general tone |
| 7. |
Hypotonicity: Hypotonic response in arms, legs, or trunk or in general tone |
| 8. |
Lethargy: Low levels of motor, state, and physiologic reactivity |
| 9. |
Quality of Movement: Attributes of motor control including smoothness, maturity, lack of startles and tremors |
| 10. |
Regulation: Capacity to modulate arousal, organize motor activity, physiology, and state in response to stimulation |
| 11. |
Non-optimal reflexes: Number of poor scores to elicited reflexes |
| 12. |
Stress/abstinence: Number of stress and abstinence signs observed during the exam. |
| 13. |
Arousal: Level of arousal maintained during the exam including state and motor |
Summary scores for the NNNS were developed by using an approach that combined conceptual and statistical (coefficient apha) aggregation of items and scores. The summary scores were first developed on a random selection of one half of the sample and then “replicated” on the second half of the sample. Scores on the summary variables indicate “higher/more” or “lower/less” of the behavior not necessarily “better” or “worse.” For example, a high score on “handling,” refers to an infant who requires substantial handling to elicit attention, indicating that they are difficult to test; a high score on hypertonia means that the infant was more hypertonic, and a high score on hypotonia means that the infant was more hypotonic. The habituation scale was not used in the present analysis because too few infants were asleep at the one month visit to the clinic.
Prenatal drug exposure
Prenatal drug exposure during pregnancy includes maternal use of cocaine, opiate, tobacco, alcohol, and marijuana. Based on previous work 5 prenatal drug exposure was as present or absent and the level of exposure (heavy vs some vs none) was recorded for cocaine, tobacco, alcohol and marijuana.
Medical outcomes
Newborn medical characteristics included gestational age divided into three groups (<=32 weeks, 33–36 weeks, and >=37 weeks), birthweight, length, and head circumference, small for gestational age (SGA), and intracranial ultrasound reading at 44 weeks PMA (in both term and preterm infants). Results of intracranial ultrasound reading (abnormal vs. normal) were based on agreement by 2 experts. Diagnosis of Cerebral Palsy (CP), chronic neurological abnormalities (e.g. hypoxic-ischemic encephalopathy, arrested hydrocephalus), and illness with clear risk to the brain (e.g. trauma involving the head, meningitis, shunt infection, shunt obstruction, convulsions, encephalopathy, near sudden infant death syndrome, or other brain disorders) were based on a physician medical/neurological exam and nurse medical history at the 1-month, 4-month, 8-month, and 1-, 2-, and 3-year visits.
Developmental Outcomes
The Bayley Scales of Infant Development II 26 were administered to the child and the Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) were computed at 1-, 2-, and 3-years of age. The Child Behavior Checklist (CBCL)27 was administered to the mother at the 3-year visit and scored for Externalizing, Internalizing, and Total Behavior Problems. School readiness was evaluated at age 4 with the Developmental Indicators for the Assessment of Learning (Dial-R)28 that yields standardized motor, concept, language, and total scores. At 4½ years, the Wechsler Preschool and Primary Scale of Intelligence(WPPSI)29 was used to measure child IQ. All of the developmental outcomes were coded into dichotomous variables to define children scoring in the problem range using clinical cutoffs according to the assessment manuals.
Data Analyses
Latent Profile Analysis (LPA), a probabilistic, model-based variant of traditional non-hierarchical cluster analysis 30 was used to classify infants into discrete categories or behavioral profiles. LPA assumes that the population consists of a number of unobserved subgroups referred to as latent profiles, or latent classes. Subjects within a latent profile share similarities in their responses on measured variables. Subjects in different profiles have different patterns of responses on measured variables. The best solutions of LPA minimize the heterogeneity of responses on measured variables within one profile and maximize the heterogeneity of responses across different profiles.
LPA was implemented on 12 NNNS summary scales using finite mixture modeling in Mplus Version 4.1.31, 32 Membership for categorical latent profiles that represent heterogeneous subgroups was inferred from the 12 NNNS variables. LPA models with different numbers of profiles were fitted. Determination of the best model fit was assessed via Bayesian Information Criteria (BIC) adjusted for sample size, where the smallest BIC value indicates the best fit as well as minimization of cross-classification probabilities. BIC has been shown to identify the appropriate number of profiles in finite mixture models 33 and penalizes the models for number of parameters that may indicate model overfit. Parameters for class probabilities were estimated using maximum likelihood solutions and assignment of class membership was also based on Bayesian probabilities. Different random start values were specified for the LPA models to avoid local maxima problems. The LPA models in the present study were specified with correlated errors for summary scores with overlapping components and with unequal variances for the summary scales across different profiles. Validity of the NNNS profiles was examined by comparing profiles on prenatal drug exposure, infant medical characteristics, and developmental outcomes up to age 4½ years using logistic regression analysis.
Results
LPA analysis
We fitted LPA models with 2 to 6 profiles to determine the optimal number of profiles. As the number of profiles increased from 2 to 6, the sample size adjusted BIC values decreased, suggesting improvement in the goodness of fit. Although the 6-profile model had the smallest BIC, one profile was composed of only 10 subjects, an indication of spurious findings. Model fit statistics are shown in Table 2. Both the size of each latent profile and the average class probabilities for the 5-profile solution suggested that the model was the best solution.
Table 2.
Model Fit Statistics for the 5 Profile Solution
| Profile | BIC Values Adjusted for sample size | P= | Average Posterior Class Probability |
|---|---|---|---|
| 1 | 34993.891 | - | .91 (CI=.90–.93) |
| 2 | 32866.523 | 0.0000 | .85 (CI =.84–.87) |
| 3 | 32108.694 | 0.0000 | .87 (CI =.84–89) |
| 4 | 31683.395 | 0.0000 | .85 (CI =.83–87) |
| 5 | 31254.125 | 0.0000 | .85 (CI =.83–87) |
The normality distribution of the 12 NNNS summary scales across the profiles was examined for potential outliers and extreme values using histograms, box plots, and Shapiro-Wilk’s test of normality. No extreme values or outliers were found on attention, handling, self regulation, arousal, quality of movement, and non-optimal reflexes. Extreme values were observed on stress abstinence, excitability, and lethargy. However, no observations were found to be consistent outliers on all of the NNNS summary scales in any profiles.
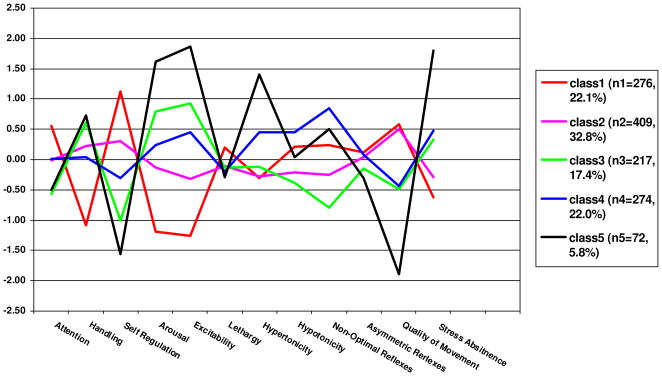
We examined whether the 5-profile solution yielded 5 unique classes by testing the differences in the NNNS summary scales across the profiles (Table 3) and plotting the 5 profiles. In Figure 1 standardized scores were used for the graph because the original scores were not on the same scale.
Table 3.
Means and Standard Deviations of NNNS Summary Scores by 5 Profiles (N=1248)
| NNNS Summary Scores | Profile 1 (n=276, 22.1%) | Profile 2 (n=409, 32.8%) | Profile 3 (n=217, 17.4%) | Profile 4 (n=274, 22.0%) | Profile 5 (n=72, 5.8%) | P< |
|---|---|---|---|---|---|---|
| Attention | 6.12 (1.29) | 5.35 (1.22) | 4.57 (1.20) | 5.37 (1.40) | 4.66 (1.49) | .001 |
| Handling | 0.23 (0.21) | 0.61 (0.24) | 0.72 (0.20) | 0.56 (0.27) | 0.76 (0.21) | .001 |
| Self Regulation | 6.00 (0.55) | 5.28 (0.50) | 4.12 (0.53) | 4.73 (0.52) | 3.63 (0.53) | .001 |
| Arousal | 3.58 (0.40) | 4.32 (0.42) | 4.98 (0.45) | 4.58 (0.50) | 5.55 (0.45) | .001 |
| Excitability | 1.27 (1.08) | 3.76 (1.32) | 7.08 (1.36) | 5.81 (1.36) | 9.61 (1.35) | .001 |
| Lethargy | 3.86 (1.82) | 3.19 (1.77) | 3.12 (2.20) | 2.98 (2.20) | 2.76 (2.15) | .001 |
| Hypertonicity | 0.28 (0.54) | 0.29 (0.58) | 0.45 (0.74) | 0.98 (1.16) | 1.88 (1.63) | .001 |
| Hypotonicity | 0.34 (0.68) | 0.11 (0.34) | 0.01 (0.10) | 0.47 (0.77) | 0.25 (0.52) | .001 |
| Non-Optimal Reflexes | 5.00 (1.72) | 3.91 (1.65) | 2.70 (1.84) | 6.35 (1.72) | 5.57 (2.56) | .001 |
| Asymmetric Reflexes | 0.98 (1.18) | 0.89 (1.17) | 0.69 (1.05) | 0.95 (1.16) | 0.51 (0.82) | .001 |
| Quality of Movement | 4.86 (0.54) | 4.79 (0.53) | 4.03 (0.64) | 4.06 (0.64) | 2.94 (0.62) | .001 |
| Stress Abstinence | 0.12 (0.07) | 0.15 (0.07) | 0.21 (0.07) | 0.22 (0.07) | 0.34 (0.11) | .001 |
Figure 1.
Five NNNS Profiles (N=1248)
Profile 1 (Figure 1) included 276 (22.1%) subjects. These infants showed the highest attention (>.5 SD) with less handling, the highest self regulation, low arousal and excitability, average lethargy, hypertonicity, hypotonicity, non-optimal and asymmetric reflexes, high quality of movement, and less stress abstinence when compared with other profiles. The 409 (32.8%) subjects in Profile 2 showed high quality of movement but otherwise showed mostly average performance on the other summary scores. Compared with Profiles 1 and 2, Profile 3 infants (N=217, 17.4%), had lower attention with more handling, lower self regulation, higher arousal and excitability, similar lethargy and hypertonicity, less hypotonicity, fewer non-optimal, and asymmetric reflexes, lower quality of movement, and more stress abstinence signs. Profile 4 infants (N=274, 22.0%), showed average performance on attention, handling, self regulation, arousal, excitability, and lethargy, but had more hypertonicity, than profiles 1–3, more asymmetric reflexes than profiles 1–3, lower quality of movement and more stress abstinence than profiles 1 and 2, and the most hypotonicity and non-optimal reflexes among the 5 profiles. Profile 5 (N=72, 5.8%), showed the most extreme negative scores. These infants showed the lowest attention with more handling, the lowest self regulation, the highest arousal, excitability, and hypertonicity, average lethargy, hypotonicity, non-optimal and asymmetric reflexes, and the lowest quality of movement and most stress abstinence signs. Given that infants in profile 5 showed the poorest performance, we hypothesized that this group would more likely have medical and developmental sequelae than infants in the other 4 profiles. Infants in profile 4 had extremes in tone (either hypertonic or hypotonic), the most non-optimal reflexes, poor quality of movement and a high number of stress signs, thus, we also predicted that infants in the profile 4 and 5 group would show more medical and developmental problems than infants in the other 3 profile groups.
Validation and Prediction
Prenatal Drug Exposure (Table 4)
Table 4.
ODDS Ratio (95% CI) Comparing Profile 5 and Profiles 4& 5 with Other Profiles as Predictors of Child Outcomes
| Variables | Profile 5 vs. Profiles 1–4 | Profiles 4 & 5 vs. Profiles 1–3 | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Prenatal Drug Exposure | ||||
| Cocaine | 1.36 (.84–2.18) | .209 | 1.13 (.88–1.45) | .327 |
| Heavy Cocaine | 1.62 (.80–3.28) | .183 | 1.71 (1.14–2.56) | .009 |
| Cocaine & Opiate | 3.86 (1.80–8.28) | .001 | 1.72 (.97–3.07) | .064 |
| Tobacco | 2.07 (1.24–3.47) | .005 | 1.33 (1.04–1.71) | .026 |
| Heavy Tobacco | 1.29 (.66–2.52) | .182 | 1.21 (.89–1.64) | .222 |
| Alcohol | 1.61 (.96–2.70) | .069 | 1.23 (.95–1.59) | .111 |
| Heavy Alcohol | 1.29 (.66–2.52) | .460 | 1.42 (.99–2.05) | .060 |
| Marijuana | 1.73 (1.04–2.87) | .035 | 1.39 (1.05–1.85) | .023 |
| Heavy Marijuana | 1.44 (.50–4.13) | .50 | 1.31 (.71–2.42) | .383 |
| Medical Outcomes | ||||
| GA <= 32 weeks | 2.07 (1.17–3.66) | .013 | 1.74 (1.26–2.42) | .001 |
| 33–36 weeks | 1.15 (.63–2.09) | .647 | 1.23 (.91–1.65) | .179 |
| BW < 1500 grams | 2.17 (1.20–3.95) | .011 | 1.77 (1.23–2.55) | .002 |
| BW < 2500 grams | 1.75 (1.09–2.82) | .022 | 1.57 (1.23–2.02) | .000 |
| Abnormal Ultrasound Reading, 1 mo | 2.77 (1.40–5.46) | .003 | 1.19 (0.74–1.91) | .478 |
| Chronic neurological abnormalities by 3 y | 2.80 (1.68–4.65) | .000 | 2.51 (1.85–3.41) | <.001 |
| Any disease related to brain risk by 3y | 1.89 (1.09–3.31) | .025 | 2.31 (1.68–3.17) | <.001 |
| CP diagnosis by age 3 yr | 3.12 (1.04–9.37) | .042 | 2.40 (1.09–5.33) | .031 |
| Behavioral Outcomes | ||||
| Bayley MDI <= 2 SD, 1 yr | 3.34 (1.22–9.10) | .019 | 1.89 (.88–4.05) | .102 |
| Bayley MDI, <= 2 SD, 2 yr | 1.92 (1.05–3.52) | .034 | 1.26 (.87–1.83) | .215 |
| Bayley MDI <= 2 SD, 3 yr | 1.57 (.76–3.24) | .225 | 1.23 (.82–1.84) | .310 |
| Bayley PDI <= 2 SD, 1 yr | 1.67 (.64–4.37) | .298 | 1.22 (.67–2.22) | .516 |
| Bayley PDI <= 2 SD, 2 yr | 1.80 (.61–5.25) | .285 | 2.18 (1.17–4.04) | .014 |
| Bayley PDI <= 2 SD, 3 yr | 1.17 (.35–3.94) | .798 | 1.24 (.69–2.20) | .474 |
| CBCL Externalizing Problems > 63, 3 yr | 2.07 (1.17–3.67) | .013 | 1.08 (0.78–1.51) | .631 |
| CBCL Internalizing Problems > 63, 3 yr | 2.70 (1.50–4.87) | .001 | 1.29 (0.9–1.85) | .173 |
| CBCL Total Problems > 63, 3 yr | 2.38 (1.34–4.20) | .003 | 1.26 (0.91–1.76) | .163 |
| Dial-R/Potential Motor Problem, 4 yr | 1.75 (0.92–3.34) | .088 | 1.43 (1.01–2.01) | .041 |
| Dial-R/Potential Concept Problem, 4 yr | 2.06 (1.09–3.90) | .027 | 1.47 (1.04–2.08) | .029 |
| Dial-R/Potential Language Problem, 4 yr | 1.99 (0.89–4.46) | .094 | 1.68 (1.06–2.67) | .027 |
| Dial-R/Potential Total Problem, 4 yr | 1.42 (0.72–2.79) | .315 | 1.36 (0.96–1.94) | .084 |
| WPPSI – IQ verbal <= 2 SD, 4 ½ yr | 1.74 (.86–3.48) | .120 | 1.10 (.74–1.64) | .627 |
| WPPSI – IQ performance <= 2 SD, 4½ | 1.01 (.44–2.34) | .977 | 1.45 (.97–2.17) | .070 |
| WPPSI – IQ full <= 2 SD, 4 ½ yr | 1.99 (1.02–3.90) | .044 | 1.26 (.86–1.83) | .235 |
Infants in the profile 5 group were more likely to be exposed to cocaine plus opiates, tobacco, and marijuana in utero than infants in the other 4 profiles. Infants in the combined profiles 4 and 5 group were more likely to be exposed to heavy cocaine use, tobacco and marijuana in utero than infants in profiles 1–3.
Medical Outcomes (Table 4)
Profile 5 infants were more likely to be preterm (<=32 weeks’ gestation), very low birthweight (<1500 grams) or low birthweight (<2500 grams). They were more likely to have an abnormal ultrasound reading at 1-month, chronic neurological abnormalities, brain related illness or diagnosis of CP by age 3. These same findings were observed comparing infants with profile 4 or 5 vs. infants with profiles 1–3 with the exception of the ultrasound finding. No differences were found in gender or SGA status related to profile 5 or profiles 4 and 5 combined.
Behavioral Outcomes (Table 4)
Profile 5 infants were more likely to have low Bayley MDI scores at 1 and 2 years of age, more CBCL externalizing, internalizing, and total problems at age 3, more DIAL-R concept problems at 4, and lower IQ at 4½ years than infants with profiles 1–4. Infants with profiles 4 or 5, compared to infants with profiles 1–3, were more likely to show poor Bayley PDI scores at age 2, and more DIAL-R motor, concept, and language problems on the DIAL-R at age 4.
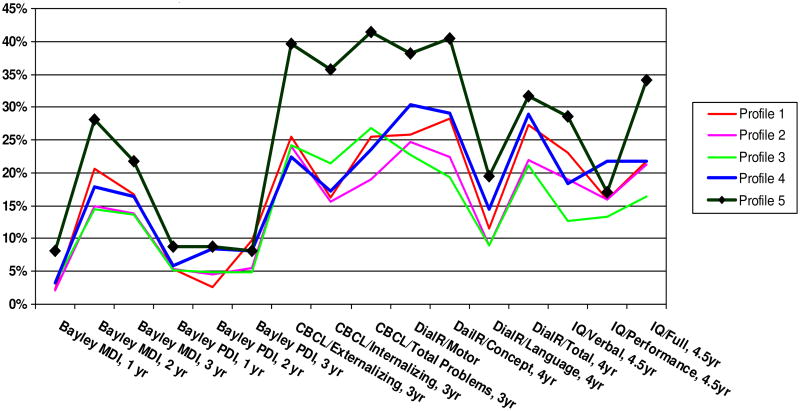
Profile 5 had the highest proportion of infants defined as clinically worrisome on 20 of the 23 outcomes that we examined (Fig. 2 and 3). Profile 4 appears to be more sensitive to medical (Fig. 2) than developmental outcomes (Fig. 3) with the exception of the Dial R/Motor.
Figure 2.
Proportions of Subjects with Non-optimal Infant Medical Characteristics by NNNS Profiles
Figure 3.
Proportions of Subjects with Non-optimal Developmental Outcomes by NNNS Profiles (Bayley and WPPSI < <=2 SD)
Adjustment for covariates
We compared the predictive validity of the NNNS with the predictive validity of the 2 most likely variables that would also be expected to predict these outcomes, gestational age and SES.34–36 Forward stepwise logistic regression analyses were implemented to determine the predictive validity of the NNNS profiles after controlling for gestational age, (<33 weeks, 33–36 weeks, and >=37 weeks) and low SES (Hollingshead Index of Social Position Level 5).
The profile 5 group, compared to children in the other profile groups after controlling for gestational age and SES, were more likely to have abnormal ultrasound reading at 1-month (OR=2.37, CI=1.17–4.80), chronic neurological abnormalities (OR=2.35, CI=.36 – 4.02), brain related illnesses (OR=1.86, CI=1.06–3.27), externalizing behavior problems (OR=2.05, CI=1.15–3.67), internalizing behavior problems (OR=2.72, CI=1.49–4.98), and total behavior problems (OR=2.37, CI=1.33–4.24) by age 3.
The profile 4 and 5 combined group compared to profile 1–3 children, after controlling for gestational age and SES, were more likely to have birthweight <2500 g (OR=1.37, CI=1.012–1.85), heavy prenatal cocaine exposure (OR=1.71, CI=1.13–2.58), any prenatal exposure to tobacco (OR=1.40, CI=1.08–1.81), alcohol (OR=1.32, CI=1.01–1.72), p=.042 or marijuana (OR=1.37, CI=1.02–1.84), chronic neurological abnormalities (OR= 2.42, CI=1.75–3.34) and brain related illnesses (OR=2.34, CI=1.70–3.23) by age 3, as well as concept problems (OR=1.43, CI=1.01–2.04) and language problems (OR=1.70, CI=1.06–2.73) at age 4. For descriptive purposes we compared the number of children with negative outcomes identified by gestational age and/or SES with the number of additional children identified by profiles 4 and 5 (Table 5). For example, of the 88 children with an abnormal ultrasound, 45.5% were identified by gestational age and/or SES; an additional 17% not identified by gestational age and/or SES were identified by the profiles 4 and 5. In 9 of the 20 outcomes in the table, the profiles identified at least 15% of infants with poor outcome not identified by low gestational age and/or SES.
Table 5.
Number and Percentage of Subjects with Developmental Problems Identified by GA<33 wks, Low SES, or NNNS Profiles
| Outcomes | N(%) of Subjects with Problems* | # (%) of subjects Identified by Either GA < 33 wks or Low SES | # (%) of additional subjects Identified by Profiles 4 & 5 Combined |
|---|---|---|---|
| Medical Outcomes | |||
| Abnormal Ultrasound Reading, 1 mo | 88 (7.1%) | 40 (45.5%) | 15 (17%) |
| Chronic neurological abnormalities by 3 yr | 213 (17.1%) | 121 (56.8%) | 40 (18.8%) |
| Any disease related to risks to brains by 3 yr | 194 (15.5%) | 75 (38.7%) | 46 (23.7%) |
| CP diagnosis by age 3 yr | 25 (2.2%) | 17 (68%) | 3 (12%) |
| Behavioral Outcomes | |||
| Bayley MDI <= 2 SD, 1 yr | 28 (3.0%) | 15 (53.6%) | 3 (10.7%) |
| Bayley MDI, <= 2 SD, 2 yr | 160 (17.6%) | 79 (49.4%) | 22 (13.8%) |
| Bayley MDI <= 2 SD, 3 yr | 134 (15.4%) | 64 (47.8%) | 16 (11.9%) |
| Bayley PDI <= 2 SD, 1 yr | 52 (5.7%) | 30 (57.7%) | 6 (11.5%) |
| Bayley PDI <= 2 SD, 2 yr | 44 (5.2%) | 23 (52.3%) | 7 (15.9%) |
| Bayley PDI <= 2 SD, 3 yr | 58 (7.1%) | 30 (51.7%) | 6 (10.3%) |
| CBCL Externalizing Problems > 63, 3 yr | 226 (25.0%) | 104 (46%) | 37 (16.4%) |
| CBCL Internalizing Problems > 63, 3 yr | 165 (18.2%) | 84 (50.9%) | 29 (17.6%) |
| CBCL Total Problems > 63, 3 yr | 218 (24.1%) | 103 (47.2%) | 38 (17.4%) |
| Dial-R/Potential Motor Problem, 4 yr | 213 (26.6%) | 107 (50.2%) | 31 (14.6%) |
| Dial-R/Potential Concept Problem, 4 yr | 204 (25.7%) | 95 (46.6%) | 33 (16.2%) |
| Dial-R/Potential Language Problem, 4 yr | 89 (11.3%) | 42 (47.2%) | 15 (16.9%) |
| Dial-R/Potential Total Problem, 4 yr | 197 (25.1%) | 98 (49.7%) | 28 (14.2%) |
| WPPSI – IQ verbal <= 2 SD, 4 ½ yr | 147 (19.3%) | 67 (45.6%) | 19 (12.9%) |
| WPPSI – IQ performance <= 2 SD, 4 ½ yr | 129 (16.9%) | 66 (51.2%) | 15 (11.6%) |
| WPPSI – IQ full <= 2 SD, 4 ½ yr | 162 (21.5%) | 79 (48.8%) | 20 (12.3%) |
Note. Denominators vary across the outcomes.
Discussion
The main findings from this study are that NNNS profiles discriminate among infants with medical and behavioral problems through 4½ years of age, including infants who would not have been identified on the basis of medical and demographic factors alone. We described a method to develop neurobehavioral profiles of 1-month old infants based on NNNS scores. Five discrete profiles were identified with the most extreme negative scores shown by 5.8% of the infants (Profile 5). These clinically worrisome infants were highly aroused, excitable, and hypertonic, had poor quality of movement, self regulation, and poor attention, required substantial handling and were highly stressed. Profile 5 was sensitive to prenatal drug exposure (cocaine and opiates, tobacco and marijuana) and prematurity/low birthweight, i.e., over 50% of these infants were <2500 grams, 20% of whom were less than 1500 grams. Profile 5 had the highest proportion of infants with ultrasound, neurological and brain disease. Upwards of 40% of these infants had clinically significant behavior and school readiness problems and approximately 35% had low IQ. Thus, infants with Profile 5 are indeed worrisome and a substantial proportion of them will go on to have clinically significant follow-up findings.
We also combined Profiles 4 and 5 because Profile 4 infants showed extremes in tone, the highest number of non-optimal reflexes, poor quality of movement and a high number of stress signs. Infants with profile 4 or 5 were more likely to be in the heavy cocaine exposure group. Although some of the outcomes were no longer statistically significant, the addition of Profile 4 increased the odds ratio for disease related to the brain and was related to all three school readiness domains. Profile 4 also had a higher proportion of infants with medical conditions than Profiles 1–3.
It is also of interest to ask if these neonatal neurobehavioral antecedents are unique. After adjusting for prematurity and SES, we found that the NNNS profiles were still related to prenatal drug exposure, low birthweight, neurological abnormalities, and diseases with risks to the brain, as well as behavior problems and delayed school readiness. Measurable antecedents of later developmental outcome can be detected in neonatal neurobehavior. These unique effects accounted for an additional 16.2 – 23.7% of the children with medical or behavioral problems. We selected gestational age and SES as covariates because of their prominence in predicting developmental outcome in high risk samples 34–36 and could therefore be confounding. They also represent essentially immutable factors (even though SES can theoretically change) whereas the development of preventive interventions would target neurobehavior which can be altered.
The presence of confounding factors does not obviate the justification for early intervention. The 5.8% prevalence rate of Profile 5 in our sample is consistent with the estimate that 5–10% of the pediatric population is at high risk for later developmental problems.37 Early identification of children with developmental delay has received more attention in recent years since children are believed to benefit the most if they participate in intervention services as early as possible.38 The American Academy of Pediatrics has called for a referral to early intervention or special education following a positive screening result and a recent commentary recommended that a diagnosis is not required for such a referral.39 The NNNS profiles specify the neurobehavioral deficits associated with poor outcome that could serve as target behaviors for the development of intervention studies. These findings can be used to guide programmatic intervention efforts targeted to those with indicated dysfunction.
It is well understood that predictive validity tests for this population are problematic because infancy is a period of rapid change. In addition to measuring a moving target, developmental outcome is also determined by postnatal factors. Many infants appear normal as neonates but develop problems later (low specificity or false negative) and many infants that appear worrisome as neonates develop normally (low sensitivity or false positive). Thus, infant neurobehavioral tests may not meet standard criteria for medical screening. However, our approach was not designed to maximize sensitivity and specificity. Profile 5 identified 72 (5.8%) infants and we demonstrated that a clinically significant number of these infants will have developmental sequelae. For example, 42% of the children with Profile 5 had deviant total behavior scores on the CBCL. However, in the sample as a whole, there were 218 children who showed deviant total behavior problem scores on the CBCL. Thus, Profile 5 is indicative of later behavior problems, but because this profile only occurred in 5.8% of the sample, it cannot identify clinical conditions with higher prevalence rates.
In addition, prediction is not the only important criteria for a neonatal assessment. Diagnosing atypical neurobehavior and early dysfunctions amenable to intervention and specifying characteristics for targeted pediatric interventions serve a primary prevention and treatment need as well. Thus, our findings could stimulate an important social policy debate. On the one hand, if used as screen, the NNNS would fail to identify many infants who will later develop behavior problems and it will identify many infants as deviant who will develop normally. The latter could suffer the negative effects of being labeled and resources would be used unnecessarily. On the other hand, the NNNS is noninvasive, early intervention is benign, and there is the ethical responsibility of offering early intervention to parents whose infants have a 40% chance of having a childhood behavior disorder or school readiness problem.
A limitation of this study is that these profiles were developed in a sample of children with prenatal exposure to cocaine and other substances which could limit the generalizability of the findings. Another limitation is that this study only followed children through 4½ years of age and it would be important to determine the predictive validity of the NNNS in older children.
Conclusion
Finding continuities in behavior between the neonatal period and early childhood provides new evidence that has implications for our understanding of developmental processes. The ability to forecast with some precision, which individual infants are most likely to show developmental deficits in early childhood opens the door for the study of intervention studies to reduce or ameliorate these deficits. This could enable us to identify, from the larger pool of infants who are already at risk, which infants are at highest risk and enable us to make better use of increasingly limited resources. NNNS profiles identify infant behavioral to be targeted in well-baby pediatric care, as well as for referrals to community based early intervention services.
Acknowledgments
Supported by the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network and an interinstitute agreement with the National Institute on Drug Abuse (NIDA) through cooperative agreements: U10 DA 024117-01, U10HD21385 (to SS), U10 DA 024128-06, U10HD2786 (to HSB), U10 DA 024119-01, U10HD27904 (to BML), and U10 DA 024118-01, U10HD21397 (to CRB); NICHD contract N01-HD-2-3159 (to BML).
Abbreviations
- BIC
Bayesian Information Criteria
- BW
Birthweight
- CBCL
Child Behavior Checklist
- CI
Confidence Interval
- CP
Cerebral Palsy
- DIAL-R
Developmental Indicators for the Assessment of Learning
- GA
Gestational Age
- IQ
Intelligence Quotient
- LPA
Latent Profile Analysis
- MDI
Mental Developmental Index
- NNNS
NICU Network Neurobehavioral Scale
- OR
Odds Ratio
- PDI
Psychomotor Developmental Index
- SES
Socioeconomic Status
- SGA
Small for Gestational Age
- WPPSI
Wechsler Preschool and Primary Scale of Intelligence
References
- 1.American Academy of Pediatrics Committee on Children with Disabilities. Developmental surveillance and screening of infants and young children. Pediatrics. 2001;108:192–196. doi: 10.1542/peds.108.1.192. [DOI] [PubMed] [Google Scholar]
- 2.Brazelton T, Nugent J, Lester B. Neonatal Behavioral Assessment Scale. In: Osofsky J, editor. Handbook of Infant Development. Oxford, England: John Wiley & Sons; 1987. pp. 780–817. [Google Scholar]
- 3.Singer L, Zeskind P. Biobehavioral Assessment of the Infant. New York: The Guilford Press; 2001. [Google Scholar]
- 4.Lester B, Tronick E. The Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS) Pediatrics. 2004;113(3 part 2 of 2):631–699. [PubMed] [Google Scholar]
- 5.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110(6):1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 6.Napiorkowski B, Lester B, Freier C, Brunner S, Dietz L, Nadra A, et al. Effects of in utero substance exposure on infant neurobehavior. Pediatrics. 1996;98(1):71–75. [PubMed] [Google Scholar]
- 7.Singer L. MDMA Use During Pregnancy and Early Infant Outcomes. Annual meeting of the XVth Biennial International Conference on Infant Studies; Kyoto, Japan. June 19th 2006. [Google Scholar]
- 8.Carvalho de Moraes Barros M, Guinsburg R, de Araujo Peres C, Mitsuhiro S, Chalem E, Laranjeira R. Exposure to marijuana during pregnancy alters neurobehavior in the early neonatal period. The Journal of Pediatrics. 2006;149(6):781–787. doi: 10.1016/j.jpeds.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 9.Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111(6 Pt 1):1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 10.Stroud LR, Paster RL, Papandonatos GD, Niaura R, Salisbury AL, Battle C, et al. Maternal Smoking during Pregnancy and Newborn Neurobehavior: Effects at 10 to 27 Days. J Pediatr. 2008 doi: 10.1016/j.jpeds.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown N, Anderson P, Howard K, Bear M, Wang H, Hunt R, et al. Clinical Validity of Early Neurobehavioural Assessments of Very Preterm Infants. Pediatric Research. 2004;55(4):582A. [Google Scholar]
- 12.Brown N, Doyle L, Bear M, Inder T. Alterations in Neurobehavior at Term Reflect Differing Perinatal Exposures in Very preterm Infants. Pediatrics. 2006;118(6):2461–2471. doi: 10.1542/peds.2006-0880. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho de Moraes Barros M, Guinsburg R, Mitsuhiro S, Chalem E, Laranjeira R. Neurobehavior of full-term small for gestational age newborn infants of adolescent mothers. Journal Pediatr (Rio J) 2008;84(3):217–223. doi: 10.2223/JPED.1796. [DOI] [PubMed] [Google Scholar]
- 14.Salisbury A, Fallone M, Lester B. Neurobehavioral Assessment From Fetus to Infant: The NICU Network Neurobehavioral Scale and the Fetal Neurobehavior Coding Scale. Mental Retardation and Developmental Disabilities. 2005;11:14–20. doi: 10.1002/mrdd.20058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagner D, Sheinkopf S, Miller-Loncar C, LaGasse L, Lester B, Liu J, et al. The Effect of Parenting Stress on Child Behavior Problems in Children Prenatally Exposed to Cocaine. Child Psychiatry and Human Development. doi: 10.1007/s10578-008-0109-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salisbury AL, Lester BM, Seifer R, Lagasse L, Bauer CR, Shankaran S, et al. Prenatal cocaine use and maternal depression: effects on infant neurobehavior. Neurotoxicol Teratol. 2007;29(3):331–340. doi: 10.1016/j.ntt.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho de Moraes Barros M, Guinsburg R, de Araujo Peres C, Mitsuhiro S, Chalem E, Laranjeira R. Neurobehavioral profile of healthy full-term newborn infants of adolescent mothers. Early Hum Dev. 2008;84(5):281–287. doi: 10.1016/j.earlhumdev.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Coyle MG, Ferguson A, Lagasse L, Liu J, Lester B. Neurobehavioral effects of treatment for opiate withdrawal. Arch Dis Child Fetal Neonatal Ed. 2005;90(1):F73–74. doi: 10.1136/adc.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boukydis CZ, Lester BM. Mother-infant consultation during drug treatment: Research and innovative clinical practice. Harm Reduct J. 2008;5:6. doi: 10.1186/1477-7517-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han K. Development of a Home-Based Parenting Intervention Program for Low Birth-weight Infants. Sigma Theta Tau International Conference; Seoul, South Korea. 2003. [Google Scholar]
- 21.Lester B, Tronick E. NICU Network Neurobehavioral Scale Manual. London: Paul H. Brookes Publishing Co., Inc; 2004. [Google Scholar]
- 22.Lester BM, ElSohly M, Wright LL, Smeriglio VL, Verter J, Bauer CR, et al. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107(2):309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 23.Bauer CR, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, et al. The Maternal Lifestyle Study: drug exposure during pregnancy and short-term maternal outcomes. Am J Obstet Gynecol. 2002;186(3):487–495. doi: 10.1067/mob.2002.121073. [DOI] [PubMed] [Google Scholar]
- 24.Bauer CR, Langer JC, Shankaran S, Bada HS, Lester B, Wright LL, et al. Acute neonatal effects of cocaine exposure during pregnancy. Arch Pediatr Adolesc Med. 2005;159(9):824–834. doi: 10.1001/archpedi.159.9.824. [DOI] [PubMed] [Google Scholar]
- 25.Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, et al. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119(2):e348–359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- 26.Bayley N. Bayley Scales of Infant Development. 2. San Antonio, Ca: The Psychological Corporation; 1993. [Google Scholar]
- 27.Achenbach T. Manual for the Child Behavior Checklist/2–3 and 1992 Profile. Department of Psychiatry, University of Vermont; 1992. [Google Scholar]
- 28.Mardell-Czudnowski C, Goldenberg D. Developmental indicators for the assessment of learning -Revised. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- 29.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) New York: The Psychological Corporation Harcourt Brace Jovanovich, Inc; 1989. [Google Scholar]
- 30.Vermunt J, Magidson J. Latent class cluster analysis. In: Hagenaars J, McCutcheon A, editors. Applied latent class analysis. Cambridge, MA: Cambridge Unviersity Press; 2002. [Google Scholar]
- 31.McLachlan G, Peel D. Finite mixture models. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 32.Muthen L, Muthen B. Mplus users’ guide. 3. Los Angels, CA: Muthen & Muthen; 1998–2006. [Google Scholar]
- 33.D’Unger A, Land K, McCall P, Nagin D. How Many Latent Classes of Delinquent/Criminal Careers? Results from Mixed Poisson Regression Analyses of the London, Philadelphia, and Racine Cohort Studies. American Journal of Sociology. 1998;103(6):1593–1630. [Google Scholar]
- 34.Korner A, Stevenson D, Kraemer H, et al. Prediction of the development of low birth weight preterm infants by a new neonatal medical index. Journal of Developmental and Behavioral Pediatrics. 1995;17:37–43. [PubMed] [Google Scholar]
- 35.Majnemer A, Rosenblatt B. Prediction of outcome at school age in neonatal intensive care unit graduates using neonatal neurologic tools. J Child Neurol. 2000;15(10):645–651. doi: 10.1177/088307380001501002. [DOI] [PubMed] [Google Scholar]
- 36.Bradley R, Corwyn R. Socioeconomic status and child development. Annual Review of Psychology. 2004;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 37.Simeonsson R, Sharp M. Developmental Delays. In: Hoekelman R, Friedman S, Neslon N, editors. Primary Pediatric Care. St. Louis, MI: Mosby-Yearbook; 1992. pp. 867–870. [Google Scholar]
- 38.Rydz D, Shevell MI, Majnemer A, Oskoui M. Developmental screening. J Child Neurol. 2005;20(1):4–21. doi: 10.1177/08830738050200010201. [DOI] [PubMed] [Google Scholar]
- 39.Marks K, Glascoe FP, Aylward GP, Shevell MI, Lipkin PH, Squires JK. The thorny nature of predictive validity studies on screening tests for developmental-behavioral problems. Pediatrics. 2008;122(4):866–868. doi: 10.1542/peds.2007-3142. [DOI] [PubMed] [Google Scholar]