Abstract
Biodiversity surveys were conducted on Saba Bank, Netherlands Antilles, to assess ichthyofaunal richness and to compare with published surveys of other Caribbean localities. The primary objective was to estimate the total species richness of the Saba Bank ichthyofauna. A variety of sampling techniques was utilized to survey the fish species of both the visually accessible megafauna and the camouflaged and small-sized species comprising the cryptic ichthyofauna.
Based on results presented herein, the number of species known on Saba Bank is increased from 42 previously known species to 270 species. Expected species-accumulation curves demonstrate that the current estimate of species richness of fishes for Saba Bank under represents the actual richness, and our knowledge of the ichthyofauna has not plateaued. The total expected fish-species richness may be somewhere between 320 and 411 species.
The Saba Bank ichthyofaunal assemblage is compared to fish assemblages found elsewhere in the Caribbean. Despite the absence of shallow or emergent shore habitats like mangroves, Saba Bank ranks as having the eighth highest ichthyofaunal richness of surveyed localities in the Greater Caribbean. Some degree of habitat heterogeneity was evident. Fore-reef, patch-reef, and lagoonal habitats were sampled. Fish assemblages were significantly different between habitats. Species richness was highest on the fore reef, but 11 species were found only at lagoonal sites.
A comprehensive, annotated list of the fishes currently known to occur on Saba Bank, Netherland Antilles, is provided and color photographs of freshly collected specimens are presented for 165 of the listed species of Saba Bank fishes to facilitate identification and taxonomic comparison with similar taxa at other localities. Coloration of some species is shown for the first time. Preliminary analysis indicates that at least six undescribed new species were collected during the survey and these are indicated in the annotated list.
Introduction
Saba Bank is the largest atoll in the Atlantic Ocean Basin and one of the three largest atolls on earth [1]. Located in the Dutch Windward Islands about 250 km east of Puerto Rico, it is a flat-topped seamount rising 1800 m from the surrounding sea floor. Except for the fact that it does not break the water surface, Saba Bank is a classic atoll consisting of a submerged mountain crowned at the summit with a ring of actively growing coral reefs [2]. Saba Bank is relatively free of the problems that are degrading many Caribbean reef systems, and the few problems it faces include anchoring and abrasion by oil tankers maneuvering off the petroleum transshipment facilities on St. Eustatius, potential petroleum spillage and subsequent use of dispersants, general vessel passage in a zone of high maritime traffic, possible overfishing for certain species, and exploration for petroleum reserves (so far unsuccessful). Saba Bank's fisheries and dive operations are economically significant to the small community on Saba Island (about 1500 residents) that has direct responsibility for its management.
The known fish fauna of Saba Bank prior to our survey consisted of 42 fish species. Most of these species were taken during fishery bottom-trawl surveys on Saba Bank, including two M/V Oregon stations in 1958 and nine stations in 1959, and two trawl hauls taken in 1969 by the R/V Pillsbury. Although four of these trawls were taken on or near the top of Saba Bank, nine were on the deep outer slopes. The habitats sampled during these surveys were restricted to relatively soft-bottom habitats due to the exclusive use of trawling techniques. These trawl samples provided valuable records of fishes living on soft bottoms and on the outer slopes of Saba Bank.
A biodiversity-assessment survey was carried out on Saba Bank during 2006 and 2007, with a major goal being to improve knowledge of the biodiversity on one of the world's most significant, though poorly known, coral-capped seamounts. In an effort to record as many fish species as possible in the short period of time available for the survey, we utilized a variety of fish sampling techniques. These techniques included visual surveys by divers, use of SCUBA to apply ichthyocide (a natural fish toxicant consisting of dried and powdered Derris root - assayed at 7.5% rotenone), hand-line fishing, by-catch from lobster and fish traps taken by local fishermen, and port sampling observations of fish landings. During the surveys, an attempt was made to obtain a photograph documenting the fresh colors of as many species as possible, a tissue sample of each species, and preserved specimen vouchers that have been archived in the National fish collection (USNM) of the National Museum of Natural History (NMNH), Smithsonian Institution (SI). Specimens representing six or more undescribed species and two rare gobies, Pycnomma roosevelti and Psilotris boehlkei, were collected.
The habitats surveyed on the Saba Bank “atoll” during this study are classified as: fore reef, patch reef, lagoonal and Small Bank. Fore-reef areas are located around the outer rim of the submerged atoll. Patch reef is an isolated portion of “reef” situated on the lagoonal (interior) side of the fore reef. Lagoonal is the central portion of the atoll interior to the fore-reefs around the atoll rim and may have a variety of bottom types. Small Bank is a small, independent “seamount-like” structure located off the northwest corner of Saba Bank with its shallowest depth at about 11 m. Depth categories were arbitrarily assigned as shallow (11–24 m), mid-depth (25–34 m), and deep (35–38 m).
The primary goals of the overall biodiversity survey were to provide data and analysis to support designation of Saba Bank as a marine protected area, to support the development of a management plan, and to contribute to a petition to the International Maritime Organization to designate appropriate parts of Saba Bank as a Particularly Sensitive Sea Area.
Results
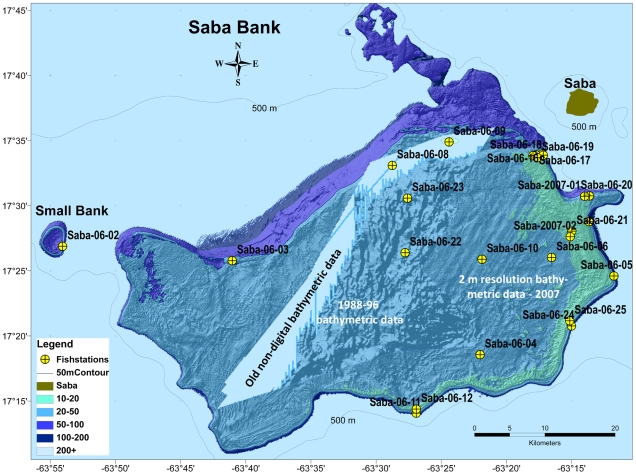
We occupied fish stations at 25 locations during a rapid assessment (RAP) survey, 2–16 January 2006; including: 20 ichthyocide stations, 12 roving visual surveys, two hook & line stations, and five by-catch stations from lobster traps; two fish-ichthyocide stations were occupied at two additional locations on 20 June 2007 (Figure 1). In 2007, Toller assessed the benthic communities and fish assemblages based in part on 40 visual surveys of the fish fauna at an area on the eastern side of Saba Bank.
Figure 1. Bathymetric map of Saba Bank with fish stations marked.
Specimens collected during the RAP survey were preserved as vouchers and processed into the fish collection (available online at http://vertebrates.si.edu/fishes/fishes_collections.html) at the NMNH (as many species as possible were also tissue sampled and photographed in the field). Toller collected and preserved vouchers from fishery landings when possible and photographed specimens representing new records. Toller's vouchers were processed into the fish collection at the NMNH and are also available in the online database.
Some species were only taken during fishery bottom trawl surveys by the M/V Oregon (1958, 1959) and the R/V Pillsbury (1969) on Saba Bank. Species represented by voucher specimens in museum collections are included in our comprehensive species list.
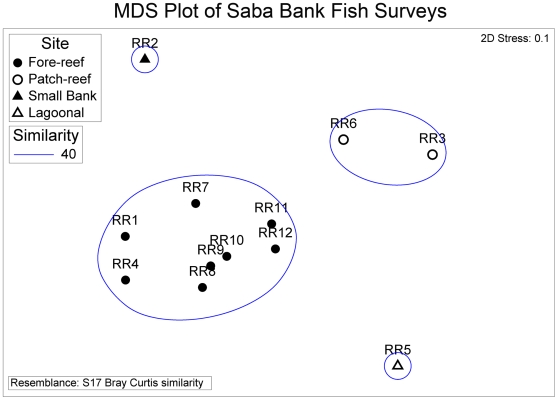
A non-metric multidimensional-scaling (MDS) ordination of the 12 rotenone and roving stations based on a Bray-Curtis similarity matrix of incidence data was used to illustrate similarities and differences of fish assemblages found on the four atoll habitat types: fore reef, patch reef, lagoonal, Small Bank (Figure 2). There were significant differences among habitat types (ANOSIM Global R = 0.96, P = 0.001). Differences between fore-reef stations and patch-reef stations were most pronounced (ANOSIM, Pairwise R = 0.92, P = 0.002). Fore-reef assemblages ranged from 39 to 60 species per station while the patch-reef stations had 26 and 32 species. Fore-reef stations were 50% similar to each other, and patch-reef stations were up to 60% similar. Fore-reef assemblages were not significantly different than Small Bank, or the lagoonal habitat (ANOSIM, Pairwise R = 0.973, P = 0.11). Low sample size (one station with 39 species) in the lagoonal habitat limits this comparison.
Figure 2. MDS ordination of fish-survey stations illustrates high similarity of assemblages within fore-reef sites and significant differences (ANOSIM, P<0.05) among habitats.
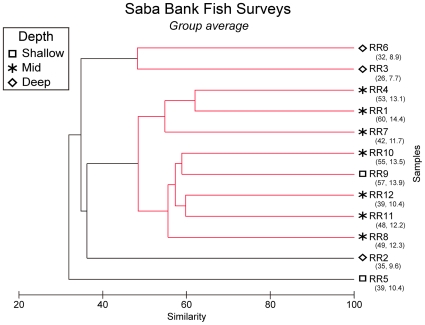
There were significant differences in the fish assemblages when classified by depth - shallow, middle, and deep (ANOSIM Global test, R = 0.618, P = 0.006). Differences in species composition were most evident between mid-depth and deep sites (ANOSIM Pairwise test, R = 0.829, P = 0.008). Fore-reef sites were typically at mid to shallow depths (20 to 34 m) whereas the patch-reef sites were typically deep (35 to 38 m). There is some evidence of habitat heterogeneity and vertical zonation for fish assemblages on Saba Bank, but more sampling is necessary to discern whether habitat or depth best explains the differences among groups.
The horizontal axis in the MDS plot represents a gradient in species richness with patch-reef and lagoonal sites having lowest richness ( Figure 2). The vertical axis in the MDS plot illustrates differences related to depth with deepest station (Small Bank) higher in the vertical axis, and shallower sites lower in the vertical axis. The two-dimensional stress value was low in the MDS (stress = 0.1) indicating a slight chance of misrepresentation.
A principal components analysis (PCA) was used to understand which species assemblages were responsible for differences among stations. The first three components explained 43.9% of the variation. The first component (18.6% of variation explained) was dominated by ubiquitous species and most common species. Strongest loadings (negative) on the first principal component (PC1) included the four species found at all stations (Halichoeres garnoti, Serranus tigrinus, Stegastes partitus, and Thalassoma bifasciatum) and 13 species found at all but one to four of the stations. These 13 common species are rarely found at the small bank, patch-reef, and lagoonal stations and, therefore, PC1 also serves to define the fore-reef sites. For example, Acanthurus bahianus, Coryphopterus glaucofraenum, Coryphopterus dicrus, and Scarus taeniopterus were found at all fore-reef sites but rarely at the Small Bank, lagoonal and at least one of the patch-reef sites. The strongest positive loadings on PC1 are from 11 species only found at the lagoonal site. Principal components 2 and 3 correspond strongly to the horizontal or species richness component on the MDS with strongest positive loadings on species typically found at the low to medium species richness stations 3, 5, 6 and 8–12 (e.g. Cryptotomus roseus, Haemulon melanurum, Halichoeres bivittatus, Astrapogon puncticulatus, and Serranus baldwini). Strongest negative scores were on species that were frequently found at high to medium species richness stations 1, 2, 4, 7 and 8 (e.g. Hypoplectrus puella, Lythrypnus elasson, Prognathodes aculeatus, Neoniphon marianus, and Gramma loreto).
An annotated list of the fishes of Saba Bank is provided below. In the list, we include the family, genus and species, author and English common name (as common names are not standardized internationally, we strove to apply the most widely used English common name based on FishBase (http://www.fishbase.org) listings). The use of “cf” before a species name indicates that the specimen photographed is similar to that species, but probably represents an undescribed species. Voucher specimens are archived at the National Museum of Natural History (USNM) and the Florida Museum of Natural History (UF) and each species with vouchers is annotated with the museum's acronym where the specimens are housed. The basis of each species record is indicated by: I – ichthyocide station, F – caught by a local fisherman and photographed, T – bottom trawl, O – visual sighting during Toller survey, V – visual sighting during RAP survey at roving and rotenone station. Lengths of specimens are recorded in mm for either standard length (SL), total length (TL), or fork length (FL). Photographs showing the color pattern of freshly collected specimens are included for as many of the species as possible. Images illustrating observed sexual and developmental (juvenile to adult) variability in color pattern are included where possible.
Ginglymostomatidae—nurse sharks
Ginglymostoma cirratum (Bonnaterre, 1788)—nurse shark; OV; Figure 3
Figure 3. Ginglymostoma cirratum, underwater photo by Juan Sanchez.
Squalidae—dogfish sharks
Squalus cubensis Howell Rivero, 1936—Cuban dogfish; F; Figure 4
Figure 4. Squalus cubensis, 475 mm TL, photo by W Toller.
Carcharhinidae—requiem sharks
Carcharhinus perezii (Poey, 1876)—reef shark; F
Galeocerdo cuvier (Péron & Lesueur, 1822)—tiger shark; F,O
Etmopteridae—lantern sharks
Etmopterus bullisi Bigelow & Schroeder, 1957—lined lantern shark; USNM, T
Dasyatidae—whiptail stingrays
Dasyatis americana Hildebrand & Schroeder, 1928—southern stingray; V
Muraenidae—morays
Anarchias similis (Lea, 1913)—pygmy moray; USNM, I
Enchelycore carychroa (Böhlke & Böhlke, 1976)—chestnut moray; USNM, I, F; Figure 5
Figure 5. Enchelychore carychroa, 175 mm TL, photo by JT Williams.
Enchelycore nigricans (Bonnaterre, 1788)—viper moray; USNM, I
Gymnothorax conspersus Poey, 1867—saddled moray; USNM, F; Figure 6
Figure 6. Gymnothorax conspersus, 730 mm TL, photo by W Toller.
Gymnothorax maderensis(Johnson, 1862)—sharktooth moray; USNM, F; Figure 7
Figure 7. Gymnothorax maderensis, 300 mm TL, photo by W Toller.
Gymnothorax miliaris (Kaup, 1856)—goldentail moray; USNM, I, O; Figure 8
Figure 8. Gymnothorax miliaris, 60.3 mm TL, photo by JT Williams.
Gymnothorax moringa (Cuvier, 1829)—spotted moray; USNM, I, O, V; Figure 9
Figure 9. Gymnothorax moringa, 189.8 mm TL, photo by JT Williams.
Gymnothorax polygonius Poey, 1876—polygon moray; USNM, F; Figure 10
Figure 10. Gymnothorax polygonius, 810 mm TL, photo by W Toller.
Gymnothorax vicinus (Castelnau, 1855)—purplemouth moray; USNM, I, O; Figure 11
Figure 11. Gymnothorax vicinus, 48.2 mm TL, photo by JT Williams.
Monopenchelys acuta (Parr, 1930)—redface moray; USNM, I; Figure 12
Figure 12. Monopenchelys acuta, 132.4 mm TL, photo by JT Williams.
Uropterygius macularius (Lesueur, 1825)—marbled moray; USNM, I
Ophichthidae—snake eels
Ahlia egmontis (Jordan, 1884)—key worm eel; USNM, I; Figures 13, 14
Figure 13. Ahlia egmontis, 183.0 mm TL, photo by JT Williams.
Figure 14. Ahlia egmontis, 183.0 mm TL, close-up of head, photo by JT Williams.
Aprognathodon platyventris Böhlke, 1967—stripe eel; USNM, I; Figures 15, 16
Figure 15. Aprognathodon platyventris, 149.2 mm TL, photo by JT Williams.
Figure 16. Aprognathodon platyventris, 149.2 mm TL, close-up of head, photo by JT Williams.
Myrichthys breviceps (Richardson, 1848) —sharptail eel; O
Myrichthys ocellatus (Lesueur, 1825)—goldspotted eel; USNM, I; Figures 17, 18
Figure 17. Myrichthys ocellatus, 383 mm TL, photo by JT Williams.
Figure 18. Myrichthys ocellatus, 383 mm TL, close-up of head, photo by JT Williams.
Congridae—conger eels
Bathycongrus thysanochilus (Reid, 1934)—conger eel; USNM, T
Conger esculentus Poey, 1866—grey conger; USNM, F
Chlopsidae—false morays
Kaupichthys hyoproroides (Strömman, 1896)—false moray; USNM, I; Figure 19
Figure 19. Kaupichthys hyoproroides, 79.4 mm TL, photo by JT Williams.
Kaupichthys nuchalis Böhlke, 1967—collared eel; USNM, I; Figure 20
Figure 20. Kaupichthys nuchalisi, 61.6 mm TL, photo by JT Williams.
Moringuidae—spaghetti eels
Moringua edwardsi (Jordan & Bollman, 1889)—spaghetti eel; USNM, I; Figure 21
Figure 21. Moringua edwardsi, 258 mm TL, photo by JT Williams.
Synodontidae—lizardfishes
Saurida brasiliensis Norman 1935—largescale lizardfish; UF, T
Saurida normani Longley 1935—shortjaw lizardfish; UF, T
Synodus intermedius (Spix & Agassiz, 1829)—sand diver; USNM, UF, F, O; Figure 22
Figure 22. Synodus intermedius, 316 mm SL, photo by W Toller.
Synodus poeyi Jordan, 1887—offshore lizardfish; UF, T
Synodus saurus (Linnaeus, 1758)—Atlantic lizardfish; UF, I, V
Synodus synodus (Linnaeus, 1758)—red lizardfish; UF, USNM, I; Figure 23
Figure 23. Synodus synodus, 42.5 mm SL, photo by JT Williams.
Trachinocephalus myops (Forster, 1801)—snakefish; V
Ophidiidae—cusk-eels
Brotula barbata (Bloch & Schneider, 1801)—bearded brotula; USNM, O; Figure 24
Figure 24. Brotula barbata, 628 mm TL, photo by W Toller.
Neobythites ocellatus Günther 1887—ocellate cusk-eel; USNM, T
Neobythites unicolor Nielsen & Retzer 1994—unicolor cusk-eel; USNM, T
Ophidion antipholus Lea & Robins, 2003—longnose cusk-eel; UF, T
Otophidium omostigma (Jordan & Gilbert, 1882)—polka-dot cusk-eel; UF, T
Parophidion schmidti (Woods & Kanazawa, 1951)—dusky cusk-eel; USNM, I; Figure 25
Figure 25. Parophidion schmidti, 69.3 mm TL, photo by JT Williams.
Petrotyx sanguineus (Meek & Hildebrand, 1928)—redfin brotula; USNM, I; Figure 26
Figure 26. Petrotyx sanguineus, 62.2 mm TL, photo by JT Williams.
Bythitidae—viviparous brotulas
Ogilbia sabaji Moller, Schwarzhans & Nielsen, 2005—Sabaj coralbrotula; USNM, I; Figure 27
Figure 27. Ogilbia sabaji, 27.2 mm SL, photo by JT Williams.
Antennariidae—frogfishes
Antennarius pauciradiatus Schultz, 1957 —dwarf frogfish; USNM, I; Figures 28, 29
Figure 28. Antennarius pauciradiatus, 24.9 mm SL, photo by JT Williams.
Figure 29. Antennarius pauciradiatus, 24.9 mm SL, close-up of head, photo by JT Williams.
Antennarius multiocellatus (Valenciennes, 1837) —longlure frogfish; USNM, I
A single juvenile specimen was collected in 2007. Although adults of this species have a very long first spine, our juvenile specimen has the first dorsal spine about the same length as the second. Böhlke and Chaplin [3] mention this allometric growth pattern in which the first spine is short in young specimens, but increases in length with growth. The juvenile exhibits the typical adult color pattern.
Chaunacidae—sea toads
Chaunax suttkusi Caruso, 1989—Suttkus sea toad; USNM, T
Ogcocephalidae—batfishes
All of the batfish records from Saba Bank are based on trawl collections with vouchered museum specimens.
Dibranchus atlanticus Peters, 1876—Atlantic batfish; UF, T
Halieutichthys aculeatus (Mitchill, 1818)—pancake batfish; UF, T
Ogcocephalus pumilus Bradbury, 1980—dwarf batfish; USNM, T
Exocoetidae—flyingfishes
Cypselurus comatus (Mitchill, 1815)—clearwing flyingfish; USNM
This specimen was probably captured at the surface using a dip net.
Syngnathidae—pipefishes
Anarchopterus tectus (Dawson, 1978) —insular pipefish; USNM, I
Bryx randalli (Herald, 1965)—ocellated pipefish; USNM, I; Figure 30
Figure 30. Bryx randalli, 24.3 mm SL, photo by JT Williams.
Micrognathus crinitus (Jenyns, 1842) —banded pipefish; USNM, I; Figures 31, 32
Figure 31. Micrognathus crinitus, 102.8 mm SL, photo by JT Williams.
Figure 32. Micrognathus crinitus, 102.8 mm SL, close-up of head, photo by JT Williams.
Aulostomidae—trumpetfishes
Aulostomus maculatus Valenciennes, 1837—trumpetfish; USNM, I, O, V
Holocentridae—squirrelfishes
Holocentrus adscensionis (Osbeck, 1765)—squirrelfish; USNM, I, O, V; Figure 33
Figure 33. Holocentrus ascensionus, 195.0 mm SL, photo by JT Williams.
Holocentrus rufus (Walbaum, 1792)—longspine squirrelfish; USNM, I, O, V; Figure 34
Figure 34. Holocentrus rufus, 153.2 mm SL, photo by JT Williams.
Myripristis jacobus Cuvier, 1829—blackbar soldierfish; USNM, I, O; Figure 35
Figure 35. Myripristis jacobus, 114.7 mm SL, photo by JT Williams.
Neoniphon marianus (Cuvier, 1829)—longjaw squirrelfish; USNM, I; Figure 36
Figure 36. Neoniphon marianus, 98.2 mm SL, photo by JT Williams.
Plectrypops retrospinis (Guichenot, 1853)—cardinal soldierfish; USNM, I; Figure 37
Figure 37. Plectrypops retrospinis, 71.5 mm SL, photo by JT Williams.
Sargocentron coruscum (Poey, 1860)—reef squirrelfish; USNM, I, O; Figures 38, 39
Figure 38. Sargocentron coruscum, juvenile, 31.1 mm SL, photo by JT Williams.
Figure 39. Sargocentron coruscum, 81.7 mm SL, photo by JT Williams.
Scorpaenidae—scorpionfishes
Scorpaena albifimbria Evermann & Marsh, 1900—coral scorpionfish; USNM, I; Figure 40
Figure 40. Scorpaena albifimbria, 37.4 mm SL, photo by JT Williams.
Scorpaena bergii Evermann & Marsh, 1900—goosehead scorpionfish; USNM, I
Scorpaena grandicornis Cuvier, 1829—plumed scorpionfish; USNM, I; Figure 41
Figure 41. Scorpaena grandicornis, 65.1 mm SL, photo by JT Williams.
Scorpaena inermis Cuvier, 1829 —mushroom scorpionfish; USNM, I, T; Figures 42, 43
Figure 42. Scorpaena inermis, 40.1 mm SL, red morph, photo by JT Williams.
Figure 43. Scorpaena inermis, 65.9 mm SL, yellow morph, photo by JT Williams.
Two color morphs were collected at Saba Bank, a red morph (Figure 42) and a yellow morph (Figure 43).
Scorpaena plumieri Bloch, 1789—spotted scorpionfish; USNM, I
Scorpaenodes caribbaeus Meek & Hildebrand, 1928—reef scorpionfish; USNM, I; Figure 44
Figure 44. Scorpaenodes caribbaeus, 30.1 mm SL, photo by JT Williams.
Triglidae—searobins
All of the searobin records from Saba Bank are based on trawl collections with vouchered museum specimens.
Bellator egretta (Goode & Bean, 1896)—streamer searobin; USNM, T
Bellator militaris (Goode & Bean, 1896)—horned searobin; UF, T
Prionotus ophryas Jordan & Swain, 1885—bandtail searobin; UF, T
Symphysanodontidae—slopefishes
Symphysanodon berryi Anderson, 1970—slope bass; USNM, F; Figure 45
Figure 45. Symphysanodon berryi, top 75 mm SL, lower 63 mm SL, photo by W Toller.
Serranidae—sea basses
Alphestes afer (Bloch, 1793)—mutton hamlet; F, O; Figure 46
Figure 46. Alphestes afer, underwater photo by W Toller.
Cephalopholis cruentata (Lacepède, 1802)—graysby; USNM, I, F, O, V; Figure 47
Figure 47. Cephalopholis cruentata, 101.1 mm SL, photo by JT Williams.
Cephalopholis fulva (Linnaeus, 1758)—coney; UF, USNM, I, F, O, V; Figure 48
Figure 48. Cephalopholis fulva, 121.1 mm SL, photo by JT Williams.
Diplectrum bivittatum (Valenciennes, 1828) —dwarf sand perch; UF, T
Epinephelus flavolimbatus Poey, 1865—yellowedge grouper; F; Figure 49
Figure 49. Epinephelus flavolimbatus, 652 mm SL, photo by W Toller.
Epinephelus guttatus (Linnaeus, 1758)—red hind; UF, USNM, F, I, O, T, V; Figure 50
Figure 50. Epinephelus guttatus, 283.2 mm SL, photo by JT Williams.
Epinephelus morio (Valenciennes, 1828)—red grouper; F
Epinephelus niveatus Valenciennes, 1828—snowy grouper; USNM, F; Figure 51
Figure 51. Epinephelus niveatus, 526 mm SL, photo by W Toller.
Epinephelus striatus (Bloch, 1792)—Nassau grouper; F
Hypoplectrus chlorurus (Cuvier, 1828) —yellowtail hamlet; O
Hypoplectrus nigricans (Poey, 1852)—black hamlet; USNM, I, O, V; Figure 52
Figure 52. Hypoplectrus nigricans, 67.2 mm SL, photo by JT Williams.
Hypoplectrus puella (Cuvier, 1828)—barred hamlet; O, V
Liopropoma rubre Poey, 1861—peppermint basslet; USNM, I; Figure 53
Figure 53. Liopropoma rubre, 30.0 mm SL, photo by JT Williams.
Mycteroperca interstitialis (Poey, 1860)—yellowmouth grouper; F; Figure 54
Figure 54. Mycteroperca interstitialis, 328 mm SL, photo by W Toller.
Mycteroperca tigris (Valenciennes, 1833)—tiger grouper; V
Mycteroperca venenosa (Linnaeus, 1758)—yellowfin grouper; F, OBS
Paranthias furcifer (Valenciennes, 1828)—Atlantic creolefish; UF, USNM, I, O, V; Figure 55
Figure 55. Paranthias furcifer, 148.4 mm SL, photo by JT Williams.
Pseudogramma gregoryi (Breder, 1927)—reef bass; USNM, I; Figure 56
Figure 56. Pseudogramma gregoryi, 43.5 mm SL, photo by JT Williams.
Rypticus bistripinus (Mitchill, 1818) —freckled soapfish; USNM, I; Figure 57
Figure 57. Rypticus bistripinus, 46.2 mm SL, photo by JT Williams.
Rypticus saponaceus (Bloch & Schneider, 1801)—greater soapfish; USNM, I; Figure 58
Figure 58. Rypticus saponaceus, 44.1 mm SL, photo by JT Williams.
Rypticus new species; USNM, I
This new species of soapfish is very similar in appearance to Rypticus subbifrenatus. The new species is being described by C Baldwin and DG Smith (pers. comm.).
Rypticus subbifrenatus Gill, 1861—spotted soapfish; USNM, I; Figure 59
Figure 59. Rypticus subbifrenatus, 54.0 mm SL, photo by JT Williams.
Schultzea beta (Hildebrand, 1940)—school bass; UF, USNM, I
Serranus baldwini (Evermann & Marsh, 1899)—lantern bass; UF, USNM, I, O, V; Figure 60
Figure 60. Serranus baldwini, 41.6 mm SL, photo by JT Williams.
Serranus maytagi Robins & Starck, 1961 —maytag bass; UF
Serranus notospilus Longley, 1935—saddle bass; USNM, F; Figure 61
Figure 61. Serranus notospilus, 150 mm SL, photo by W Toller.
Serranus tabacarius (Cuvier, 1829)—tobaccofish; UF, O, V
Serranus tigrinus (Bloch, 1790)—harlequin bass; USNM, I, O, V; Figure 62
Figure 62. Serranus tigrinus, 71.1 mm SL, photo by JT Williams.
Serranus tortugarum Longley, 1935—chalk bass; UF, USNM, I, O; Figure 63
Figure 63. Serranus tortugarum, 51.8 mm SL, photo by JT Williams.
Grammatidae—basslets
Gramma loreto Poey, 1868—fairy basslet; USNM, I, O, V; Figure 64
Figure 64. Gramma loreto, 38.4 mm SL, photo by JT Williams.
Opistognathidae—jawfishes
Opistognathus aurifrons (Jordan & Thompson, 1905)—yellowhead jawfish; USNM, I, O, V; Figure 65
Figure 65. Opistognathus aurifrons, 44.1 mm SL, photo by JT Williams.
Opistognathus whitehursti (Longley, 1927)—dusky jawfish; USNM, I; Figures 66, 67
Figure 66. Opistognathus whitehursti, juvenile with large black spot in dorsal fin, 22.1 mm SL, photo by JT Williams.
Figure 67. Opistognathus whitehursti, adult, 44.0 mm SL, photo by JT Williams.
Priacanthidae—bigeyes
Heteropriacanthus cruentatus (Lacepède, 1801)—glasseye snapper; O
Priacanthus arenatus Cuvier, 1829—bigeye; USNM, I; Figure 68
Figure 68. Priacanthus arenatus, 67.4 mm SL, photo by JT Williams.
Apogonidae—cardinalfishes
Apogon aurolineatus (Mowbray in Breder, 1927)—barred cardinalfish; USNM, I
Apogon binotatus (Poey, 1867)—barred cardinalfish; USNM, I
Apogon maculatus (Poey, 1860)—flamefish; USNM, I; Figure 69
Figure 69. Apogon maculatus, 21.1 mm SL, photo by JT Williams.
Apogon pillionatus Böhlke and Randall, 1968 —broadsaddle cardinalfish; UF, USNM, I
Apogon cf quadrisquamatus Longley, 1934 —sawcheek cardinalfish; USNM, I; Figure 70
Figure 70. Apogon cf quadrisquamatus, 21.1 mm SL, photo by JT Williams (this specimen represents a new undescribed species.
The specimens identified here as A.cf quadrisquamatus have been found to represent a new undescribed species closely related to A. quadrisquamatus (C. Baldwin & D.G. Smith, pers. comm. 2009).
Apogon robinsi Böhlke & Randall, 1968 —roughlip cardinalfish; USNM, I; Figure 71
Figure 71. Apogon robinsi, 35.5 mm SL, photo by JT Williams.
Apogon townsendi (Breder, 1927)—belted cardinalfish; USNM, I; Figure 72
Figure 72. Apogon townsendi, 39.1 mm SL, photo by JT Williams.
Astrapogon puncticulatus (Poey, 1867) —blackfin cardinalfish; USNM, I; Figure 73
Figure 73. Astrapogon puncticulatus, 40.0 mm SL, photo by JT Williams.
Phaeoptyx conklini (Silvester, 1916)—freckled cardinalfish; USNM, I; Figure 74
Figure 74. Phaeoptyx conklini, 40.0 mm SL, photo by JT Williams.
Phaeoptyx pigmentaria (Poey, 1860)—dusky cardinalfish; USNM, I
Malacanthidae—tilefishes
Caulolatilus cyanops Poey, 1866—blackline tilefish; USNM, F; Figure 75
Figure 75. Caulolatilus cyanops, 288 mm SL, photo by W Toller.
Malacanthus plumieri (Bloch, 1786)—sand tilefish; UF, O, V
Coryphaenidae-dolphinfishes
Coryphaena hippurus Linnaeus, 1758—common dolphinfish; F
Rachycentridae-cobias
Rachycentron canadum (Linnaeus, 1766)—cobia
Our record for this species is based on an underwater video recently filmed by Yap Films Inc (Toronto, Canada) at the shipwreck on Saba Bank. The video clearly shows a cobia swimming along the side of the shipwreck.
Carangidae—jacks
Alectis ciliaris (Bloch, 1787)—African pompano; O; Figure 76
Figure 76. Alectis ciliaris, underwater photo by W Toller.
Caranx bartholomaei Cuvier, 1833—yellow jack; O, V
Caranx crysos (Mitchill, 1815) —blue runner; O
Caranx latus Agassiz, 1831—horse-eye jack; O, V
Caranx lugubris Poey, 1860—black jack; O, V
Caranx ruber (Bloch, 1793)—bar jack; UF, F, O, V; Figure 77
Figure 77. Caranx ruber, 260.8 mm SL, photo by JT Williams.
Decapterus macarellus (Cuvier, 1833)—mackerel scad; O; Figure 78
Figure 78. Decapterus macarellus, underwater photo by W Toller.
Elagatis bipinnulata (Quoy & Gaimard, 1825)—rainbow runner; F, OBS
Selar crumenophthalmus (Bloch, 1793)—bigeye scad; F; Figure 79
Figure 79. Selar crumenophthalmus, 240 mm SL, photo by W Toller.
Seriola dumerili (Risso, 1810)—greater amberjack; F; Figure 80
Figure 80. Seriola dumerili, large specimen at top; Seriola rivoliana, two smaller specimens below, photo by W Toller.
Seriola rivoliana Valenciennes, 1833—almaco jack; F; Figure 80
Lutjanidae—snappers
Apsilus dentatus Guichenot, 1853—black snapper; USNM, F; Figure 81
Figure 81. Apsilus dentatus, 234 mm SL, photo by W Toller.
Etelis oculatus (Valenciennes, 1828) —queen snapper; F
Lutjanus apodus (Walbaum, 1792)—schoolmaster; O, VIS
Lutjanus buccanella (Cuvier, 1828)—blackfin snapper; USNM, F, O; Figure 82
Figure 82. Lutjanus buccanella, 249 mm SL, photo by W Toller.
Lutjanus cyanopterus (Cuvier, 1828)—cubera snapper; USNM, I
Lutjanus mahogoni (Cuvier, 1828) —mahogany snapper; O
Lutjanus purpureus (Poey, 1866)—Caribbean red snapper; USNM, F; Figure 83
Figure 83. Lutjanus purpureus, 207 mm SL, photo by W Toller.
Lutjanus synagris (Linnaeus, 1758)—lane snapper; USNM, F; Figure 84
Figure 84. Lutjanus synagris, 208 mm SL, photo by W Toller.
Lutjanus vivanus (Cuvier, 1828)—silk snapper; USNM, F; Figure 85
Figure 85. Lutjanus vivanus, 311 mm SL, photo by W Toller.
Ocyurus chrysurus (Bloch, 1791)—yellowtail snapper; UF, O, V
Pristipomoides aquilonaris (Goode & Bean, 1896)—wenchman; USNM, F; Figure 86
Figure 86. Pristipomoides aquilonaris, 263 mm SL, photo by W Toller.
Rhomboplites aurorubens (Cuvier, 1829) —vermilion snapper; USNM, F; Figure 87
Figure 87. Rhomboplites aurorubens, 227.3 mm SL, photo by JT Williams.
Lobotidae—tripletails
Lobotes surinamensis (Bloch, 1790)—Atlantic tripletail; F; Figure 88
Figure 88. Lobotes surinamensis, 300 mm SL, photo by W Toller.
Haemulidae—grunts
Haemulon album Cuvier, 1830—margate; F; Figure 89
Figure 89. Haemulon album, 342 mm SL, photo by W Toller.
Haemulon aurolineatum Cuvier, 1830—tomtate; F, O; Figure 90
Figure 90. Haemulon aurolineatum, 175 mm SL, photo by W Toller.
Haemulon carbonarium Poey, 1860—caesar grunt; F; Figure 91
Figure 91. Haemulon carbonarium, approximately 200 mm SL, photo by W Toller.
Haemulon flavolineatum (Desmarest, 1823)—French grunt; O, V
Haemulon melanurum (Linnaeus, 1758)—cottonwick; UF, USNM, F, I, O, V; Figure 92
Figure 92. Haemulon melanurum, 213.8 mm SL, photo by JT Williams.
Haemulon plumierii (Lacepède, 1801)—white grunt; USNM, F, I, O, V; Figure 93
Figure 93. Haemulon plumierii, 244.7 mm SL, photo by JT Williams.
Haemulon striatum (Linnaeus, 1758)—striped grunt; F; Figure 94
Figure 94. Haemulon striatum, 154 mm SL, photo by W Toller.
Inermiidae—bonnetmouths
Inermia vittata Poey, 1860—boga; O
Sparidae—porgies
Calamus calamus (Valenciennes, 1830)—saucereye porgy; V
Sciaenidae—drums and croakers
Equetus punctatus (Bloch & Schneider, 1801)—spotted drum; USNM, I; Figure 95
Figure 95. Equetus punctatus, 146.8 mm SL, photo by JT Williams.
Pareques acuminatus (Bloch & Schneider, 1801)—high-hat; USNM, I; Figure 96
Figure 96. Pareques acuminatus, 150.0 mm SL, photo by JT Williams.
Mullidae—goatfishes
Mulloidichthys martinicus (Cuvier, 1829)—yellow goatfish; USNM, F, I, O; Figure 97
Figure 97. Mulloidichthys martinicus, 105 mm SL, photo by JT Williams.
Pseudupeneus maculatus (Bloch, 1793)—spotted goatfish; USNM, I, O, V; Figure 98
Figure 98. Pseudupeneus maculatus, 218.8 mm SL, photo by JT Williams.
Chaetodontidae—butterflyfishes
Chaetodon capistratus Linnaeus, 1758—foureye butterflyfish; USNM, I, O, V; Figure 99
Figure 99. Chaetodon capistratus, 99.4 mm SL; when younger, the specimen was apparently injured in the region of the fourth dorsal-fin spine (missing) and the area healed leaving a gap in the fin; photo by JT Williams.
Chaetodon ocellatus Bloch, 1787—spotfin butterflyfish; USNM, F, O, V
Chaetodon sedentarius Poey, 1860—reef butterflyfish; UF, O, V
Chaetodon striatus Linnaeus, 1758—banded butterflyfish; USNM, I, O, V; Figure 100
Figure 100. Chaetodon striatus, 111.3 mm SL, photo by JT Williams.
Prognathodes aculeatus (Poey, 1860)—longsnout butterflyfish; USNM, I, O, V; Figure 101
Figure 101. Prognathodes aculeatus, 62.9 mm SL, photo by JT Williams.
Pomacanthidae—angelfishes
Centropyge argi Woods & Kanazawa, 1951—cherubfish; USNM, I, O, V; Figure 102
Figure 102. Centropyge argi, 33.8 mm SL, photo by JT Williams.
Holacanthus ciliaris (Linnaeus, 1758)—queen angelfish; USNM, F, I, O, V
Holacanthus tricolor (Bloch, 1795)—rock beauty; USNM, I, O, V; Figures 103, 104
Figure 103. Holacanthus tricolor, 19.3 mm SL, juvenile color pattern, photo by JT Williams.
Figure 104. Holacanthus tricolor, 40.1 mm SL, intermediate color pattern, photo by JT Williams.
Pomacanthus arcuatus (Linnaeus, 1758)—gray angelfish; V
Pomacanthus paru (Bloch, 1787)—French angelfish; O, V
Kyphosidae—sea chub
Kyphosus incisor (Cuvier, 1831)—yellow chub; V
Kyphosus sectatrix (Linnaeus, 1766)—Bermuda chub; O, V
Cirrhitidae—hawkfishes
Amblycirrhitus pinos (Mowbray, 1927)—redspotted hawkfish; USNM, I, O; Figure 105
Figure 105. Amblycirrhitus pinos, 22.6 mm SL, photo by JT Williams.
Pomacentridae-damselfishes
Chromis cyanea (Poey, 1860)—blue chromis; USNM, I, O, V; Figure 106
Figure 106. Chromis cyanea, 67.5 mm SL, iridescent blue colors on body faded immediately after death, photo by JT Williams.
Chromis multilineata (Guichenot, 1853)—brown chromis; USNM, I, O, V; Figure 107
Figure 107. Chromis multilineata, 56.3 mm SL, photo by JT Williams.
Microspathodon chrysurus (Cuvier, 1830)—yellowtail damselfish; O, V
Stegastes adustus (Troschel, 1865) —dusky damselfish; O
Stegastes leucostictus (Müller & Troschel, 1848) —beaugregory; O
Stegastes partitus (Poey, 1868)—bicolor damselfish; USNM, I, O, V; Figures 108, 109
Figure 108. Stegastes partitus, 58.6 mm SL, black-tailed color morph, photo by JT Williams.
Figure 109. Stegastes partitus, 58.6 mm SL, yellow-tailed color morph, photo by JT Williams.
This species has a variable color pattern throughout its range. Two color morphs were found at Saba Bank. Both morphs have a yellow pectoral fin and a reduced yellowish white area covering the caudal peduncle. One morph has a black caudal fin (Figure 108) and the other morph has a pale yellowish caudal fin with a dusky brown area in the middle of the upper and lower lobes (Figure 109).
Stegastes planifrons (Cuvier, 1830)—threespot damselfish; USNM, I, O; Figure 110
Figure 110. Stegastes planifrons, 82.7 mm SL, photo by JT Williams.
Labridae—wrasses
Bodianus rufus (Linnaeus, 1758)—Spanish hogfish; O, V
Clepticus parrae (Bloch & Schneider, 1801)—creole wrasse; USNM, I, O, V; Figure 111
Figure 111. Clepticus parrae, 59.8 mm SL, photo by JT Williams.
Doratonotus megalepis Günther, 1862—dwarf wrasse; USNM, I, V; Figure 112
Figure 112. Doratonotus megalepis, 44.1 mm SL, photo by JT Williams.
Halichoeres bivittatus (Bloch, 1791)—slippery dick; USNM, I, O, V; Figures 113, 114
Figure 113. Halichoeres bivittatus, 37.9 mm SL, juvenile/initial color phase, showing the black spot in the dorsal fin and a somewhat unusual orangish anal fin, photo by JT Williams.
Figure 114. Halichoeres bivittatus, 103.0 mm SL, terminal male color phase, with a distinctive red blotch on the side of the body above the pectoral fin, photo by JT Williams.
Halichoeres cyanocephalus (Bloch, 1791)—yellowcheek wrasse; O, V
Halichoeres garnoti (Valenciennes, 1839)—yellowhead wrasse; UF, USNM, I, O, V; Figures 115, 116, 117
Figure 115. Halichoeres garnoti, 46.4 mm SL, juvenile color phase, showing the characteristic blue stripe on a yellow body, photo by JT Williams.
Figure 116. Halichoeres garnoti, 118.1 mm SL, initial/terminal color phase, this specimen has almost completed the transition from the female initial phase into a terminal male, photo by JT Williams.
Figure 117. Halichoeres garnoti, 125.3 mm SL, terminal male color phase, this specimen has completed the transition from the female initial phase into a terminal male and shows the characteristic black bar and dark area over the caudal peduncle, photo by JT Williams.
These figures show portions of the transitional color phases as individuals transform from juveniles (Figure 115) into initial phase females (Figure 116) and finally into terminal phase males (Figure 117).
Halichoeres maculipinna (Müller & Troschel, 1848)—clown wrasse; USNM, I, O
Halichoeres pictus (Poey, 1860)—rainbow wrasse; USNM, I, O; Figures 118, 119
Figure 118. Halichoeres pictus, 70.7 mm SL, juvenile color phase, photo by JT Williams.
Figure 119. Halichoeres pictus, 85.0 mm SL, terminal male color phase, photo by JT Williams.
Halichoeres poeyi (Steindachner, 1867)—blackear wrasse; UF, USNM, I, O, V; Figure 120
Figure 120. Halichoeres poeyi, 87.9 mm SL, terminal male color phase, photo by JT Williams.
Halichoeres radiatus (Linnaeus, 1758)—puddingwife; O, V
Lachnolaimus maximus (Walbaum, 1792)—hogfish; USNM
Thalassoma bifasciatum (Bloch, 1791)—bluehead; USNM, I, O, V; Figures 121, 122
Figure 121. Thalassoma bifasciatum, 39.3 mm SL, juvenile/initial color phase, photo by JT Williams.
Figure 122. Thalassoma bifasciatum, 63.5 mm SL, terminal male color phase, photo by JT Williams.
Xyrichtys splendens Castelnau, 1855—green razorfish; USNM, I, O; Figures 123, 124, 125, 126
Figure 123. Xyrichtys splendens, 15.1 mm SL, young juvenile color phase, photo by JT Williams.
Figure 124. Xyrichtys splendens, 22.1 mm SL, juvenile color phase, photo by JT Williams.
Figure 125. Xyrichtys splendens, 52.4 mm SL, initial color phase of female, photo by JT Williams.
Figure 126. Xyrichtys splendens, 72.5 mm SL, terminal male color phase, photo by JT Williams.
Scaridae—parrotfishes
Cryptotomus roseus Cope, 1871—bluelip parrotfish; UF, USNM, I, O; Figure 127
Figure 127. Cryptotomus roseus, 61.5 mm SL, terminal male color phase, photo by JT Williams.
Scarus coelestinus Valenciennes, 1840—midnight parrotfish; V
Scarus guacamaia Cuvier, 1829—rainbow parrotfish; F; Figure 128
Figure 128. Scarus guacamaia, 647 mm SL, terminal male color phase, photo by W Toller.
Scarus iseri (Bloch, 1789)—striped parrotfish; USNM, O, V
Scarus taeniopterus Desmarest, 1831—princess parrotfish; USNM, I, O, V; Figure 129, 130
Figure 129. Scarus taeniopterus, 174.9 mm SL, initial female color phase, photo by JT Williams.
Figure 130. Scarus taeniopterus, 231.7 mm SL, terminal male color phase, photo by JT Williams.
Scarus vetula Bloch & Schneider, 1801—queen parrotfish; O
Sparisoma atomarium (Poey, 1861)—greenblotch parrotfish; USNM, I, O; Figure 131
Figure 131. Sparisoma atomarium, 13.7 mm SL, juvenile color phase, photo by JT Williams.
Sparisoma aurofrenatum (Valenciennes, 1840)—redband parrotfish; USNM, I, O, V; Figures 132, 133, 134
Figure 132. Sparisoma aurofrenatum, 53.8 mm SL, juvenile color phase, photo by JT Williams.
Figure 133. Sparisoma aurofrenatum, 164.0 mm SL, initial female color phase, photo by JT Williams.
Figure 134. Sparisoma aurofrenatum, 164 mm SL, terminal male color phase, photo by JT Williams.
Sparisoma chrysopterum (Bloch & Schneider, 1801)—redtail parrotfish; USNM, I, O; Figure 135
Figure 135. Sparisoma chrysopterum, 274 mm SL, terminal male color phase, photo by JT Williams.
Sparisoma radians (Valenciennes, 1840)—bucktooth parrotfish; USNM, I, O, V; Figure 136
Figure 136. Sparisoma radians, 51.6 mm SL, juvenile color phase, photo by JT Williams.
Sparisoma viride (Bonnaterre, 1788)—stoplight parrotfish; USNM, I, O, V; Figure 137
Figure 137. Sparisoma viride, 222.9 mm SL, initial female color phase, photo by JT Williams.
Tripterygiidae—triplefins
Enneanectes altivelis Rosenblatt, 1960 —lofty triplefin; USNM, I; Figure 138
Figure 138. Enneanectes altivelis, 18.3 mm SL, photo by JT Williams.
Enneanectes atrorus Rosenblatt, 1960—redeye triplefin; USNM, I
Enneanectes jordani (Evermann and Marsh, 1899) —mimic triplefin; USNM, I
Dactyloscopidae-sand stargazers
Dactyloscopus tridigitatus Gill, 1859—sand stargazer; USNM, I; Figures 139, 140
Figure 139. Dactyloscopus tridigitatus, 50.8 mm SL, photo by JT Williams.
Figure 140. Dactyloscopus tridigitatus, 50.8 mm SL, dorsal view, photo by JT Williams.
Gillellus uranidea Böhlke, 1968—warteye stargazer; USNM, I; Figure 141
Figure 141. Gillellus uranidea, 27.7 mm SL, photo by JT Williams.
Platygillellus rubrocinctus (Longley, 1934)—saddle stargazer; USNM, I; Figures 142, 143, 144
Figure 142. Platygillellus rubrocinctus, 24.1 mm SL, adult, photo by JT Williams.
Figure 143. Platygillellus rubrocinctus, 12.1 mm SL, lateral view of juvenile, photo by JT Williams.
Figure 144. Platygillellus rubrocinctus, 12.1 mm SL, dorsal view of juvenile, photo by JT Williams.
Blenniidae—combtooth blennies
Parablennius marmoreus (Poey, 1876)—seaweed blenny; USNM, I
Labrisomidae—scaly blennies
Labrisomus gobio (Valenciennes, 1836)—palehead blenny; USNM, I; Figure 145
Figure 145. Labrisomus gobio, 36.2 mm SL, photo by JT Williams.
Labrisomus haitiensis Beebe &Tee-Van, 1918—longfin blenny; USNM, I; Figure 146
Figure 146. Labrisomus haitiensis, 18.8 mm SL, photo by JT Williams.
Malacoctenus boehlkei Springer, 1959—diamond blenny; USNM, I; Figure 147
Figure 147. Malacoctenus boehlkei, 36.3 mm SL, photo by JT Williams.
Paraclinus grandicomis (Rosen, 1911)—horned blenny; UF, USNM, I; Figure 148
Figure 148. Paraclinus grandicomis, 27.1 mm SL, photo by JT Williams.
Starksia atlantica Longley, 1934—smootheye blenny; USNM, I; Figures 149, 150
Figure 149. Starksia atlantica, 9.5 mm SL, juvenile, photo by JT Williams.
Figure 150. Starksia atlantica, 15.1 mm SL, adult male, photo by JT Williams.
The Saba Bank population may be a distinct species in the species complex currently referred to as Starksia atlantica. This complex requires additional taxonomic study.
Starksia cf lepicoelia Böhlke & Springer, 1961—blackcheek blenny; USNM, I; Figures 151, 152
Figure 151. Starksia lepicoelia, 20.2 mm SL, adult female, photo by JT Williams.
Figure 152. Starksia lepicoelia, 19.8 mm SL, adult male, photo by JT Williams.
Females (Figure 151) lack the black spot on the cheek that is characteristic of mature males (Figure 152). The Saba Bank population may be a distinct species in the species complex currently referred to as Starksia lepicoelia. This complex requires additional taxonomic study.
Starksia melasma Williams & Mounts, 2003—black spot blenny; USNM, I
Starksia nanodes Böhlke & Springer, 1961—dwarf blenny; USNM, I; Figure 153
Figure 153. Starksia nanodes, 10.6 mm SL, adult male, photo by JT Williams.
The Saba Bank population may be a distinct species in the species complex currently referred to as Starksia nanodes. This complex requires additional taxonomic study.
Chaenopsidae—tube blennies
Acanthemblemaria aspera (Longley, 1927)—roughhead blenny; USNM, I; Figures 154, 155, 156
Figure 154. Acanthemblemaria aspera, 14.0 mm SL, juvenile/female, photo by JT Williams.
Figure 155. Acanthemblemaria aspera, 19.7 mm SL, adult male, photo by JT Williams.
Figure 156. Acanthemblemaria aspera, 20.4 mm SL, yellow color morph (female?), photo by JT Williams.
Three different color patterns were observed at Saba Bank: juvenile/female (Figure 154), adult male (Figure 155), and a distinctive yellow, probably female, color morph (Figure 156). The adult male and the yellow morph were taken together at the same collecting station.
Emblemaria pandionis Evermann & Marsh, 1900—sailfin blenny; USNM, I; Figure 157
Figure 157. Emblemaria pandionis, 37.0 mm SL, adult female, photo by JT Williams.
Emblemariopsis cf signifer (Ginsburg, 1942)—flagfin blenny; USNM, I; Figure 158
Figure 158. Emblemariopsis cf signifer, 16.2 mm SL, adult male, this specimen represents an undescribed species in the signifer species complex, photo by JT Williams.
The signifer species complex ranges from Brazil throughout the Caribbean and includes a number of undescribed species in the Caribbean region. Additional taxonomic study is required to resolve the taxa.
Gobiesocidae—clingfishes
Acyrtus artius Briggs, 1955—papillate clingfish; USNM, I; Figures 159, 160, 161
Figure 159. Acyrtus artius, 20.4 mm SL, dorsal view, photo by JT Williams.
Figure 160. Acyrtus artius, 20.4 mm SL, lateral view, photo by JT Williams.
Figure 161. Acyrtus artius, 20.4 mm SL, ventral view showing pelvic disk, photo by JT Williams.
Callionymidae—dragonets
Paradiplogrammus bairdi Jordan, 1888—lancer dragonet; USNM, I, O; Figures 162, 163
Figure 162. Paradiplogrammus bairdi, 39.8 mm SL, lateral view of an adult male, photo by JT Williams.
Figure 163. Paradiplogrammus bairdi, 29.8 mm SL, dorsal view of a smaller adult male, photo by JT Williams.
Gobiidae-gobies
Coryphopterus dicrus Böhlke & Robins, 1960—colon goby; USNM, I; Figure 164
Figure 164. Coryphopterus dicrus, 31.8 mm SL, photo by JT Williams.
Coryphopterus eidolon Böhlke & Robins, 1960—pallid goby; USNM, I; Figure 165
Figure 165. Coryphopterus eidolon, 18.3 mm SL, photo by JT Williams.
Coryphopterus glaucofraenum Gill, 1863—bridled goby; USNM, I, V; Figure 166
Figure 166. Coryphopterus glaucofraenum, 33.7 mm SL, photo by JT Williams.
Coryphopterus personatus (Jordan & Thompson, 1905)—masked goby; USNM, I; Figure 167
Figure 167. Coryphopterus personatus, 19.0 mm SL, photo by JT Williams.
Coryphopterus thrix Böhlke & Robins, 1960—bartail goby; USNM, I; Figure 168
Figure 168. Coryphopterus thrix, 26.8 mm SL, photo by JT Williams.
Elacatinus chancei (Beebe & Hollister 1933)—shortstripe goby; USNM, I; Figure 169
Figure 169. Elacatinus chancei, 28.1 mm SL, photo by JT Williams.
Elacatinus evelynae (Böhlke & Robins, 1968)—sharknose goby; USNM, I; Figure 170
Figure 170. Elacatinus evelynae, 27.7 mm SL, photo by JT Williams.
Elacatinus genie (Böhlke & Robins, 1968)—cleaner goby; USNM, I
Evermannichthys metzelaari Hubbs, 1923 —roughtail goby; USNM, I; Figure 171
Figure 171. Evermannichthys metzelaari, 21.0 mm SL, photo by JT Williams.
Gnatholepis thompsoni Jordan, 1904—goldspot goby; USNM, I, V; Figure 172
Figure 172. Gnatholepis thompsoni, 31.5 mm SL, photo by JT Williams.
Lythrypnus elasson Böhlke & Robins, 1960—dwarf goby; USNM, I; Figure 173
Figure 173. Lythrypnus elasson, 9.2 mm SL, adult male, photo by JT Williams.
Lythrypnus minimus Garzón & Acero P., 1988 —pygmy goby; USNM, I; Figure 174
Figure 174. Lythrypnus minimus, 10.6 mm SL, adult male, photo by JT Williams.
Lythrypnus nesiotes Böhlke & Robins, 1960—island goby; USNM, I; Figure 175
Figure 175. Lythrypnus nesiotes, 11.9 mm SL, adult male, photo by JT Williams.
Priolepis hipoliti (Metzelaar, 1922)—rusty goby; USNM, I; Figures 176, 177
Figure 176. Priolepis hipoliti, 12.2 mm SL, female, photo by JT Williams.
Figure 177. Priolepis hipoliti, 18.7 mm SL, adult male, photo by JT Williams.
Psilotris batrachodes Böhlke, 1963—toadfish goby; USNM, I; Figure 178
Figure 178. Psilotris batrachodes, 13.3 mm SL, adult, photo by JT Williams.
Psilotris boehlkei Greenfield, 1993—yellowspot goby; I; Figure 179
Figure 179. Psilotris boehlkei, 26.5 mm SL, adult, photo by JT Williams.
The fresh colors of P. boehlkei are presented (Figure 179) for the first time. This very rare species was previously known from only five specimens taken at St. Barthelemey in 1965. The single specimen we collected extends the known distribution of P. boehlkei to Saba Bank. We have named this species the yellowspot goby in reference to the yellow spots on the head and body.
Pycnomma roosevelti Ginsburg, 1939 —Roosevelt's goby; USNM, I; Figure 180
Figure 180. Pycnomma roosevelti, 14.6 mm SL, adult, photo by JT Williams.
Although P. roosevelti has previously been taken from a several scattered localities around the Caribbean (Isla Providencia, Guadeloupe, Belize and Puerto Rico), there are fewer than 10 specimens known and its fresh colors (Figure 180) have not been published previously.
Risor ruber (Rosén, 1911) —tusked goby; USNM, I; Figure 181
Figure 181. Risor ruber, 18.7 mm SL, adult, photo by JT Williams.
Acanthuridae—surgeonfishes
Acanthurus bahianus Castelnau, 1855—ocean surgeon; USNM, I, O, V; Figure 182
Figure 182. Acanthurus bahianus, 128.2 mm SL, photo by JT Williams.
Acanthurus chirurgus (Bloch, 1787)—doctorfish; USNM, I, O, V; Figure 183
Figure 183. Acanthurus chirurgus, 168.0 mm SL, photo by JT Williams.
Acanthurus coeruleus Bloch & Schneider, 1801—blue tang; USNM, I, O, V; Figure 184
Figure 184. Acanthurus coeruleus, 146.2 mm SL, photo by JT Williams.
Sphyraenidae—barracudas
Sphyraena barracuda (Walbaum, 1792)—great barracuda; O, V
Scombridae—mackerels
Acanthocybium solandri (Cuvier, 1832)—wahoo; F
Euthynnus alletteratus (Rafinesque, 1810)—little tunny; USNM; Figure 185
Figure 185. Euthynnus alletteratus, 355.1 mm FL, photo by JT Williams.
Scomberomorus regalis (Bloch, 1793)—cero; USNM
Thunnus atlanticus (Lesson 1831)—blackfin tuna; F; Figure 186
Figure 186. Thunnus atlanticus, 516 mm FL, photo by W Toller.
Caproidae—boarfishes
Antigonia capros Lowe, 1843—deepbody boarfish; USNM, F; Figure 187
Figure 187. Antigonia capros, 37 mm SL, close-up of head, photo by W Toller.
Antigonia combatia Berry & Rathjen 1959—shortspine boarfish; UF, T
The shortspine boarfish record from Saba Bank is based on a trawl collection with vouchered museum specimens at UF.
Bothidae—lefteye flounders
Bothus lunatus (Linnaeus, 1758)—peacock flounder; O
Bothus ocellatus (Agassiz, 1831)—eyed flounder; UF, USNM, I; Figure 188
Figure 188. Bothus ocellatus, 95.8 mm SL, photo by JT Williams.
Trichopsetta ventralis (Goode & Bean, 1885)—sash flounder; UF, T
Paralichthyidae—sand flounders
Citharicthys dinoceros Goode & Bean, 1886—spined whiff; USNM, T
Cynoglossidae—tonguefishes
Symphurus arawak Robins & Randall, 1965—Caribbean tonguefish; USNM, I; Figure 189
Figure 189. Symphurus arawak, 30.7 mm SL, photo by JT Williams.
Symphurus ommaspilus Böhlke, 1961—ocellated tonguefish; USNM, I; Figure 190
Figure 190. Symphurus ommaspilus, 42.9 mm SL, photo by JT Williams.
Balistidae—triggerfishes
Balistes vetula Linnaeus, 1758—queen triggerfish; USNM, O, V
Canthidermis sufflamen (Mitchill, 1815)—ocean triggerfish; O, V
Melichthys niger (Bloch, 1786)—black durgon; USNM, O, V
Xanthichthys ringens (Linnaeus, 1758)—sargassum triggerfish; USNM, I; Figure 191
Figure 191. Xanthichthys ringens, 87.3 mm SL, photo by JT Williams.
Monacanthidae—filefishes
Aluterus scriptus (Osbeck, 1765)—scrawled filefish; USNM, F, V
Cantherhines macrocerus (Hollard, 1853)—whitespotted filefish; USNM, F; Figure 192
Figure 192. Cantherhines macrocerus, 273 mm SL, image flipped horizontally – right side is shown, photo by W Toller.
Cantherhines pullus (Ranzani, 1842)—orangespotted filefish; O, V
Monacanthus ciliatus (Mitchill, 1818)—fringed filefish; USNM, I, V; Figure 193
Figure 193. Monacanthus ciliatus, 33.4 mm SL, photo by JT Williams.
Monacanthus tuckeri Bean, 1906—slender filefish; USNM, I; Figure 194
Figure 194. Monacanthus tuckeri, 32.3 mm SL, photo by JT Williams.
Ostraciidae—boxfishes
Acanthostracion polygonia Poey, 1876—honeycomb cowfish; USNM, F, O, V
Acanthostracion quadricornis (Linnaeus, 1758)—scrawled cowfish; USNM, F, O
Lactophrys bicaudalis (Linnaeus, 1758)—spotted trunkfish; F
Lactophrys trigonus (Linnaeus, 1758)—trunkfish; USNM, I, V; Figure 195
Figure 195. Lactophrys trigonus, 293.1 mm SL, photo by JT Williams.
Lactophrys triqueter (Linnaeus, 1758)—smooth trunkfish; USNM, F, OBS, VIS; Figure 196
Figure 196. Lactophrys triqueter, 111.7 mm SL, photo by JT Williams.
Tetraodontidae—puffers
Canthigaster rostrata (Bloch, 1786)—sharpnose puffer; USNM, I, O, V; Figure 197
Figure 197. Canthigaster rostrata, 50.9 mm SL, photo by JT Williams.
Sphoeroides spengleri (Bloch, 1785)—bandtail puffer; USNM, I, O; Figure 198
Figure 198. Sphoeroides spengleri, 73.1 mm SL, photo by JT Williams.
Diodontidae—porcupinefishes
Chilomycterus antillarum Jordan & Rutter, 1897—web burrfish; USNM, F; Figure 199
Figure 199. Chilomycterus antillarum, 180.0 mm SL, photo by JT Williams.
Diodon holocanthus Linnaeus, 1758—balloonfish; USNM, I, V; Figure 200
Figure 200. Diodon holocanthus, 104.9 mm SL, photo by JT Williams.
Diodon hystrix Linnaeus, 1758—porcupinefish; O
Discussion
We document the occurrence of 270 species of fishes at Saba Bank. The diversity of fishes at Saba Bank is comparable (Table 1) to that of the oceanic atolls of Colombia (273 species), the islands in the Mona Passage of Puerto Rico (261 species) and Buck Island Reef National Monument (BIRNM; 262 species). The relatively high diversity of fishes at Saba Bank exists despite the lack of emergent land at the bank. There is no shallow-water shore-fish fauna represented on the bank due to the absence of a high-energy shoreline. These habitats typically add significantly to the fish diversity of Caribbean habitats. For example, the tube blennies (family Chaenopsidae) are a group of shorefishes typically found in fairly shallow coastal waters. According to Williams [4], there are approximately 22 recognized species of tube blennies known to occur in the central Caribbean, but only three of these species were found on Saba Bank. In addition, the absence of mangrove vegetation and apparent lack of sea-grass beds also limits the fish fauna of Saba Bank. In their quantitative study of a number of Saba Bank habitats, Toller et al. [5] attribute the apparent lack or rarity of a number of fish species found on Saba Bank to the absence of those habitats as nurseries for the juvenile stages of these fish species. Nevertheless, diverse habitat types exist on Saba Bank ranging from coral reefs and algal flats to soft-bottom lagoon areas and scoured hard, flat, pavement-like zones. These diverse habitats support a highly diverse, fish fauna and include a number of undescribed, new species along with species rarely encountered elsewhere in the Caribbean.
Table 1. Number of fishes recorded at well sampled sites in the Greater Caribbean.
| Site | No. fish species | Source |
| Alligator Reef, FL | 517 | Starck [12] |
| Dry Tortugas, FL | 442 | Longley & Hildebrand [13] |
| Bermuda | 433 | Smith-Vaniz et al [14] |
| St. Croix | 400 | Clavijo et al [15] |
| Barrier Reef, Belize | 339 | C.L. Smith et al [9] |
| Offshore Banks, Belize | 293 | C.L. Smith et al [9] |
| Oceanic Atolls, Colombia | 273 | Mejia et al [16] |
| Saba Bank | 270 | This paper |
| Buck Island Reef | 262 | Smith-Vaniz et al [8] |
| Mona Passage Islands, Puerto Rico | 261 | Dennis et al [7] |
| Flower Garden Bank Nat. Mar. Sanctuary, Texas, USA | 240 | E. Hickerson, NOAA, pers. comm., Dec. 2009 |
| Navassa Island, USA | 237 | Collette et al [6] |
| Rhomboidal Cays, Belize | 193 | C.L. Smith et al [9] |
| Core Pelican Cays, Belize | 168 | C.L. Smith et al [9] |
| Peripheral Rhomboidal Cays, Belize | 123 | C.L. Smith et al [9] |
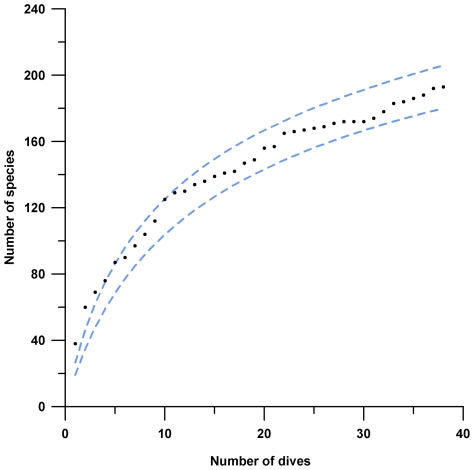
The highly diverse, fish fauna so far reported appears to substantially under represent the species richness of fishes for Saba Bank. The actual species-accumulation curve of rotenone collections and visual surveys combined do not reach an asymptote (Figure 201). The expected species-accumulation curves (Chao2 and Jack1 estimators in EstimateS) predict total fish-species richness somewhere between 320 and 411 species.
Figure 201. Actual species-accrual curve (black dots) for 38 dives collecting and identifying fish species on Saba Bank.
Sobs (Mao Tau) 95% confidence intervals [8] are shown as light blue dashed lines.
Of the 270 species we report (Table 1) from Saba Bank, 132 (49%) were observed during visual surveys. This result is comparable to the findings for Navassa Island where 41% of the fish species were detected by visual surveys [6], for the Mona Passage islands with 43% of the fish species detected visually [7], and for BIRNM with 44% of the fish species detected visually [8]. A higher percentage of fish species was detected visually at Saba Bank than at Navassa, Mona, or BIRNM. This could possibly be a result of the higher number of visual surveys carried out at Saba Bank than at Navassa or Mona. Although the BIRNM results are based on a higher number of visual surveys (70), a comparable percentage of the fauna was detected visually at BIRNM. BIRNM ichthyocide surveys (58) appear to demonstrate the ability of ichthyocide collections to more thoroughly sample the fauna (see below). Visual censuses have numerous biases as discussed in the methods for the Pelican Cays study [9]. Despite the slight differences in methods and sampling designs between the published fish species-richness studies in different parts of the Caribbean, the methods utilized produce consistent and comparable results.
Ichthyocide collections at Saba Bank yielded specimens representing 155 fish species (57% of the total fish fauna). At Navassa Island where there were fewer visual surveys than at Saba Bank, over 70% of the fish species were collected with ichthyocide [6], at the Mona Passage islands, 61% of the fish species were collected with ichthyocide [7], and at BIRNM, 87% of the fish species were collected with ichthyocide despite their being more visual surveys in the BIRNM study [8]. By occupying 58 (27 at Saba Bank) ichthyocide stations covering a broader diversity of ecological habitats, the BIRNM study appears to have obtained a more thorough sampling of the resident species. The lower number of species taken at Saba Bank with ichthyocide is at least partially due to the absence of shallow habitat. Shallow habitat is required by many of the small cryptic fish species that are normally detected only with the use of ichthyocide and these species have not been found at Saba Bank. The prevalence of strong currents across Saba Bank further limits the effectiveness of ichthyocide, which is only effective when relatively high concentrations of ichthyocide remain in one place (preferably in a confined area) for more than 15 to 20 minutes. The ichthyocide-collecting methods employed at Saba Bank were the same as those used at Navassa Island and Belize. The BIRNM ichthyocide collecting methods differed from ours only in that the BIRNM study used a block net in addition to collecting outside the net. Nevertheless, the BIRNM methods were comparable to ours because the BIRNM study included those species collected outside the block nets in their results. There are inherent biases in ichthyocide collecting due to unpredictability of environmental parameters, such as currents, temperature, degree of confinement of the sampling area, ability of larger species to swim away, and variable assays of active rotenone in the powdered Derris root (batches often vary from 5% to over 11% active rotenone due to natural variation in rotenone concentration among the roots of different plants). Despite these biases, ichthyocide collections yield comparable results as described above.
Neither visual surveying nor ichthyocide collecting alone are capable of providing a comprehensive survey of coastal (or submerged atoll in the case of Saba Bank) fish species. A combination of visual surveys, ichthyocide sampling using SCUBA, and various fishing techniques must be employed to effectively assess fish-species richness in marine habitats shallow enough to be accessible to SCUBA divers.
The number of fish species living on Saba Bank is undoubtedly higher than 270 as indicated by Chao2 and Jack1 estimators. As most parts of Saba Bank are deeper than 25 m, sampling with ichthyocides using SCUBA is limited by the reduced bottom time at these depths. As a result we focused primarily on the rim (shallowest parts) of the submerged atoll to maximize bottom time for collecting. Future sampling with ichthyocides applied by divers utilizing rebreathers, supplemented with trawl and dredge sampling would allow collecting from the outer slopes and would certainly yield additional new and interesting species of fishes from this submerged atoll.
Methods
The NMNH/SI Animal Care and Use Committee approved the methods and procedures utilized during the course of this biodiversity assessment project. All Saba Bank projects had collecting permits through the Convention on Trade in Endangered Species (CITES, where necessary) and the Saba Conservation Foundation (where CITES was not required).
Roving surveys were completed using SCUBA and lasted 60 minutes, bottom time permitting. All species encountered were listed on a slate while swimming in a haphazard pattern covering all bottom depths possible down to a maximum of 38 m. Other visual surveys are described in Toller et al [5]. Collecting methods follow Collette et al [6]. Species were photographed in aquaria after the fins were pinned out and brushed with formaldehyde solution. Tissue samples were taken from fresh specimens and the voucher specimens were preserved in a formaldehyde solution diluted with water to 3.75% formaldehyde. Large specimens were also injected with 37.5% formaldehyde before being soaked in the 3.75% formaldehyde solution.
After arrival at the Museum Support Center (MSC), National Museum of Natural History, specimens were transferred sequentially through water-diluted solutions of 25% ethanol, 50% ethanol, and finally into 75% ethanol for permanent archival storage. Specimens were then processed and cataloged into the USNM at the MSC in Suitland, MD.
To generate species-accumulation curves, a data matrix of presence/absence was constructed from 38 combined roving surveys, rotenone collections, and fish-habitat transects using species as variables and dives as observations (Table 2 provides details for each survey and collecting station). The matrix was employed for actual and expected species-accumulation curves (Mao Tau) in EstimateS v.8.0 software [10]. Total expected richness was estimated using Chao2 and Jack1 estimators.
Table 2. Collecting stations occupied on Saba Bank, 2006-2007.
| Station number | Roving & Rotenone | Coordinates | Date | Depth (m) | Local name | Description of habitat |
| Saba-06-01 | RR01 | 17o28.778N; 63o13.663W | 04 Jan 2006 | 30 | North East Reef | N.E. reefs, just S of Poison Bank. Spur and groove reef, low relief, many algae (Dictyota)-rotenoned a groove in reef with sand at bottom, walls of groove with low corals |
| Saba-06-02 | RR02 | 17o26.883N; 63o54.055W | 05 Jan 2006 | 38 | Small Bank South | Small Bank South. Scattered corals, many Xestospongia. Nassau grouper, yellowfin grouper, and black grouper sighted. Nurse shark-rotenoned area with encrusting corals |
| Saba-06-03 | RR03 | 17o25.778N; 63o41.037W | 05 Jan 2006 | 35 | Rhodolith Reef | Western patch reef, many rodoliths-rotenoned a flat area with sponges and rubble bottom |
| Saba-06-04 | 17o18.557N; 63o22.019W | 05 Jan 2006 | 35 | Fish Trap- Local Fishermen | ||
| Saba-06-05 | RR04 | 17o24.602N; 63o11.748W | 06 Jan 2006 | 27 | Redman Bulge | Eastern reef, spur and groove, medium height, many algae (dictyota) |
| Saba-06-06 | RR05 | 17o26.028N; 63o16.536W | 06 Jan 2006 | 22 | Seaweed city | pavement covered with ca. 10 cm sand layer, Pseudopterogorgia, many algae species |
| Saba-06-07 | 17o33.697N; 63o46.949W | 0-1 | Caught on hook and line while trolling over Saba Bank | |||
| Saba-06-08 | RR06 | 17o33.092N; 63o28.758W | 07 Jan 2006 | 37 | Grouper Bank | bare pavement, occasional solution holes, Manicinias, many conch (old) |
| Saba-06-09 | RR07 | 17o34.893N; 63o24.400W | 07 Jan 2006 | 30 | Rendezvous Hill | pavement with corals, low relief-rotenoned a low-relief coral reef with soft and hard corals, and sponges |
| Saba-06-10 | 17o25.883N; 63o21.874W | 08 Jan 2006 | 0-1 | Caught on hook and line while trolling over Saba Bank | ||
| Saba-06-11 | RR08 | 17o14.070N; 63o26.915W | 08 Jan 2006 | 25 | Butterfly Reef | Southern outer reef. Spur and groove, medium height (2-3 ft) |
| Saba-06-12 | RR09 | 17o14.380N; 63o26.915W | 08 Jan 2006 | 18 | Brain coral reef | Southern inner reef, rock pavement, scattered corals |
| Saba-06-13 | RR10 | 17o33.801N; 63o17.806W | 09 Jan 2006 | 32 | Fishpot surprise | sloping pavement with ledges, sand patches and large rubble, algae, scattered corals-rotenoned an area with sand and loose rock at base of rocky slope with brown algae abundant. |
| Saba-06-14 | 17o33.970N; 63o17.227W | 09 Jan 2006 | 40 | Fish Trap | ||
| Saba-06-15 | 17o33.897N; 63o17.730W | 09 Jan 2006 | 40 | Fish Trap | ||
| Saba-06-16 | 17o33.878N; 63o17.168W | 10 Jan 2006 | 40 | Fish Trap | ||
| Saba-06-17 | RR11 | 17o33.849N; 63o17.872W | 10 Jan 2006 | 26 | Lost anchor | Sloping pavement, patches of large rubble, algae, scattered corals; red hind spawning area |
| Saba-06-18 | 17o33.837N; 63o17.962W | 10 Jan 2006 | 30 | Fish Trap | ||
| Saba-06-19 | RR12 | 17o33.686N; 63o17.629W | 10 Jan 2006 | 26 | Moonfish Bank | Sloping pavement, sand patches, large rubble, algae, scattered corals; red hind spawning area |
| Saba-06-20 | 17o30.751N; 63o13.632W | 12 Jan 2006 | 29 | Poison Bank | Rotenone | |
| Saba-06-21 | 17o28.046N; 63o14.978W | 12 Jan 2006 | 19 | Rotenone-northeastern shallow flats | ||
| Saba-06-22 | 17o26.390N; 63o27.776W | 13 Jan 2006 | 26 | Rotenone-flat bottom in central area of bank | ||
| Saba-06-23 | 17o30.580N; 63o27.595W | 13 Jan 2006 | 28 | Rotenone-flat bottom in central area of bank | ||
| Saba-06-24 | 17o20.766N; 63o15.008W | 14 Jan 2006 | 31 | Coral Garden | Rotenone-coral groove at top of steep outer dropoff on SE side of Saba Bank, rock, coral, and sponges, sand at bottom of groove. | |
| Saba-06-25 | 17o21.162N; 63o15.138W | 14 Jan 2006 | 18 | Rotenone-near Coral Garden at SE edge of Saba Bank, small ledge on edge of sand and rubble flat, gorgonians, coral and rock | ||
| Saba-06-26 | No label with these-specimens taken at one or more of the other stations, but their locality labels were lost | |||||
| Saba 2007-01 | 17.51206 N; 63.2332 W | 20 Jun 2007 | 29-38 | Poison Bank- rotenone-dead and live coral channel with rubble on deep reef. | ||
| Saba 2007-02 | 17.46028 N; 63.2517 W | 20 Jun 2007 | 15-19 | Twin Peaks- rotenone-algae covering rock and some sand on a low ridge |
A Bray-Curtis resemblance matrix was generated from a matrix of presence/absence data from 12 combined roving surveys and rotenone collections in order to produce a non-metric multidimensional scaling (MDS) ordination and group averaged hierarchical clustering dendrogram in PRIMER v. 6.1 software [11]. The ANOSIM statistic was employed to test for a priori differences between habitat types and depth classes. A dendrogram based on group averaged hierarchical cluster techniques was used to illustrate the differences among depth classes (Figure 202). Color codes are derived from the similarity profile (SIMPROF) statistic in Primer 6.1. SIMPROF is less powerful than ANOSIM, intended for a posteriori tests of structure in the data. The test was employed here for representational purposes. A second data matrix of species presence and absence was introduced for an a posteriori test of genuine data structure among Caribbean localities, using the Bray-Curtis resemblance measure to determine the level of similarity among localities. Principal Components Analysis (PCA) was also conducted in PRIMER 6.1 in order to determine the fish species primarily responsible for differences among habitats.
Figure 202. Hierarchical clustering dendrogram of stations showing significant differences (black bars) among fish assemblages at deep stations versus middle-depth and shallow stations (SIMPROF, P<0.05).
Acknowledgments
This program was a joint initiative of the Department of Environment and Nature of the Netherlands Antilles and Conservation International. We are especially grateful to the many people who made the biodiversity survey possible, including Andy Caballero of Nature Foundation St. Maarten; David Kooistra, Stanley Peterson, and Jan Den Dulk of Saba Conservation Foundation; Shelley Lundvall, Saba Bank project manager; fishermen Leroy Peterson, Nicky Johnson and Armand Simmons. We thank Jerome Finan, Kris Murphy, and David G. Smith, National Museum of Natural History, Smithsonian Institution, for their help with this project. We thank Menno Van der Velde and Wim Schutten for collecting fishes at several ichthyocide stations. Jill Leger, Yap Films Inc, provided a video clip of fishes filmed at the shipwreck on Saba Bank. Emma L. Hickerson, Flower Garden Banks National Marine Sanctuary (FGBNMS), NOAA, kindly provided information on the number of fish species known to occur at the FGBNMS. Dr. JA Sanchez, University de los Andes, allowed us to use his photograph for Figure 3.
Footnotes
Competing Interests: Dr. Peter Etnoyer co-authored the Deep Sea News blog with PLoS One editor Dr. Craig McClain 2005–2009. Dr. McClain has recused himself from the Saba Bank Biodiversity Assessment volume for this reason.
Funding: The Department of Environment and Nature of the Netherlands Antilles and Conservation International provided funding for this project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Meesters EH, Nijkamp H, Bijvoet L. Towards sustainable management of the Saba Bank. 1996. 42. Unpublished report to the Department of Public Health and Environment, Curacao, Netherlands Antilles.
- 2.Van der Land J. The Saba Bank, a large atoll in the northeastern Caribbean. FAO Fisheries Reports. 1977;200:469–481. [Google Scholar]
- 3.Böhlke JE, Chaplin CCG. Fishes of the Bahamas and adjacent tropical waters. 1968. 771. Academy of Natural Sciences of Philadelphia.
- 4.Williams JT. In K.E. Carpenter (ed) 2003. 2003. pp. 1761–1767. The living marine resources of the Western Central Atlantic. Volume 3: Bony fishes part 2 (Opistognathidae to Molidae, sea turtles and marine mammals. FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5. Rome, FAO.
- 5.Toller W, Debrot AO, Vermeij MJA, Hoetjes PC. Reef Fishes of Saba Bank, Netherlands Antilles: Assemblage Structure across a Gradient of Habitat Types. 2010. In press. This volume of PLoS ONE. [DOI] [PMC free article] [PubMed]
- 6.Collette BB, Williams JT, Thacker CE, Smith ML. Shore fishes of Navassa Island, West Indies: a case study on the need for rotenone sampling in reef fish biodiversity studies. Aqua J Ichthyol Aquatic Biol. 2003;6(3):89–131. [Google Scholar]
- 7.Dennis GD, Smith-Vaniz WF, Colin PL, Hensley DL, McGehee MA. Marine fishes from Islands of the Mona Passage, Greater Antilles. Caribbean Journal of Science. 2005;41(4):716–743. [Google Scholar]
- 8.Smith-Vaniz WF, Jelks HL, Rocha LA. Relevance of cryptic fishes in biodiversity assessments: A case study at Buck Island Reef National Monument, St. Croix. Bulletin of Marine Science. 2006;79(1):17–48. [Google Scholar]
- 9.Smith, CL, Tyler JC, Davis WP, Jones RS, Smith DG, et al. Fishes of the Pelican Cays, Belize. Atoll Research Bulletin. 2003;497:1–88. [Google Scholar]
- 10.Colwell RK. EstimateS: statistical estimation of species richness and shared species from samples 2006.
- 11.Clarke KR, Warwick RM. Change in Marine Communities: An approach to statistical analysis and interpretation. 2001. PRIMER-E: Plymouth, UK.
- 12.Starck WA., II A list of fishes of Alligator Reef, Florida with comments on the nature of the Florida reef fish fauna. Undersea Biology. 1968;1(1):5–36. [Google Scholar]
- 13.Longley WH, Hildebrand SF. Systematic catalogue of the fishes of Tortugas, Florida, with observations on color, habits, and local distribution. 1941. 331. Papers from Tortugas Laboratory No. 34 (Carnegie Institution of Washington Publ. 535)
- 14.Smith-Vaniz WF, Collette BB, Luckhurst B. Fishes of Bermuda: history, zoogeography, annotated checklist, and identification keys. 1999. 424. American Society of Ichthyologists and Herpetologists, Special Publication No. 4.
- 15.Clavijo IE, Yntema JA, Ogden JC. An annotated list of the fishes of St. Croix, U.S. Virgin Islands. 1980. 49. West Indies Lab, Special Publication (2nd ed.)
- 16.Mejia LS, Garzon-Ferreira J, Acero PA. Peces registrados en los complejos arrecifales de los cayos Courtown, Albuquerque, Serrana, y Rancodor, Caribe occidental, Colombia. Boletin Ecotropica: Ecosistemas Tropicales. 1998;32:25–42. [Google Scholar]