Abstract
An increased risk of HIV-1 associated dementia (HAD) has been observed in patients abusing methamphetamine (METH). Since both HIV viral proteins (gp120, Tat) and METH induce oxidative stress, drug abusing patients are at a greater risk of oxidative stress-induced damage. The objective of this study was to determine if N-acetylcysteine amide (NACA) protects the blood brain barrier (BBB) from oxidative stress-induced damage in animals exposed to gp120, Tat and METH. To study this, CD-1 mice pre-treated with NACA/saline, received injections of gp120, Tat, gp120 + Tat or saline for 5 days, followed by three injections of METH/saline on the fifth day, and sacrificed 24 h after the final injection. Various oxidative stress parameters were measured, and animals treated with gp120+Tat+Meth were found to be the most challenged group, as indicated by their GSH and MDA levels. Treatment with NACA significantly rescued the animals from oxidative stress. Further, NACA-treated animals had significantly higher expression of TJ proteins and BBB permeability as compared to the group treated with gp120+Tat+METH alone, indicating that NACA can protect the BBB from oxidative stress-induced damage in gp120, Tat and METH exposed animals, and thus could be a viable therapeutic option for patients with HAD.
Keywords: HIV associated dementia, Methamphetamine, Oxidative stress, Blood brain barrier
Introduction
HIV-1-associated-dementia (HAD), a neurological syndrome characterized by cognitive deficits and motor and behavioral dysfunctions, is one of the most common complications associated with human immunodeficiency virus (HIV-1) infection (1-4). A third of the adults and half of the children with HIV infection have been reported to develop HAD (5). HAD is one of the most common causes of dementia worldwide among people aged 40 years or less, and is a significant independent risk factor in death due to HIV infection (6). Though the clinical and pathological conditions of HAD have been well characterized, the pathogenesis of the progression of the disease is not well understood.
The blood-brain barrier (BBB), defining an interface between the central nervous system and the blood, performs the essential function of shielding the brain from toxic substances and is believed to play an important role in the development of HAD (7-8). Studies have shown that disruption of the BBB is more frequent in HAD patients when compared with non-demented HIV patients or control patients (9). Furthermore, the HIV-1 envelope glycoprotein (gp120) and transregulatory protein (Tat) of HIV-1 are neurotoxic and cytotoxic and have been implicated in the development of HAD (10-11). Previous studies have reported that oxidative stress induced by gp120 and Tat leads to the disruption of the BBB (12). A dose-dependent increase in oxidative stress and decrease in intracellular glutathione have been observed in brain endothelial cells treated with Tat (9).
In addition to this, many HIV-positive patients use addictive drugs like methamphetamine (METH), which is a well known neurotoxicant (13-15). METH has been reported to promote dopamine release in the nucleus accumbens, leading to degeneration of the striatal dopamine terminals (16-17). Further, dopamine oxidation leads to the formation of reactive oxygen species, which disturbs the antioxidant defense mechanism in the body leading to oxidative stress-induced damage (18). Overproduction of superoxide radicals and a decrease in antioxidant enzyme activity have been observed in mice treated with METH. Degeneration of various regions of the brain, particularly the BBB, has also been reported due to METH abuse. Brain degeneration is associated with modifications of the BBB (19). Disruption of the tight junctions (TJ) is one of the common causes of BBB dysfunction. TJs are composed of the tight junction proteins (Occludin, Claudin, Zona Occludens), and play an important role in maintaining the structural integrity and low permeability of the BBB (20). Disruption of the BBB has been reported to contribute to the progression of various neurological diseases like multiple sclerosis, Alzheimer’s and Parkinson’s disease (21). Further, oxidative stress has also been reported to be an important factor in BBB dysfunction (22). Under physiological conditions, the integrity of the BBB is protected from oxidative stress because the BBB has high levels of antioxidant enzymes. However, under oxidative stress, depletion of these antioxidant enzymes leads to the increase in permeability and loss of integrity of these endothelial cells (23). Supplementation of antioxidants is becoming increasingly popular in oxidative stress-related disorders. Thiol antioxidants like cysteine, glutathione and N-acetylcysteine (NAC) have been shown to provide a protective effect against stress-related disorders (24-27). However, some of these thiols, such as NAC, have been reported to have several side effects and toxicities such as suppressing respiratory burst, and causing toxic accumulation of ammonia in the liver (28-29). In addition, bioavailability of NAC is very low because its carboxylic group loses its proton at physiological pH, making the compound negatively charged and consequently less permeable. N-acetylcysteine amide (NACA), a modified form of NAC, where the carboxyl group has been replaced by an amide group, has been found to be more effective in neurotoxic cases because of its ability to permeate cell membranes and the BBB (29).
Since METH has been shown to induce oxidative stress, it was of significant interest to understand if METH potentiated the oxidative stress induced by HIV-1 proteins gp120 and Tat at the BBB. Also, the efficacy of the thiol antioxidant NACA to confer protection to animals exposed to gp120, Tat and METH, and to abrogate the oxidative stress-induced damage at the BBB was investigated.
Materials and Methods
Materials
CD-1 mice were obtained from the in-house colony at the VA medical center-St. Louis. N-acetylcysteine amide (NACA) was provided by Dr. Glenn Goldstein (David Pharmaceuticals, New York, NY, USA). N-(1-pyrenyl)-maleimide (NPM) was purchased from Sigma (St. Louis, MO). High-performance liquid chromatography (HPLC) grade solvents were purchased from Fisher Scientific (Fair Lawn, NJ). All other chemicals were purchased from Sigma (St. Louis, MO), unless stated otherwise.
Animal experiments
Male CD-1 mice (30-35 g, 7 weeks old) were obtained from the in-house breeding colony at the VA Medical Center-St. Louis and were housed at the University of MO-Rolla in a controlled-temperature (20°-23°C) and controlled-humidity (~55%) animal facility, with a 12 h light and dark cycle. The animals had unlimited access to rodent chow and water, and were used after 1 week of acclimatization. All animal procedures were conducted under an animal protocol approved by the Institutional Animal Care and Use Committee of the Missouri University of Science and Technology. The mice were divided into two major groups: an experimental and a control group. The animals in the experimental group were further divided into seven groups (n=8 each): (1) gp20 (2) Tat (3) gp120+Tat (4) METH (5) gp120+Tat+METH (6) heat inactivated gp120 (7) heat inactivated Tat. The animals in the control group (n=4 each) were divided into (1) control and (2) NACA only-treated group. All animals in the control and experimental groups were injected (i.p) with either saline or NACA (250mg/kg body weight), 30 min before exposure to gp120, Tat or METH. The animals in the experimental group were injected (i.v) with either gp120 (200ng) or Tat (50ng) or a combination of both, for 5 consecutive days (Fig 1). The animals in the METH-treated group were injected (i.v) with 3 doses of METH (10mg/kg body weight), 2 h apart on the 5th or the last day of the treatment. {The dosage and route of METH used in this experiment have been based on previous studies (30, 31, 32). In the literature, 10 mg/ kg body weight dose of METH has been reported to have the most consistent evidence of METH-induced CNS pathology (33). Further, METH abusers vary widely in their dosage/ frequency of use. Studies suggest that the doses of METH used by humans range from 5-1000 mg over a period of 24 h (34). Further, a study conducted by Zule and Desmond (35), indicated that among METH users, the most common pattern was to use 2-3 injections per day on 1-2 days per week.} No evidence of toxicity was observed in the animals treated with gp120 and Tat proteins. However, the animals treated with METH experienced hyperactivity and aggression. The mice were sacrificed 24 h after the last METH injection by urethane injection. All mice were weighed at the beginning and at the end of the study. Following sacrifice, each brain was harvested and divided into two parts, of which, one was snap frozen in liquid nitrogen and the remaining tissue was stored in an antioxidant buffer [8.6 mM sodium phosphate dibasic (Na2HPO4), 26.6 mM sodium phosphate monobasic (NaH2PO4), 50 μM butylhydroxytoluene (BHT), 10 mM aminotriazole, 0.1 mM diethyltriaminepentaacetic acid (DTPA)] at −80°C for further analysis.
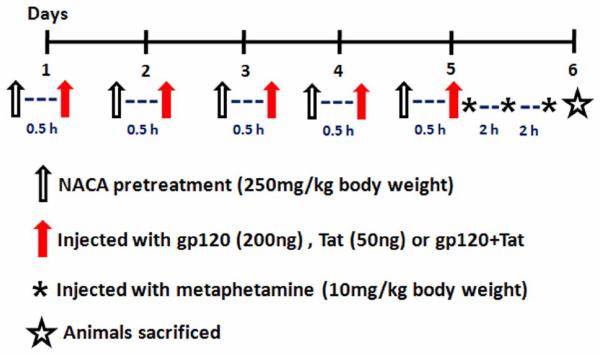
Fig 1.
Schematic representation of the study protocol. Male CD1 mice were injected with gp120 and Tat for 5 consecutive days. Animals in the methamphetamine treated group were injected with 3 doses, at 2 h intervals on the fifth day. All of the animals were pretreated with either NACA or saline, 30 min before exposure to HIV viral proteins and methamphetamine, as mentioned in the Materials and Methods Section. The mice were sacrificed by urethane injection 24 h after the last methamphetamine injection.
Determination of GSH levels
The levels of GSH in the brain were determined by RP-HPLC, according to the method developed in our laboratory (36). The HPLC system (Thermo Electron Corporation) consisted of a Finnigan Spectra System vacuum membrane degasser (model SCM1000), a gradient pump (model P2000), autosampler (model AS3000), and a fluorescence detector (model FL3000) with λex=330 nm and λem=376 nm. The HPLC column used was a Reliasil ODS-1 C18 column (5-μm packing material) with 250×4.6 mm i.d (Column Engineering, Ontario, CA). The mobile phase (70% acetonitrile and 30% water) was adjusted to a pH of 2 with acetic acid and o-phosphoric acid. The NPM derivatives of GSH were eluted from the column isocratically at a flow rate of 1 ml/min. The tissue samples were homogenized in a serine borate buffer, centrifuged, and 250 μl of the supernatant were added to 750 μl of 1 mM NPM. The resulting solution was incubated at room temperature for 5 min, and the reaction was stopped by adding 10 μl of 2N HCl. The samples were then filtered through a 0.45-μm filter and injected into the HPLC system.
Determination of malondialdehyde (MDA)
The MDA levels were determined according to the method described by Draper et al., (37). Briefly, 550 μl of 5% tricholoroacetic acid (TCA) and 100 μl of 500 ppm butylated hydroxytoluene (BHT) in methanol were added to 350 μl of the tissue homogenates, and boiled for 30 min in a water bath. After cooling on ice, the mixtures were centrifuged, and the supernatant collected was mixed 1:1 with saturated thiobarbituric acid (TBA). The mixture was again heated in a water bath for 30 min, followed by cooling on ice. 500 μl of the mixture was extracted with 1 ml of n-butanol and centrifuged to facilitate the separation of phases. The resulting organic layers were first filtered through 0.45 μm filters and then injected into the HPLC system (Shimadzu, US), which consisted of a pump (model LC-6A), a Rheodyne injection valve and a fluorescence detector (model RF 535). The column was a 100 × 4.6 mm i.d C18 column (3 μm packing material, Astec, Bellefonte, PA). The mobile phase used contained 69.4% sodium phosphate buffer, 30% acetonitrile, and 0.6% tetrahydrofuran. The fluorescent product was monitored at λex= 515 nm and λem= 550 nm. Malondialdehyde bis (dimethyl acetal), which gives malondialdehyde on acid treatment, was used as a standard.
Determination of Glutathione Peroxidase (GPx) Activity
Glutathione peroxidase (GPx) protects mammals against oxidative damage by catalyzing the reduction of a variety of ROOH or H2O2 using GSH as the reducing substance. The GPx-340™ assay (Oxis International, Beverly Hills, CA) is an indirect measure of the activity of GPx. Oxidized glutathione (GSSG), produced upon reduction of an organic peroxide by GPx, was recycled to its reduced state by the enzyme glutathione reductase (GR): The oxidation of NADPH to NADP+ was accompanied by a decrease in absorbance at 340 nm (A340), providing a spectrophotometric means for monitoring GPx enzyme activity. The molar extinction coefficient for NADPH is 6220 M−1cm−1 at 340 nm. To measure the activity of GPx, tissue homogenate was added to a solution containing glutathione, glutathione reductase, and NADPH. The enzyme reaction was initiated by adding the substrate, tert-butyl hydroperoxide, and the absorbance was recorded at A340. The rate of decrease in the A340 was directly proportional to the GPx activity in the sample.
Determination of Protein Carbonyl
The protein carbonyl levels were determined according to the method described by Dalle-Donne et al., (38). This assay measures protein carbonyls, as an indicator of protein oxidation, using 2,4-dinitrophenylhydrazine (DNPH). DNPH reacts with protein carbonyls to form hydrazones that can be measured spectrophotmetrically. Briefly, 500μl of 10 mM DNPH (dissolved in 2.5 M HCl) was mixed with 1mg of protein samples. Equal amounts of protein samples without DNPH were used as controls. Both the control and DNPH-treated samples were then incubated in the dark for 1 h and vortexted every 10 min. After the incubation, 500 μl of 20% trichloroacetic acid (TCA) solution were added to each tube and the tubes were placed on ice for 5 min after vortexing. The tubes were then centrifuged at 10,000 × g for 5 min at 4°C. The supernatant was discarded and the pellet was again resuspended, first in 20% TCA and later in ethanol/ethyl acetate mixture (1:1), to remove any free DNPH. This procedure was repeated three times, and the sample was resuspended in 6M guanidine hydrochloride (dissolved in 2N HCl, pH 2.3) at 37°C for 15 min with vortexing. The protein carbonyl content was determined from the absorbance at 366 nm using a molar absorption coefficient of 22,000 M−1cm−1.
Determination of protein
Protein levels of the tissue samples were measured by the Bradford method (39). Concentrated Coomassie Blue (Bio-Rad, Hercules, CA) was diluted 1:5 (v/v) with distilled water. 20 μl of the diluted tissue homogenate were then added to 1.5 ml of this diluted dye, and absorbance was measured at 595 nm using a UV spectrophotometer (Shimadzu Scientific Instruments, Columbia, MD). Bovine serum albumin (BSA) was used as the protein standard.
Western Blot Analysis
Brain homogenates were prepared in lysis buffer (1% triton-x-100, 50 mM NaCl, 10 mM Tris, 1 mM EDTA, 1 mM EGTA, 2 mM sodium vanadate, 0.2 mM PMSF, 1 mM HEPES, 1 μg/ml leupeptin, and 1 μg/ml aprotinin) and protein concentration was estimated using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) as mentioned before. Briefly, 50 μg of tissue homogenate were resolved by electrophoresis on a 12% sodium dodecyl sulfate (SDS) polyacrylamide gel (120v, 1.5 h) in a running gel buffer containing 25 mM Tris, pH 8.3, 162 mM glycine, and 0.1% SDS. The samples were transferred to nylon membrane for 1 h and 20 min at 350 mA. The membranes were incubated overnight in a mixture of T-TBS with 0.1% tween in 2% milk and the respective antibodies {ZO1, ZO2, Claudin 5, Occludin antibody (Invitrogen Corporation, Carlsbad, CA) and GAPDH (Cell Signaling Technology, Inc. Danvers, MA)} in 1:1000 dilution. Subsequently the membrane was incubated in the respective secondary antibody (1:10,000) for 1 h at room temperature. Final visualization was carried out with the enhanced chemiluminescence kit (Bio-Rad, Hercules, CA). The protein bands were quantitated by densitometry, where band intensity ratio of the treated group over the untreated group or control was calculated (40).
Evaluation of BBB permeability
Changes in the permeability on the BBB were assessed using the fluorescent tracer, sodium-fluorescein (NA-F), as described previously (40, 41, 42). Briefly, mice were injected with 100 μl of 2% Na-F in PBS, after which (30 mins later) they were anesthetized with urethane, and then transcardially perfused with PBS until colorless perfusion was visualized. The animals were then decapitated and the brains isolated, were weighed and homogenized in 10 times volume of 50% trichloroacetic acid. The homogenate was then centrifuged for 10 min at 13000 ×g and the supernatant collected was neutralized with 5M NaOH (1:0.8). Measurement of Na-F was monitored at λex= 440 nm and λem= 525 nm using a microplate reader (FLOUstar, BMG Labtechnologies, Durham, NC, USA). The concentration of Na-F in the brain was calculated using external standards with a range of 10-200 ng/ml, and the data was expressed as amount of Na-F /gm of the brain tissue.
Immunoprecipitation
Total brain homogenates were centrifuged for 15 min at 40,000 × g and the supernatant was used for the experiment. The supernatant was collected and protein concentrations were estimated using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA), by Bradford method as mentioned before. For immunoprecipitation of both Occludin and Claudin 5, 3 mg of protein were incubated overnight with either 10 μg of anti-mouse Occludin antibody or anti-mouse Claudin-5 antibody, with rocking at 4°C. Mice IgG was used as a negative control. The antibody protein mixtures were then incubated with 20 μl of Protein A/G plus beads (Santa Cruz Biotechnology, Santacruz, CA) for an additional 3 h with rocking (4°C), after which the beads were pelleted. For washing, the beads were centrifuged at 400 ×g for 3 min, supernatant was removed and 1 ml of ice-cold PBS was added and rocked for 10 min per wash (3X). The bound proteins were then eluted with an equal volume of sample buffer and resolved on 12% SDS-PAGE. Proteins were transferred and blotted against anti-rabbit 4-Hydroxy-2-nonenal (Alpha Diagonostic International, San Antonio, TX), anti-rabbit nitrotyrosine antibody (Chemicon Inc., Temecula, CA), anti-mouse Occludin (positive control), and anti-mouse Claudin 5 (positive control) antibody as mentioned previously.
Statistical Analysis
Group comparisons were performed using the one-way analysis of variance (ANOVA) test and the TUKEYS post hoc test. Statistical analyses were made using GraphPad Prism 5.01 (GraphPad Software Inc., La Jolla, CA). Statistical significance was set at p < 0.05.
Results
Effects of HIV proteins, METH and NACA on GSH levels in the brain
The effects of HIV proteins gp120 and Tat in the brain were studied. Compared to the controls and the NACA-alone treated group, the gp120- and METH-treated animals had decreases (~ 20%) in the GSH levels in their brains. A significant and drastic decrease (~ 85%) in the levels of GSH was observed in animals treated with Tat protein alone. In this study, animals treated with gp120+Tat and gp120+Tat+METH, also experiences significant decrease in GSH levels, as compared to the controls or the NACA-alone treated group. In addition, animals in the gp120+Tat+METH treated group had lower GSH levels as compared to the gp120+Tat-alone treated group (though not signficant), pointing to the fact that METH may be potentiating the oxidative stress induced by gp120 and Tat (Fig 2A) in the brain. However, animals in the gp120+Tat+METH group, pretreated with NACA, had a significant increase in the GSH levels, as compared to the gp120+Tat+METH- alone treated group, indicating that NACA was protecting the brain from HIV proteins and METH-induced oxidative stress. No difference in the GSH levels in the brains of control, gp120 -heat inactivated group and Tat heat-inactivated group were observed (Fig 2B).
Fig 2.
Glutathione levels in the brain. The GSH levels in the brain of CD1 mice exposed to (A) HIV viral proteins (gp120 and Tat), methamphetamine and the antioxidant NACA for 5 days as described in the Materials and Methods section. (B) Comparison of the GSH levels in the brain of animals treated with only NACA and heat inactivated gp120 and Tat.* Values significantly different from the control. # Values significantly different from the gp120+Tat+METH treated group.
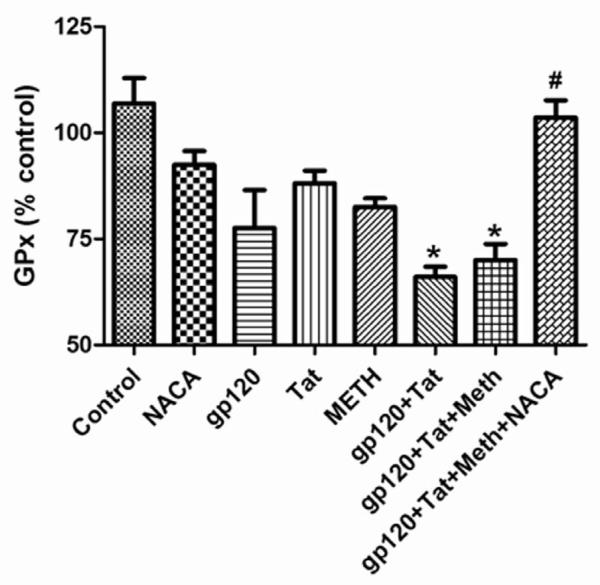
Effects of HIV proteins, METH and NACA on Glutathione peroxidase (GPx) levels in the brain
Antioxidant enzymes like GPx, are involved in the detoxification of organic peroxides in the body. Animals injected with gp120, Tat and METH alone had lower levels of GPx in the brain, as compared to the animals in the control group and NACA alone-treated group. Animals treated with gp120+Tat and gp120+Tat+METH had significant decreases in their GPx levels, as compared to that of the control animals. However, a complete reversal in the GPx levels was observed in animals of the gp120+Tat+METH group, pretreated with NACA (Fig 3).
Fig 3.
Glutathione peroxidase (GPx) levels in the brain. GPx levels in the brain of mice treated with HIV viral proteins (gp120 and Tat), methamphetamine and the antioxidant NACA for 5 days as described in the Materials and the Methods section. * Values significantly different from the control. # Values significantly different from the gp120+Tat+METH treated group.
Effects of HIV proteins, METH and NACA on lipid peroxidation in the brain
Lipid peroxidation is an important consequence of oxidative stress, and can be estimated by measuring the levels of malondialdehyde (MDA), a stable by-product of lipid peroxidation. A significant increase in the level of MDA was observed in the brain tissue of animals treated with gp120, Tat and METH, as compared to that of the control and NACA-alone treated group. Animals treated with gp120+Tat had a higher MDA level than that of the gp120, Tat-alone treated group. Further, animals treated with gp120+Tat+METH experienced the highest level of lipid peroxidation, as compared to all other groups (Fig 4). However, animals in the gp120+Tat+METH group, pretreated with NACA, had a significantly lower MDA level than that of the untreated group, indicating that NACA was protecting the animals from oxidative stress-induced damage. Animals in the gp120 heat-inactivated group and Tat heat-inactivated group, had similar MDA levels as those of the control animals (data not shown).
Fig 4.
Lipid peroxidation in the brain. MDA levels in the brain of mice treated with HIV viral proteins (gp120 and Tat), methamphetamine and the antioxidant NACA for 5 days as described in the Materials and the Methods section. * Values significantly different from the control. # Values significantly different from the gp120+Tat+METH treated group.
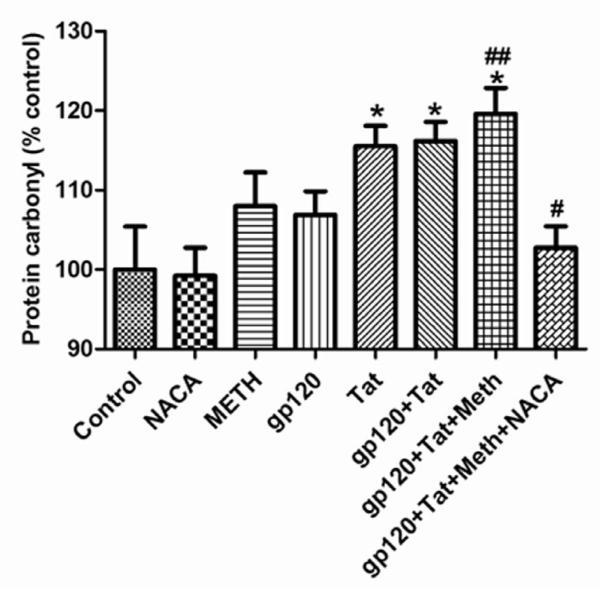
Effects of HIV proteins, METH and NACA on protein carbonyl levels in the brain
Compared to the control and NACA-alone treated group, gp120 and METH treated animals had increased protein carbonyl levels in their brains. A significant increase in the levels of protein carbonyl was observed in animals treated with Tat and gp120+Tat. In addition, the protein carbonyl levels in the brains of mice treated with gp120+Tat+METH were significantly greater than those of the gp120+Tat treated animals, indicating that METH was potentiating the oxidative stress induced by gp120+Tat alone (Fig 5). Pretreatment of animals in the gp120+Tat+METH group with NACA, significantly lowered the protein carbonyl levels in their brains.
Fig 5.
Protein carbonyl levels in the brain. Protein carbonyl was measured as an indicator of protein oxidation in the brain of mice treated with HIV viral proteins (gp120 and Tat), methamphetamine and the antioxidant NACA for 5 days as described in the Materials and the Methods section. * Values significantly different from the control. # Values significantly different from the gp120+Tat+METH treated group. ## Values significantly different from the gp120+Tat treated group.
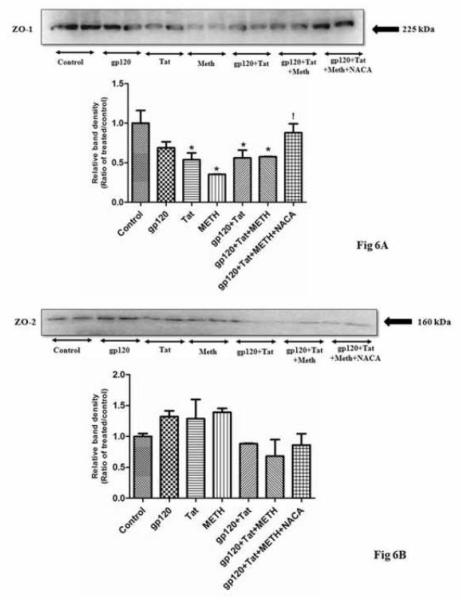
Western blotting of tight junction proteins
To understand if HIV viral proteins and METH alter the permeability of the BBB, the levels of TJ proteins were evaluated. A significant decrease in the expression on ZO1 and Occludin protein, were observed in the brain of animals treated with gp120+Tat+METH, as compared to the controls (Fig 6A, C). However, no significant change in the expression of ZO2 and Claudin 5 protein, was observed in the gp120+Tat+METH treated group (Fig 6B, D), though a trend towards decrease in the expression of these proteins were observed when compared to controls. Interestingly, animals in the gp120+Tat+METH group, pretreated with NACA had a significant increase in the expression of ZO1, Occludin proteins suggesting that the thiol antioxidant NACA was protecting the BBB from oxidative stress induced damage.
Fig 6.
Effect of HIV viral proteins and methamphetamine on tight junction proteins. Shown are the representative western blots of ZO1 (A), ZO2 (B), Occludin (C) and Claudin5 (D) proteins in total brain homogenates of CD1 mice treated with HIV viral proteins (gp120 and Tat), Methamphetamine and the thiol antioxidant NACA. The graphs represent relative densitometric analysis of treated animals over the controls. The results are representative of three individual experiments. * Values significantly different from the control. ! Values significantly different from the gp120+Tat+METH treated group.
Effects of HIV proteins, METH and NACA on blood brain barrier permeability
Changes in the permeability of the BBB were further confirmed using Na-F fluorescent tracer. A significant increase in the Na-F levels (about 2-fold) were observed in the brains of animals treated with gp120+Tat+METH as compared to the controls and the NACA treated group (Fig 7), indicating that HIV proteins and METH were affecting the permeability of the BBB. However, the animals in the gp120+Tat+METH group pretreated with NACA, had Na-F levels in the brains that were similar to that of the control group, indicating that NACA was protecting the BBB from gp120+Tat+METH induced damage.
Fig 7.
Effect of HIV viral proteins and methamphetamine on blood brain barrier permeability. CD1 mice pretreated with NACA or saline, were injected with HIV viral proteins (gp120 and Tat) and methamphetamine for 5 days. BBB permeability was evaluated by administration of the sodium fluorescein (Na-F) tracer (i.p), as described in the Materials and Methods section. After perfusion, the level of Na-F in the brain tissue was measured. The results are expressed as ng of Na-F/ gm of brain tissue. * Values significantly different from the control and NACA-alone group. # Values significantly different from the gp120+Tat+METH+NACA treated group.
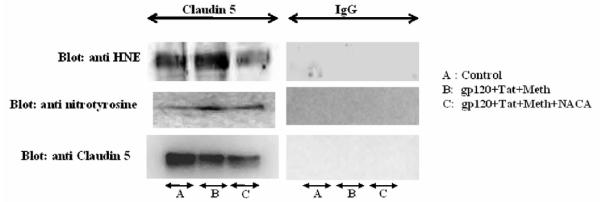
Immunoprecipitation to assess oxidative modification of tight junction proteins
Immunoprecipitation assay was conducted to assess if tight junction proteins like Occludin and Claudin 5 have been oxidatively modified. Total brain homogenates immunoprecipitated with Occludin and Claudin 5 antibodies, were immunoblotted with anti-HNE (to detect Michael’s adducts) and anti-nitrotyrosine (to detect 3-NT) antibodies. For these experiments, Occludin and Claudin 5 were used as a positive control, and IgG as a negative control. As evident by the immunoblotting, both in Occludin and Claudin 5 protein, a higher expression of Michael’s adduct was observed in animals treated with gp120+Tat+METH, when compared to the controls (Fig 8,9). Animals in the gp120+Tat+METH group pretreated with NACA, had lower expression of the adducts as compared to the untreated group, indicating that the tight junction proteins underwent higher protein modification in the gp120+Tat+METH group, as compared to the control or the NACA pretreated group. Similarly, higher expression of 3-NT adducts were observed in Claudin 5 of animals treated with gp120+Tat+METH as compared to the animals in the control or NACA pretreated group (Fig 9). However, no such changes were evident in the Occludin protein, and animals in the control, gp120+Tat+METH and gp120+Tat+METH pretreated with NACA had similar levels of 3-NT (Fig 8).
Fig 8.
Immunoprecipitation assay to detect the oxidative modification of the tight junction protein Occludin. Total brain homogenates were immunoprecipitated with Occludin antibody, separated by SDS-PAGE and immunoblotted with anti rabbit HNE and 3NT antibodies as mentioned in the Materials and Methods section. IgG was used as a negative control, and Occludin served as the positive control. Data shown are representative of three independent experiments.
Fig 9.
Immunoprecipitation assay to detect the oxidative modification of the tight junction protein Claudin 5. Total brain homogenates were immunoprecipitated with Claudin 5 antibody, separated by SDS-PAGE and immunoblotted with anti rabbit HNE and 3NT antibodies as mentioned in the Materials and Methods section. IgG was used as a negative control, and Claudin 5 served as the positive control. Data shown are representative of three independent experiments.
Discussion
In the recent years, METH use has been implicated in worsening of HIV associated neurological impairments, especially HAD (43-47). The neurotoxic effect of METH increases dopamine and glutamate formation in the brain that, in turn, mediates damage to the dopamine neurons through the formation of toxic ROS (48-51). The HIV viral proteins (gp120 and Tat) have also been reported to increase oxidative stress in the brain (12). Although both HIV viral proteins and METH are known to induce oxidative stress, nothing is known about whether METH potentiates oxidative stress induced by gp120 and Tat in concert or how it affects the functioning of the BBB. In this study we determined the oxidative stress parameters (GSH, MDA, GPx, protein carbonyl, modification of TJ proteins by 4-HNE, 3-nitrotyrosine) in the brains of animals exposed to gp120, Tat and METH. We also investigated the role of the antioxidant NACA in protecting the BBB from oxidative stress-induced damage.
The human brain uses more than 20% of the oxygen consumed by the body (52), as a result of which, the potential for the generation of ROS during oxidative phosphorylation in the brain increases to a great extent (53). For proper functioning of the brain, the ROS has to be counter-balanced by an antioxidant defense system. Glutathione (L-γ-glutamyl-L-cysteinylglycine, GSH) is the key low molecular thiol antioxidant involved in the defense of brain cells against oxidative stress (54-55). In the literature, a decrease in GSH levels has been connected to physiological processes such as aging (56) and neurological disorders like schizophrenia (57), Alzheimer’s disease (58), and epilepsy (59). In patients with Parkinson’s disease, the GSH content in the brain region has been reported to decrease by 40-50%, as compared to that of controls (60-61). Similar results were observed in our current study, where animals exposed to HIV viral proteins (especially gp120+Tat) had significant decreases in GSH levels in their brains as compared to controls. The greatest decline in the level of GSH was observed in animals treated with both HIV viral protein (gp120+Tat) and METH, indicating that METH was potentiating the oxidative stress-induced by the viral proteins. However, pretreatment of the animals (in the gp120+Tat+METH group) with NACA, increased the GSH levels significantly, indicating that the antioxidant NACA was able to partially abrogate oxidative stress induced damage in these animals. Although GSH is the primary molecule involved in detoxification of ROS in the body, antioxidant enzymes like GPx, are also known to play a role in this process. During detoxification of peroxides, the enzyme GPx converts GSH to GSSG (glutathione disulphide). A significant decrease in the activity of GPx was observed in animals treated with gp120+Tat and gp120+Tat+METH, as compared to the controls, indicating that the overwhelming oxidative stress induced by these toxins deplete the antioxidant enzyme in the brain. However, animals in the gp120+Tat+METH group, pretreated with NACA, had GPx levels similar to that of the control. This is in agreement with previous studies from our laboratory, where NACA has been reported to inhibit METH-induced oxidative stress, in an in vitro model of BBB (62).
Free radicals, produced by oxidative stress, damage different biological molecules like protein, lipid and DNA. Membrane lipids form an important constituent of the BBB, providing a large surface area across which lipid-soluble molecules undergo diffusion by the transcellular pathway (63). Membrane lipids undergo oxidation, producing cytotoxic lipid peroxidation products like MDA and 4-hydroxynonenal (4-HNE), which adversely affect the integrity of the BBB (64). Conversely, treatment of cells with inhibitors of lipid peroxidation products decreased the BBB permeability by modulating the passage of transcellular substances (65-66). Further, MDA has also been reported to be neurotoxic. In addition to this, reactive oxygen species (ROS) are also known to convert amino groups of proteins to carbonyl moieties (67-68), which leads to the loss of their functional activities (69-70). Increases in protein carbonyl levels have been reported in the brains of patients suffering from amyotrophic lateral sclerosis (71). Further, modifications of key enzymes and structural proteins have also been demonstrated to lead to neurobiliary degeneration of neurons in patients suffering from Alzheimer’s disease (72). Our results are in good agreement with these studies where animals treated with gp120+Tat+METH experience significant increases in lipid peroxidation and protein carbonylation, as compared to the controls, thereby pointing to the role of oxidative stress induced damage in our model.
In addition, HIV patients abusing addictive drugs like METH have been reported to have exacerbated neurodegenerative changes (73-77), and one of the most critical factors in the development and progression of these changes is the loss of integrity of the BBB (78-80). The BBB, composed primarily of the brain microvascular endothelelial cells, forms a tight seal due to the presence of well developed tight junctions (TJ) that restrict the entrance of circulating molecules and immune cells into the brain (81). The major component of the TJ includes transmembrane proteins, occludin and claudins, and the submembranous peripheral ZO proteins (82-83). These TJ proteins are not only involved in paracellular transport (84), but also play a role as signaling molecules involved in actin cytoskeleton reorganization (85). TJ proteins are also highly sensitive, and respond to the changes in their microenvironment by alteration and dissociation of the occludin/ZO complex, leading to impairment of the BBB (86). In the current study, a decrease in the expression of ZO1 and occludin protein has been observed in animals treated with gp120+Tat+METH, pointing to the alteration of BBB permeability in our model. However, no change in the expression of ZO2 and claudin 5 was observed in our model. Pretreatment of the animals with the antioxidant NACA, increases the expression of these TJ proteins. An increase in BBB permeability was further confirmed by the Na-F tracer experiment, where animals pretreated with NACA in the gp120+Tat+METH group had significant decrease in the Na-F levels in their brain as compared to the gp120+Tat+METH alone treated group, indicating the role of oxidative stress in altering the permeability of the BBB in our model.
In addition to this, TJ proteins like occludin and claudin 5 were also found to be modulated by 4-HNE in our model. As mentioned before, 4-HNE, one of the major biologically active aldehydes generated from peroxidation of membrane lipids (87), and has been implicated in actin cytoskeleton remodeling and disruption of endothelial cell barrier in the lungs (88). One of the initial reactions of 4-HNE in the cells is the protein modification by the formation of Michael adducts (89-92) which, in turn, are capable of invoking a wide range of biological activities by modulation of different cell signaling pathways (88). These adducts have also been reported to increase paracellular transport of albumin across the human umbilical endothelial cell monolayer (93) and permeability of the BBB (94). In the current study, an increase in the expression of Michael’s adducts was observed in the TJ proteins (occludin and claudin 5) of animals treated with gp120+Tat+METH, indicating altered permeability of the BBB in our model. Further, studies by Usatyuk et al., (88), have shown that Michaels adducts induce oxidative stress by depleting intracellular GSH level, modulate MAPK activation, and alter the endothelial cell barrier function. This is in agreement with our studies, where Michael adduct formations in TJ proteins (claudin 5 and occludin), were partially blocked by pretreatment with the thiol protectant NACA.
In addition to Michaels adduct, damage to the BBB have also been linked to 3-NT, a specific and stable marker for peroxynitrite formation (95-96). An intense and widespread deposition of 3-NT has been observed in the autopsy CNS tissues of patients with AIDS-associated dementia. However, no 3-NT was detected in patients who had died with HIV encephalitis not associated with dementia (97). Further, the presence of 3-NT was also detected in patients suffering from multiple sclerosis (98). These findings are similar in lines with our current study, where the TJ protein claudin 5 were found to be modulated by 3-NT in animals treated with gp120+Tat+METH, pointing to the role of 3-NT in the alteration of BBB permeability in HAD.
In summary, results from the present study indicates that in our animal model, addictive drugs like METH potentiate oxidative stress induced by gp120 and Tat in concert (Fig. 10), by decreasing the levels of the antioxidants GSH and GPx in the brain. The free radicals also modify the lipid and protein molecules in the brain, as indicated by the increase in MDA and protein carbonyl levels. Treatment of animals with gp120+Tat+METH, also alters the expression of the TJ proteins, in addition to modulating it, leading to a compromised BBB integrity. However, pretreatment of these animals with the thiol antioxidant NACA, confers protection to these animals and, thus, could be considered as a viable therapeutic option for patients suffering from HAD and other neurodegenerative diseases.
Fig 10.
Schematic representation of the mechanism of HIV viral proteins and methamphetamine-induced oxidative damage to the blood brain barrier, and the protective role of the thiol antioxidant N-acetylcysteineamide (NACA). HIV viral protein gp120 and Tat, along with methamphetamine, synergistically increases oxidative stress induced damage by lowering the level of antioxidant enzymes GSH and GPx and increasing oxidative modification of proteins and lipids in the brain. This leads to decrease in the expression of tight junction proteins in the blood brain barrier (BBB), as a result of which the BBB permeability increases. Further, oxidative modification of the tight junction proteins also aids in increase in permeability of the BBB, leading to increased passage of toxins and leukocytes in to the brain leading to severe dementia in HIV patients abusing methamphetamine. However, pretreatment with the novel antioxidant, NACA partially restores the oxidative balance in the brain and maintains the BBB permeability, thus protecting the brain from toxins and inflammation.
Acknowledgements
Dr. Ercal is supported by 1 R15DA023409-01A2 from the NIDA, NIH. The contents of this paper are solely the responsibility of the authors and do not necessarily represent official views of the NIDA or NIH. Dr. Banks is supported by VA Merit Review and R01 AG029839. The authors appreciate the efforts of Barbara Harris in editing the manuscript. HIV-1 Tat and HIV-1 Bal gp120 protein was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Methamphetamine was obtained from NIDA.
List of abbreviations
- HAD
HIV-1 associated dementia
- METH
Methamphetamine
- NACA
N-acetylcysteine amide
- BBB
Blood brain barrier
- HIV-1
Human immunodeficiency virus
- gp120
HIV-1 envelope glycoprotein (gp120)
- Tat
Transregulatory protein
- TJ
Tight junctions
- NAC
N-acetylcysteine
- GSH
Glutathione
- MDA
Malondialdehyde
- GPx
Glutathione peroxidase
- ROS
Reactive oxygen species
- 4-HNE
4-hydroxynonenal
- 3-NT
3-nitrotyrosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Atrayee Banerjee, Department of Chemistry, Missouri University of Science and Technology, Rolla, MO, USA.
Xinsheng Zhang, Department of Chemistry, Missouri University of Science and Technology, Rolla, MO, USA.
Kalyan Reddy Manda, Department of Chemistry, Missouri University of Science and Technology, Rolla, MO, USA.
Nuran Ercal, Department of Chemistry, Missouri University of Science and Technology, Rolla, MO, USA.
References
- 1.Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of the AIDS dementia complex. AIDS. 1997;11:S35. [PubMed] [Google Scholar]
- 2.Krebs FC, Ross H, McAllister J, Wigdahl B. HIV-1-associated central nervous system dysfunction. Adv.Pharmacol. 2000;49:315. doi: 10.1016/s1054-3589(00)49031-9. [DOI] [PubMed] [Google Scholar]
- 3.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J. Infect. Dis. 2002;186:S193. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 4.Kolson DL. Neuropathogenesis of central nervous system HIV-1 infection. Clin. Lab. Med. 2002;22:703. doi: 10.1016/s0272-2712(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 5.Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;7:411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- 6.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 7.Banks WA. Physiology and pathophysiology of the blood–brain barrier: implications for microbial pathogenesis, drug delivery and neurodegenerative disorders. J. Neurovirol. 1999;5:538–555. doi: 10.3109/13550289909021284. [DOI] [PubMed] [Google Scholar]
- 8.Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood–brain barrier during HIV-1 encephalitis. J. Immunol. 1997;158:3499–3510. [PubMed] [Google Scholar]
- 9.Toborek M, Lee YW, Pu H, Malecki A, Flora G, Garrido R, Hennig B, Bauer HC, Nath A. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J Neurochem. 2003;84:169–179. doi: 10.1046/j.1471-4159.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res. 2005;8:119–134. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- 11.Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J. Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- 12.Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045:57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 14.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am. J. Physiol. Renal. Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 15.Bradbury MW. The blood-brain barrier. Exp. Physiol. 1993;78:453–472. doi: 10.1113/expphysiol.1993.sp003698. [DOI] [PubMed] [Google Scholar]
- 16.Cho A. Ice: a new dosage form of an old drug. Science. 1990;249:631–634. doi: 10.1126/science.249.4969.631. [DOI] [PubMed] [Google Scholar]
- 17.Wise R, Hoffman DC. Localization of drug reward mechanisms by intracranial injections. Synapse. 1992;10:247–263. doi: 10.1002/syn.890100307. [DOI] [PubMed] [Google Scholar]
- 18.Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res. Brain Res. Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 19.Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am.J. Pathol. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardridge WM. Brain metabolism: a perspective from the bloodbrain barrier. Physiol. Rev. 1983;63:1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- 21.Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur J Clin Invest. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 22.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinase’s leading to blood-brain barrier dysfunction. J Neurochemistry. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 23.Plateel M, Dehouck MP, Torpier G, Cecchelli R, Teissier E. Hypoxia increases the susceptibility to oxidant stress and the permeability of the blood-brain barrier endothelial cell mono layer. J Neurochem. 1995;65:2138–2145. doi: 10.1046/j.1471-4159.1995.65052138.x. [DOI] [PubMed] [Google Scholar]
- 24.Penugonda S, Mare S, Goldstein G, Banks WA, Ercal N. Effects of N-acetylcysteine amide (NACA), a novel thiol antioxidant against glutamate-induced cytotoxicity in neuronal cell line PC-12. Brain. Res. 2005;1056:132–138. doi: 10.1016/j.brainres.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Wu W, Abraham LS, Ogony J, Matthews R, Goldstein G, Ercal N. Effects of N-acetylcysteine amide, a novel thiol antioxidant on radiation induced cytotoxicity in chinese hamster ovary cells. Life Sci. 2008;82:1122–1130. doi: 10.1016/j.lfs.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Price TO, Uras F, Banks WA, Ercal N. A novel antioxidant, N-acetylcysteine amide prevents gp120 and tat-induced oxidative stress in brain endothelial cells. Exp. Neurol. 2006;201:193–202. doi: 10.1016/j.expneurol.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Amer J, Atlas D, Fibach E. N-acetylcysteine amide (AD4) attenuates oxidative stress in beta-thalassemia blood cells. Biochim. Biophys. Acta. 2008;1780:249–255. doi: 10.1016/j.bbagen.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv. Pharmacol. 1997;38:205–227. [PubMed] [Google Scholar]
- 29.Atlas D, Melamed E, Offen D. Brain targeted low molecular weight hydrophobic antioxidant compounds. No. 5 U.S. Patent. 1999;874:468.
- 30.Broening HW, Pu C, Vorhees CV. Methamphetamine selectively damages dopaminergic innervations to the nucleus accumbens core while sparing the shell. Synapse. 1997;27:153–160. doi: 10.1002/(SICI)1098-2396(199710)27:2<153::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- 32.Gluck MR, Moy LY, Jayatilleke E, Hogan KA, Manzino L, Sonsalla PK. Parallel increases in lipid and protein oxidative markers in several mouse brain regions after methamphetamine treatment. J Nuerochem. 2001;79:152–160. doi: 10.1046/j.1471-4159.2001.00549.x. [DOI] [PubMed] [Google Scholar]
- 33.Madden LJ, Flynn CT, Zandonatti MA, May M, Parsons LH, Katner SN, Henrikson SJ, Fox HS. Modelling human methamphetamine exposure in nonhuman primates: chronic dosing in the rhesus macaque leads to behavioral and physiological abnormalities. Neuropsychopharmacology. 2005;30:350–359. doi: 10.1038/sj.npp.1300575. [DOI] [PubMed] [Google Scholar]
- 34.Mitler MM, Hajdukovic R, Erman MK. Treatment of narcolepsy with methamphetamine. Sleep. 1993;16:306–317. [PMC free article] [PubMed] [Google Scholar]
- 35.Zule WA, Desmond DP. An ethnographic comparison of HIV risk behaviors among heroin and methamphetamine injectors. Am J Drug Alcohol Abuse. 1999;25:1–23. doi: 10.1081/ada-100101843. [DOI] [PubMed] [Google Scholar]
- 36.Winters R, Zukowski J, Ercal N, Matthews D, Spitz DR. Analysis of glutathione, glutathione disulphide, cysteine, homocysteine and other biological thiols by HPLC following derivatization with N-(1-pyrenyl) malemide. Anal Biochem. 1995;227:14–21. doi: 10.1006/abio.1995.1246. [DOI] [PubMed] [Google Scholar]
- 37.Draper HH, Squires EJ, Mahmoodi H, Wu J, Agarwal M, Hadley M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993;15:353–363. doi: 10.1016/0891-5849(93)90035-s. [DOI] [PubMed] [Google Scholar]
- 38.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clinica. Chimica. Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 39.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez SH, Potula R, Fan S, Eidem T, Papugani A, Reichenbach N, Dykstra H, Weksler BB, Romero IA, Couraud PO, Persidsky Y. Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:1933–1945. doi: 10.1038/jcbfm.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenzser G, Kis B, Bari F, Busija DW. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res. 2005;1051:72–80. doi: 10.1016/j.brainres.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 42.Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permability changes during a central nervous system inflammatory response: TNF- alpha is neither necessary nor sufficient. J Immunol. 2007;178:7334–7343. doi: 10.4049/jimmunol.178.11.7334. [DOI] [PubMed] [Google Scholar]
- 43.Piot P, Bartos M, Ghys PD, Walker N, Schwartlander B. The Global Impact of HIV/AIDS. Nature. 2001;410:968–973. doi: 10.1038/35073639. [DOI] [PubMed] [Google Scholar]
- 44.Denkar BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:1–9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 45.Conant K, St Hillaire C, Anderson C, Galey D, Wang J, Nath A. Human immunodeficiency virus type 1 Tat and methamphetamine affect the release and activation of matrix-degrading proteinases. J Neurovirol. 2004;10:21–28. doi: 10.1080/13550280490261699. [DOI] [PubMed] [Google Scholar]
- 46.Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- 47.Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- 48.Cadet JL, Sheng P, Ali S, Rothman R, Carlson E, Epstein C. Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J Neurochem. 1994;62:380–383. doi: 10.1046/j.1471-4159.1994.62010380.x. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto BK, Zhu W. The Effects of methamphetamine on the production of free radicals and oxidative stress. J. Pharmacol. Exp. Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- 50.LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J. Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res. Brain Res. Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 52.Sigel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Lippincott-Raven; Philadelphia: 1999. [Google Scholar]
- 53.Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- 54.Cooper AJ, Kristal BS. Multiple roles of glutathione in the central nervous system. Biol. Chem. 1997;378:793–802. [PubMed] [Google Scholar]
- 55.Dringen R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 56.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. (2000) [DOI] [PubMed] [Google Scholar]
- 57.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuenod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur. J. Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 58.Gu M, Owen AD, Toffa SE, Cooper JM, Dexter DT, Jenner P, Marsden CD, Schapira AH. Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J. Neurol. Sci. 1998;158:24–29. doi: 10.1016/s0022-510x(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 59.Mueller SG, Trabesinger AH, Boesiger P, Wieser HG. Brain glutathione levels in patients with epilepsy measured by in vivo 1H-MRS. Neurology. 2001;57:1422–1427. doi: 10.1212/wnl.57.8.1422. [DOI] [PubMed] [Google Scholar]
- 60.Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci. Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- 61.Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glu-514 R. Dringen and J. Hirrlinger tathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, Banerjee A, Banks WA, Ercal N. N-acetylcysteineamide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized brain endothelial cells. Brain Res. 2009;1275:87–95. doi: 10.1016/j.brainres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pun PB, Lu J, Moochhala S. Involvement of ROS in BBB dysfunction. Free Radic Res. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- 64.Mertsch K, Blasig I, Grune T. 4-hydroxynonenal impairs the permeability of an in vivo rat blood-brain barrier. Neurosci Lett. 2001;314:135–138. doi: 10.1016/s0304-3940(01)02299-6. [DOI] [PubMed] [Google Scholar]
- 65.Shi F, Cavitt J, Audus KL. 21-aminosteroid and 2-amino-methyl chromans inhibition of arachnoid acid-induced lipid peroxidation and permeability enhancement in bovine brain microvessel endothelail cell monolayers. Free Radic Biol Med. 1995;19:349–387. doi: 10.1016/0891-5849(95)00049-4. 1995. [DOI] [PubMed] [Google Scholar]
- 66.Smith SL, Scherzh HM, Hall ED. Protective effects of tirilazad mesylate and metabolite -89678 against blood brain barrier damage after subarachnoid haemorrhage and lipid peroxidative neuronal injury. Neurosurgery. 1996;84:229–233. doi: 10.3171/jns.1996.84.2.0229. [DOI] [PubMed] [Google Scholar]
- 67.Chavko M, Harabin AL. Regional lipid peroxidation and protein oxidation in rat brain after hyperbaric oxygen exposure. Free Radic. Biol. Med. 1996;20:973–978. doi: 10.1016/0891-5849(95)02181-7. [DOI] [PubMed] [Google Scholar]
- 68.Perry G, Raina AK, Nunomura A, Wataya T, Sayre LM, Smith MA. How important is oxidative damage? Lessons from Alzheimer’s disease. Free Radical Biol. Med. 2000;28:831–834. doi: 10.1016/s0891-5849(00)00158-1. [DOI] [PubMed] [Google Scholar]
- 69.Davies KJ, Goldberg AL. Proteins damaged in extracts of red blood cells. J Biol Chem. 1987;262:8220–8226. [PubMed] [Google Scholar]
- 70.Rivett AJ, Levine RL. Metal-catalyzed oxidation of Escherichia coli glutamine synthetase: correlation of structural and functional changes. Arch. Biochem. Biophys. 1990;278:26–34. doi: 10.1016/0003-9861(90)90226-o. [DOI] [PubMed] [Google Scholar]
- 71.Bowling AC, Schulz JB, Brown RH, Beal MF. Superoxide dismutase activity, oxidative damage and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J. Neurochem. 1993;61:2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 72.Aksenov MY, Aksenova MV, Butterfield DA, Gedded JW, Markesbery WR. Protein oxidation in the brain in Alzheimer’s disease. Neurosci. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 73.Theodore S, Cass WA, Nath A, Steiner J, Young K, Maragos WF. Inhibition of tumor necrosis factor-alpha signaling prevents human immunodeficiency virus-1 protein Tat and methamphetamine interaction. Neurobiol of Dis. 2006;23:663–668. doi: 10.1016/j.nbd.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Theodore S, Cass WA, Maragos WF. Involvement of cytokines in human immunodeficiency virus-1 protein Tat and Methamphetamine interactions in the striatum. Experimental Neurol. 2006;199:490–498. doi: 10.1016/j.expneurol.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 75.Theodore S, Cass WA, Maragos WF. Methamphetamine and human immunodeficiency virus protein Tat synergize to destroy dopaminergic terminals in the rat striatum. Neurosci. 2006;137:925–935. doi: 10.1016/j.neuroscience.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 76.Theodore S, Cass WA, Nath A, Maragos WF. Progress in understanding basal ganglia dysfunction as a common target for methamphetamine abuse and HIV-1 neurodegeneration. Curr. HIV Res. 2007;5:301–313. doi: 10.2174/157016207780636515. [DOI] [PubMed] [Google Scholar]
- 77.Mahajan SD, Hu Z, Reynolds JL, Aalinkeel R, Schwartz SA, Nair MP. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells: implications for HIV-1 pathogenesis. Mol Diagn Ther. 2006;10:257–269. doi: 10.1007/BF03256465. [DOI] [PubMed] [Google Scholar]
- 78.Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood–brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am. J. Pathol. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood–brain barrier. Eur. J. Clin. Invest. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 80.Bar-Or A, Nuttall RK, Duddy M, Alter A, Kim HJ, Ifergan I, Pennington CJ, Bourgoin P, Edwards DR, Yong VW. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–2749. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]
- 81.Pachter JS, de Vries HE, Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62:593–604. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- 82.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aurrand-Lions M, Johnson-Leger C, Lamagna C, Ozaki H, Kita T, Imhof BA. Junctional adhesion molecules and interendothelail junctions. Cells Tissues Organs. 2002;172:152–160. doi: 10.1159/000066967. [DOI] [PubMed] [Google Scholar]
- 84.Balda MS, Flores-Maldonado C, Cereijido M, Matter K. Multiple domains of occludin are involved in the regulation of paracellular permeability. J Cell Biochem. 2000;78:85–96. [PubMed] [Google Scholar]
- 85.Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, Meade G, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arterioscler Thromb Vasc Biol. 2006;26:62–68. doi: 10.1161/01.ATV.0000194097.92824.b3. [DOI] [PubMed] [Google Scholar]
- 87.Hammer A, Ferro M, Tillian HM, Tatzber F, Zollner H, Schauenstein E, Schaur RJ. Effect of oxidative stress by iron on 4-hydroxynonenal formation and proliferative sctivity in hepatomas of different degrees of differentiation. Free Radic. Biol. Med. 1997;23:26–33. doi: 10.1016/s0891-5849(96)00630-2. [DOI] [PubMed] [Google Scholar]
- 88.Usatyuk PV, Parinandi NL, Natarajan V. Redox regulation of 4-hydroxy-2-nonenal mediated endothelial barrier dysfunction by focal adhesion, adherens and tight junction proteins. J Biol Chem. 2006;281:35554–35566. doi: 10.1074/jbc.M607305200. [DOI] [PubMed] [Google Scholar]
- 89.Eaton P, Li JM, Hearse DJ, Shattock MJ. Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat hearts. Am J Physiol. 1999;276:H935–H943. doi: 10.1152/ajpheart.1999.276.3.H935. [DOI] [PubMed] [Google Scholar]
- 90.Hamilton RF, Li L, Eschenbacher WL, Szweda L, Holian A. Potential involvement of 4-hydroxynonenal in the response of human lung cells to ozone. Am J Physiol. 1998;274:L8–L16. doi: 10.1152/ajplung.1998.274.1.L8. [DOI] [PubMed] [Google Scholar]
- 91.Usatyuk PV, Natarajan V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J Biol Chem. 2004;279:11789–11797. doi: 10.1074/jbc.M311184200. [DOI] [PubMed] [Google Scholar]
- 92.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential producer of intracellulaqr peroxide production. J Biol Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 93.Herbst U, Toborek M, Kaiser S, Mattson MP, Hennig B. 4-hydroxynonenal induces dysfunction and apoptosis of cultures endothelial cells. J Cell Physiol. 1999;181:295–303. doi: 10.1002/(SICI)1097-4652(199911)181:2<295::AID-JCP11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 94.Mertsch K, Blasig I, Grune T. 4-Hydroxynonenal impairs the permeability of an in vitro rat blood brain barrier. Neurosci. Lett. 2001;314:135–138. doi: 10.1016/s0304-3940(01)02299-6. [DOI] [PubMed] [Google Scholar]
- 95.Boven LA, Gomes L, Hery C, Gray F, Verhoef J, Portegies P, Tardieu M, Nottet HS. Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV-1 infection. J Immunol. 1999;162:4319–4327. [PubMed] [Google Scholar]
- 96.Liu JSH, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158:2057–2067. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 98.Cross AH, Manning PT, Keeling RM, Schmidt RE, Misko TP. Peroxynitrite formation within the central nervous system in active multiple sclerosis. J Neuroimmunol. 1998;88:45–56. doi: 10.1016/s0165-5728(98)00078-2. [DOI] [PubMed] [Google Scholar]