Abstract
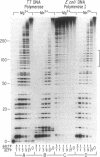
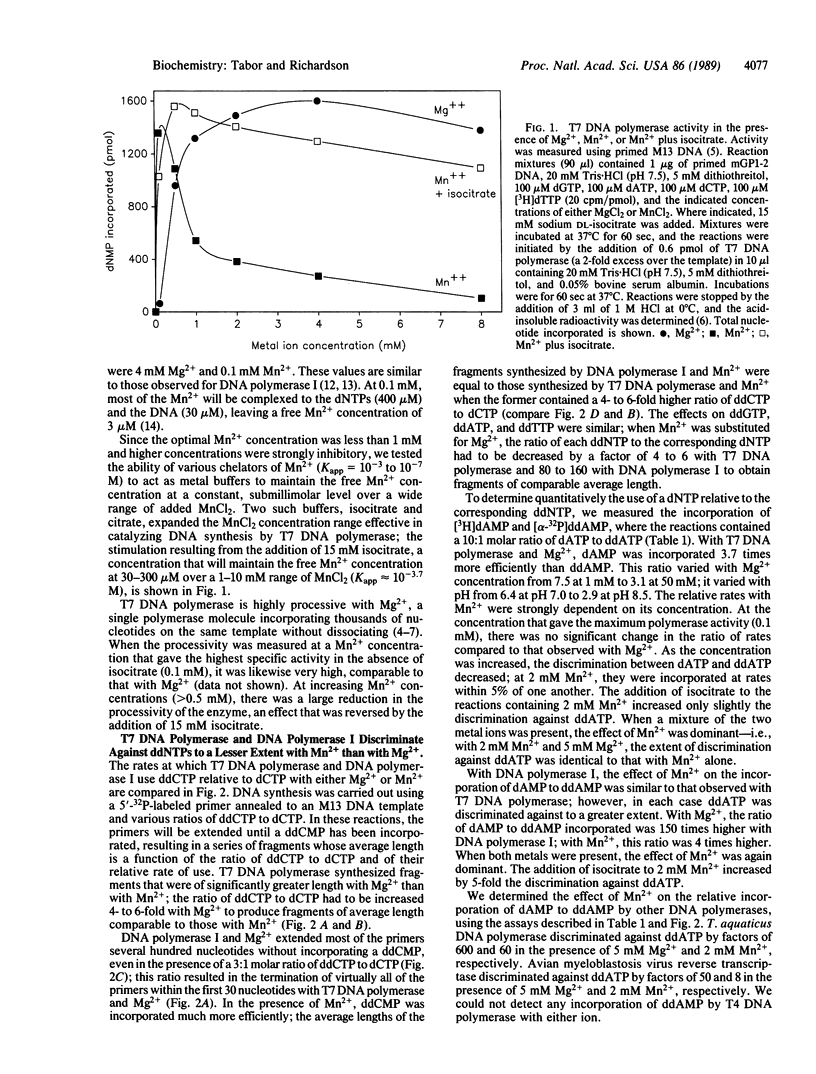
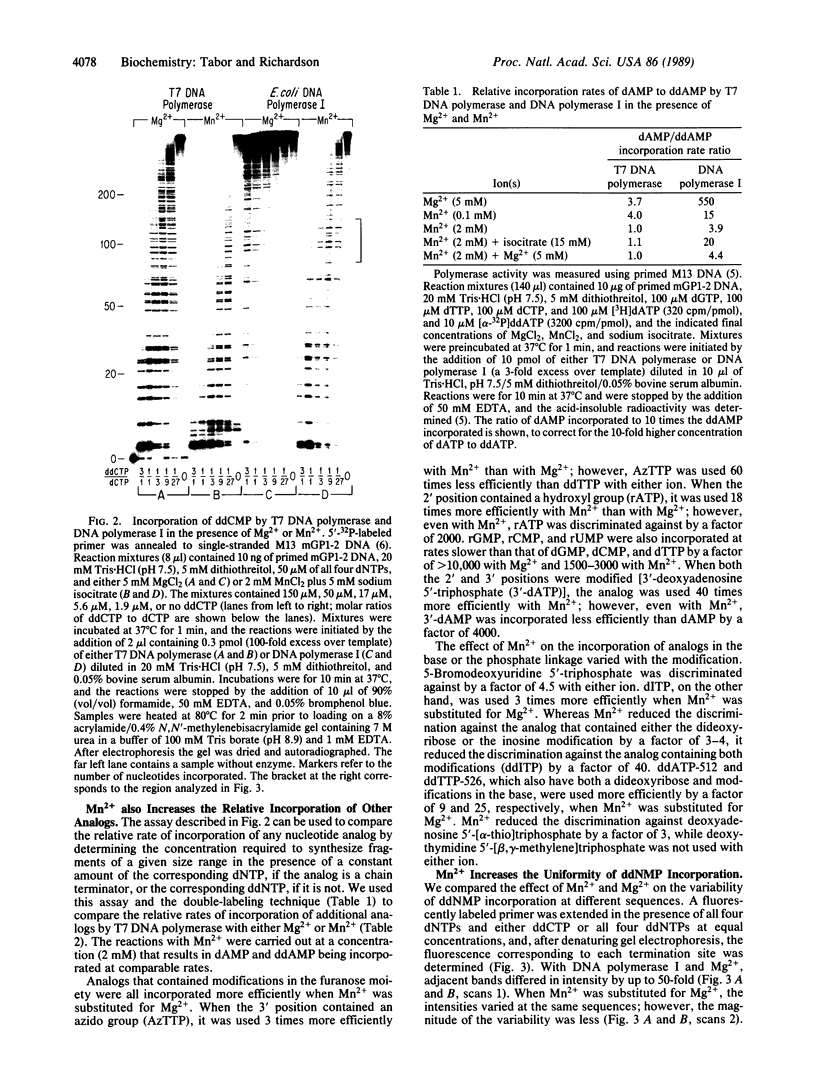
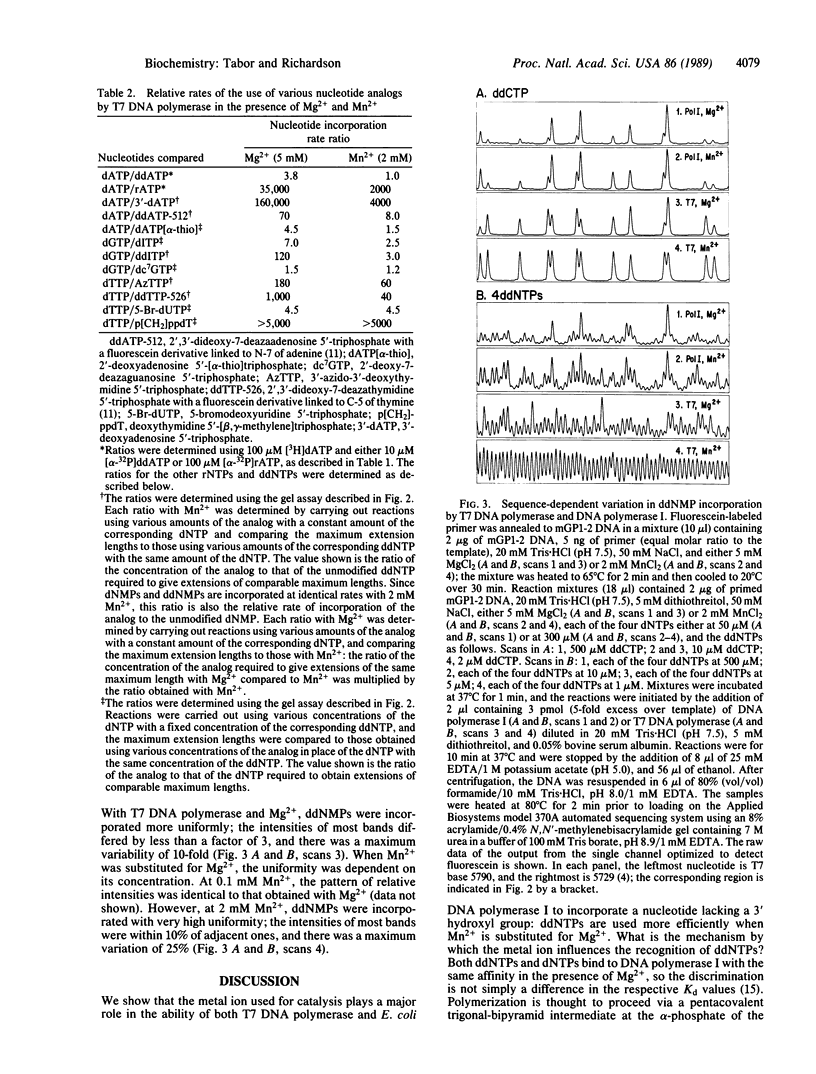
Incorporation of dideoxynucleotides by T7 DNA polymerase and Escherichia coli DNA polymerase I is more efficient when Mn2+ rather than Mg2+ is used for catalysis. Substituting Mn2+ for Mg2+ reduces the discrimination against dideoxynucleotides approximately 100-fold for DNA polymerase I and 4-fold for T7 DNA polymerase. With T7 DNA polymerase and Mn2+, dideoxynucleotides and deoxynucleotides are incorporated at virtually the same rate. Mn2+ also reduces the discrimination against other analogs with modifications in the furanose moiety, the base, and the phosphate linkage. A metal buffer, isocitrate, expands the MnCl2 concentration range effective in catalyzing DNA synthesis. The lack of discrimination against dideoxynucleoside triphosphates using T7 DNA polymerase and Mn2+ results in uniform terminations of DNA sequencing reactions, with the intensity of adjacent bands on polyacrylamide gels varying in most instances by less than 10%.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. R., Deutscher M. P., Kornberg A., Russell A. F., Moffatt J. G. Enzymatic synthesis of deoxyribonucleic acid. XXXIV. Termination of chain growth by a 2',3'-dideoxyribonucleotide. Biochemistry. 1969 Dec;8(12):4897–4904. doi: 10.1021/bi00840a037. [DOI] [PubMed] [Google Scholar]
- Beckman R. A., Mildvan A. S., Loeb L. A. On the fidelity of DNA replication: manganese mutagenesis in vitro. Biochemistry. 1985 Oct 8;24(21):5810–5817. doi: 10.1021/bi00342a019. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F. A study of the mechanism of DNA polymerase I from Escherichia coli with diastereomeric phosphorothioate analogs of deoxyadenosine triphosphate. J Biol Chem. 1979 Aug 10;254(15):6889–6893. [PubMed] [Google Scholar]
- Englund P. T., Huberman J. A., Jovin T. M., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXX. Binding of triphosphates to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3038–3044. [PubMed] [Google Scholar]
- Hillebrand G. G., Beattie K. L. Template-dependent variation in the relative fidelity of DNA polymerase I of Escherichia coli in the presence of Mg2+ versus Mn2+. Nucleic Acids Res. 1984 Apr 11;12(7):3173–3183. doi: 10.1093/nar/12.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H. E., Tabor S., Richardson C. C. Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J Biol Chem. 1987 Nov 25;262(33):16224–16232. [PubMed] [Google Scholar]
- Joyce C. M., Grindley N. D. Construction of a plasmid that overproduces the large proteolytic fragment (Klenow fragment) of DNA polymerase I of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1830–1834. doi: 10.1073/pnas.80.7.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildvan A. S., Loeb L. A. The role of metal ions in the mechanisms of DNA and RNA polymerases. CRC Crit Rev Biochem. 1979;6(3):219–244. doi: 10.3109/10409237909102564. [DOI] [PubMed] [Google Scholar]
- Ollis D. L., Kline C., Steitz T. A. Domain of E. coli DNA polymerase I showing sequence homology to T7 DNA polymerase. 1985 Feb 28-Mar 6Nature. 313(6005):818–819. doi: 10.1038/313818a0. [DOI] [PubMed] [Google Scholar]
- Prober J. M., Trainor G. L., Dam R. J., Hobbs F. W., Robertson C. W., Zagursky R. J., Cocuzza A. J., Jensen M. A., Baumeister K. A system for rapid DNA sequencing with fluorescent chain-terminating dideoxynucleotides. Science. 1987 Oct 16;238(4825):336–341. doi: 10.1126/science.2443975. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirover M. A., Dube D. K., Loeb L. A. On the fidelity of DNA replication. Metal activation of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Jan 10;254(1):107–111. [PubMed] [Google Scholar]
- Sloan D. L., Loeb L. A., Mildvan A. S. Conformation of deoxynucleoside triphosphate substrates on DNA polymerase I from Escherichia coli as determined by nuclear magnetic relaxation. J Biol Chem. 1975 Dec 10;250(23):8913–8920. [PubMed] [Google Scholar]
- Smith L. M., Sanders J. Z., Kaiser R. J., Hughes P., Dodd C., Connell C. R., Heiner C., Kent S. B., Hood L. E. Fluorescence detection in automated DNA sequence analysis. Nature. 1986 Jun 12;321(6071):674–679. doi: 10.1038/321674a0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Huber H. E., Richardson C. C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J Biol Chem. 1987 Nov 25;262(33):16212–16223. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Selective inactivation of the exonuclease activity of bacteriophage T7 DNA polymerase by in vitro mutagenesis. J Biol Chem. 1989 Apr 15;264(11):6447–6458. [PubMed] [Google Scholar]
- Van de Sande J. H., Loewen P. C., Khorana H. G. Studies on polynucleotides. 118. A further study of ribonucleotide incorporation into deoxyribonucleic acid chains by deoxyribonucleic acid polymerase I of Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6140–6148. [PubMed] [Google Scholar]