Summary
Potential predators emit uncharacterized chemosignals that warn receiving species of danger. Neurons that sense these stimuli remain unknown. Here we show that detection and processing of fear-evoking odors emitted from cat, rat, and snake require the function of sensory neurons in the vomeronasal organ. To investigate the molecular nature of the sensory cues emitted by predators, we isolated the salient ligands from two species using a combination of innate behavioral assays in naïve receiving animals, calcium imaging, and cFos induction. Surprisingly, the defensive behavior-promoting activity released by other animals is encoded by species-specific ligands belonging to the major urinary protein (Mup) family, homologs of aggression-promoting mouse pheromones. We show that recombinant Mup proteins are sufficient to activate sensory neurons and initiate defensive behavior similar to native odors. This co-option of existing sensory mechanisms provides a molecular solution to the difficult problem of evolving a variety of species-specific molecular detectors.
Introduction
The ability of prey to innately recognize the odor of a potential predator provides a strong selective advantage; however, the neural mechanisms that permit chemical eavesdropping on other species, interpret the cues, and initiate a defensive response are unknown. Inbred rodents, that have been isolated in the laboratory from other species for hundreds of generations, are known to respond with fear-like defensive behavior to cat odors (Dielenberg et al., 2001; Dielenberg and McGregor, 2001; Takahashi et al., 2005; Vyas, 2007). This innate response suggests that the neural mechanisms of detection in the receiving animals are genetically determined. Evolving an innate capacity to respond to chemosignals from a variety of species is a mechanistic challenge. To maximize the specificity of the warning, the receiver may sense potential threats by detecting specific ligands from all other animals. Given the sensory circuitry needed to detect and process each cue and the probability that each individual may only encounter a small subset of potential predator odors in its lifetime, this strategy would require a significant genetic investment that may go largely unutilized. An alternate, simpler, mechanism may involve other animals emitting a common odor, perhaps as a consequence of carnivore metabolism, that activates a general predator sensory circuit in the receiving prey (Fendt, 2006). Identifying the signaling ligands from multiple distantly related species is an essential step towards elucidating general mechanisms generating inter-species communication.
Kairomones, such as those that elicit fear behavior, are cues transmitted between species that selectively disadvantage the signaler and advantage the receiver (Wyatt, 2003). Known kairomones have mainly been identified in insect communication, though these models have not provided insight to the organization of the neural response in the receiving animals (Stowe et al., 1995). It is thought that subsets of sensory neurons are genetically determined to mediate innate behavior. These are likely to be distinguished from canonical olfactory neurons by distinctive locations in the nasal cavity, alternate projections to the brain, and/or expression of atypical molecular features. The vomeronasal organ (VNO), a specialized chemosensory epithelium of terrestrial vertebrates, contains sensory neurons displaying all three of these unique olfactory characteristics and is confirmed to function in the detection of pheromones (Tirindelli et al., 2009). In addition to detecting pheromones, VNO neurons have been shown to respond to regular chemical odorants in vitro, but the biological significance of this activity has not been determined (Sam et al., 2001; Trinh and Storm, 2003). In reptiles, the VNO initiates a defensive response to predators and facilitates the tracking of prey (Halpern and Frumin, 1979; Miller and Gutzke, 1999; Wang et al., 1993). In mammals, the identity of kairomones and detecting sensory neurons remains mostly unknown. An exception to this is trimethyl-thiazoline (TMT), the prominent pungent compound isolated from fox feces that causes aversion in rodents (Buron et al., 2007). Detection of TMT occurs through unidentified neurons in the main olfactory epithelium (MOE) (Kobayakawa et al., 2007). It is not known whether the MOE neural response is specific to TMT or represents a common model for the processing of other innate cues that promote inter-species behavior.
The identities of receptors and circuits that initiate innate behavior in response to olfactory ligands are largely unknown. The isolation of ligands of known function would provide the means to precisely stimulate brain circuits leading to specific behaviors. Recent progress has been made towards identifying the molecular nature of pheromone cues by purifying individual ligands. Small volatile molecules, sulfated steroids, peptides, and small proteins all display hallmarks of mammalian pheromones (Nodari et al., 2008; Tirindelli et al., 2009). Among these ligands, the Major urinary proteins (Mups) are abundantly excreted (milligram quantities per milliliter) in mouse urine and are additionally secreted by mammary, salivary, and lachrymal glands (Finlayson et al., 1965; Szoka and Paigen, 1978). Mups emitted by mice have been demonstrated to act as pheromone carrier proteins, environmental pheromone stabilizers, and as genetically encoded pheromones themselves (Chamero et al., 2007; Hurst et al., 1998; Marchlewska-Koj et al., 2000; Mucignat-Caretta et al., 1995). In several mammals (such as mouse, rat, horse, and lemur) there is evidence for lineage specific Mup gene expansion consistent with a function in intra-species communication; however, genome analyses have shown that most other mammalian species encode a single ancestral Mup of unknown biological function (Logan et al., 2008; Mudge et al., 2008). Interestingly, these Mup orthologs are primary sources of animal allergens, indicating that they are both highly stable and eminently transmissible between species in the environment (Virtanen et al., 1999).
In order to investigate the neural code that warns of danger, we first devised a robust and quantifiable odor-based behavioral assay and then used a combination of genetic and cellular analyses to identify the responding sensory organ and neural activity. Importantly, we studied mouse odor responses to five different animal species (rat, cat, snake, rabbit, and mouse), which enabled us the comparative means to identify general mechanisms of kairomone information coding. We show that VNO defective animals, TrpC2−/−, do not sense the olfactory ligands that initiate defensive behavior. We purified and identified the kairomone activities from rat and cat and find that they each encode species-specific Major urinary protein (Mup) homologs. Previously, intra-species Mups have been shown to function as pheromones (Chamero et al., 2007). Our findings suggest that the stabilization and expansion of Mup chemosensation resulted in the co-option of function to include both inter- and intra-species communication.
Results
The vomeronasal organ mediates predator odor elicited defensive behavior
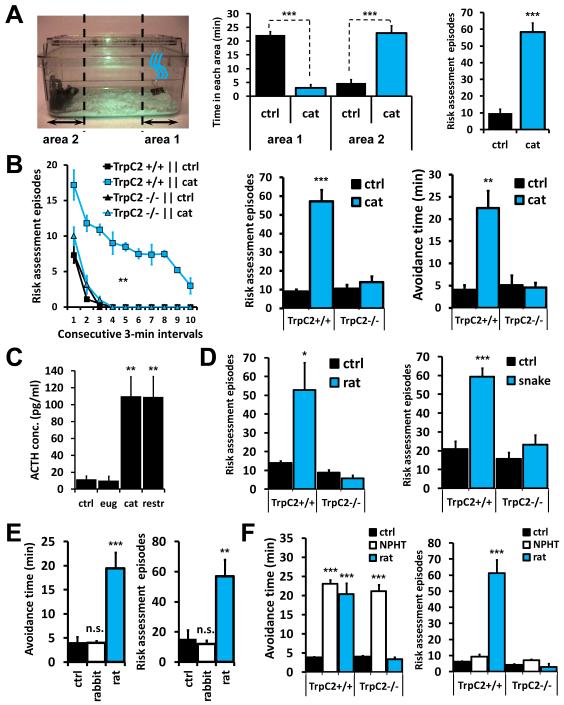
Field and laboratory studies have shown that predator odors elicit a range of defensive behaviors from prey species (Apfelbach et al., 2005; Blanchard et al., 2001; Dielenberg and McGregor, 2001). We first utilized a simple and robust odor-driven behavioral assay to quantify the effect of odors from multiple predator species on inbred, C57BL/6J, mice. All natural odors and control odors were presented in a single trial to naïve mice not previously exposed to other species. Mice were analyzed to determine if exposure to odors elicited the combination of three outputs: avoidance behavior; risk assessment behavior, which is a stereotypical cautious investigative approach characterized by a low-lying extended body posture (see Suppl. Exp. Procedures and Video S1 for a detailed description of behavior analysis); and the release of the stress response adrenocorticotropic hormone, ACTH. Together, these defensive behaviors and neuroendocrine change are considered to model responses associated with fear in rodents (Apfelbach et al., 2005; Blanchard et al., 2001; Rosen, 2004). The novelty of control odors evoked investigation without significant risk assessment behavior or release of ACTH (Fig. 1A, C). We next investigated the innate response to native odors obtained from three species that are natural mouse predators: cat (neck swab), snake (shed skin), and rat (urine). In contrast to the controls, we found that wild-type mice displayed significant innate avoidance and risk assessment behaviors as well as an increase in the stress hormone, ACTH, when exposed to odors from all three species (Figs. 1A-D, S1A-B). When similarly assayed, another complex natural odor, rabbit urine, did not induce avoidance or risk assessment behavior (Fig. 1E), nor did it increase ACTH levels (Fig. S1C), indicating that the defensive responses we observed are not generally directed to all complex novel odor stimuli. Together, our behavioral assays confirm that mice show robust defensive behavior upon first encounter with complex natural odors from three diverse species. This innate response suggests that cognate neurons that sense odors from multiple potential predators in the receiving animal are genetically hardwired to activate a fixed action pattern of defensive behavior.
Figure 1. VNO function is necessary for the display of innate behavior induced by predator odors.
(A) Left: behavioral arena, odor stimulus is indicated by blue swirls in area 1. Middle: naïve mice are attracted to area 1 containing control odors but avoid cat odors in the same area. Right: quantification of risk-assessment behavior (see Suppl. Exp. Procedures and Video S1 for description of risk assessment behavior and scoring details). (B) TrpC2 function is necessary for the display of risk assessment and avoidance behaviors stimulated by cat odors. (C) Plasma ACTH concentration increases in response to physical restraint (restr) and cat odor but not to control odor eugenol (eug). (D) Risk assessment behavior in TrpC2+/+ and −/− littermates exposed to rat (left) or snake (right) odors. (E) Behavioral outputs in wild-type animals exposed to an ethologically relevant complex stimulus (rabbit urine, white bars). (F) Exposure to the generally aversive odorant naphthalene (NPHT) induces robust avoidance behavior independent of TrpC2 function, and no risk assessment. Black bars in (E) and (F) indicate animals exposed to control odors, blue bars show animals exposed to kairomone odors. n=8-20; *P<0.05; **P<0.01; ***P<0.001; n.s., non-significant; Student’s one-tailed t-test (A, bar graphs in B & D) or ANOVA followed by Tukey-Kramer HSD post-hoc analysis (C, E, F and time course in B). Mean ± SEM. Control odors (ctrl) are PBS-soaked gauze (rat bar graph in D, E, and F) or clean dry gauze (all other panels). See also Fig. S1.
The neurons that eavesdrop on the presence of other species have not been identified but are expected to be a novel subset of chemosensory neurons in the nasal cavity. Mammalian pheromones that mediate other innate behaviors, such as male-male aggression, are detected by the vomeronasal organ (VNO) (Chamero et al., 2007; Leypold et al., 2002; Stowers et al., 2002) and the snake VNO is required to sense and respond to predators (Miller and Gutzke, 1999); therefore, we next investigated the extent to which the mammalian VNO is involved in the innate response towards kairomones. We analyzed the ability of mice lacking TrpC2, the primary signal transduction channel of VNO sensory neurons (VNs), to detect and respond to predator cues. Strikingly, these mutant animals showed no significant defensive behavior responses to any of the three odors from other species (Figs. 1B, D, S1A-B). Instead, TrpC2−/− animals investigated predator odors similar to control odors, behavior expected if they were unable to detect the ligand(s) that signal caution. To determine the sufficiency of olfactory cues in sensing other animals, we additionally investigated the response of wild-type and TrpC2−/− mutant animals in a more natural environment (Fig. S1D). When placed in the presence of a live (anesthetized) rat, wild-type mice spent the majority of the assay retreating in a hiding box and displaying numerous risk assessment episodes. In contrast, the TrpC2−/− animals approached and investigated the rat without significant defensive behaviors (Fig. S1D; Video S2). Remarkably, this suggests that, in the context of our assays, other sensory cues from the rat are not sufficient to signal danger to the mouse and those detected by the VNO are necessary to induce fear responses between species. When exposed to the generally aversive odorant naphthalene (released from burning wood), mice display strong avoidance behavior, which is independent of VNO function, but no risk assessment response (Fig. 1F). This suggests that VNO activity, while necessary for avoidance to kairomones, is not indiscriminately required for avoidance to generally aversive odors. Others have shown that a high concentration of TMT from fox feces promotes avoidance through activation of an unknown subset of MOE neurons in rodents (Kobayakawa et al., 2007). This prompted us to determine if the VNO is also required to detect TMT. We exposed wild-type and TrpC2−/− animals to TMT and quantified the resulting defensive behavior (Fig. S1E). Cat, rat, and snake odors require a functional VNO to initiate both avoidance and risk assessment behaviors. In contrast, TMT does not initiate risk assessment behavior, and the display of avoidance is not dependent on the VNO (Fig. S1E). This analysis indicates that TMT does not transmit the same sensory information as cat, rat, and snake odors. Moreover, these additional assays with naphthalene and TMT further demonstrate that TrpC2−/− mutants have intact central circuits that are capable of generating a wild-type display of avoidance behavior. This indicates that the lack of a behavioral response from TrpC2−/− mutants to our kairomones is not due to developmental defects that may affect associated pathways in the brain necessary to execute behavior (Figs. 1, S1).
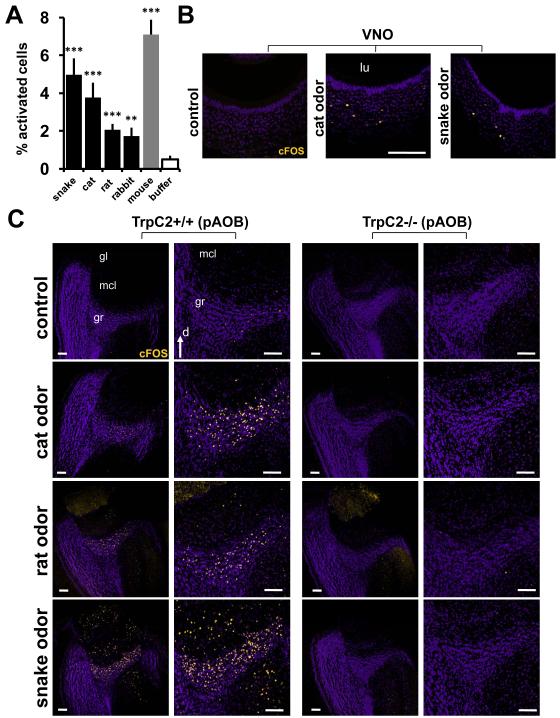
We used two different methods to investigate the extent to which VNs detect odors emitted between species. First we determined that kairomones directly activate VNs by observing calcium influx in individual dissociated VNs in response to natural odors from other species. We found odorants from all species analyzed to directly induce calcium transients in a subset of VNs (1.7-7.1% of >1500 neurons analyzed; Fig. 2A, Table S1). Second, since this ex vivo analysis cannot accurately approximate the biological concentration of ligands at the sensory dendrite, we additionally exposed freely-moving behaving mice to predator odor, observed the defensive response, and confirmed corresponding neuronal activation in the VNO epithelium by immunostaining to detect increases in the expression of the immediate early gene cFos (Fig. 2B) (Morgan and Curran, 1991). The punctate expression of cFos throughout the vomeronasal epithelium is consistent with specific activation of a subset of cognate sensory neurons. Together, these results indicate that mammalian VNs directly detect odors from a variety of other species.
Figure 2. Accessory olfactory system detects and responds to predator odors.
(A) Percent dissociated VNs showing calcium transients following perfusion with complex odors. Mean ± SEM of 1586-4315 sampled neurons (n=7-21 expts). (B) Increase in cFos expression in the VNO of freely moving behaving animals following exposure to control and kairomone odors. (C) TrpC2 function is necessary for cFos induction in the posterior AOB (pAOB) following exposure to kairomone odors (see Fig. S2 for cFos response to predator odors in the anterior part of the AOB). n=8-20; lu, VNO lumen; gr, granule cell layer of the AOB; mcl, mitral cell layer of the AOB; gl, glomerular layer of the AOB; d, dorsal; m, medial; bar=100μm. Blue labeling = nuclear stain, yellow labeling = anti-cFos immunoreactivity. **P<0.01; ***P<0.001; ANOVA followed by Tukey-Kramer HSD post-hoc analysis (A). Mean ± SEM. Control odor in B and C is clean dry gauze.
In animals exposed to predator odors, we additionally observed a striking amount of cFos expression in the accessory olfactory bulb (AOB), to which VNO axons directly project (Figs. 2C, S2A-H). This activity was entirely absent in the TrpC2−/− mutants. In wild-type animals, immunostaining was concentrated in the granule cell layer, although snake odor reliably induced higher levels of activity and additionally evoked cFos expression in the glomerular and mitral cell layers (Fig. 2C). All three odor sources consistently evoked AOB activity that appears in excess of that observed in the VNO (Fig. 2B-C, S2A-H). Such a strong AOB activation in response to kairomones is not simply a secondary result of elevated arousal levels in mice subjected to stressful conditions, since the AOB cFos expression is negligible in animals that have been conditioned to respond aversively to a regular odorant (pentyl acetate; Fig. S2I-N). The AOB is a heterogeneous nucleus with at least two different zones, anterior and posterior, each receiving axons from molecularly distinct neurons in the VNO. Purified cues have been shown to activate sensory neurons that project to either one of the AOB zones (Tirindelli et al., 2009). Interestingly, all three predator odors from our study induced robust activity in both zones, suggesting that they are composed of several ligands capable of activating distinct VNO receptors.
Purification of a single ligand that evokes defensive behavior
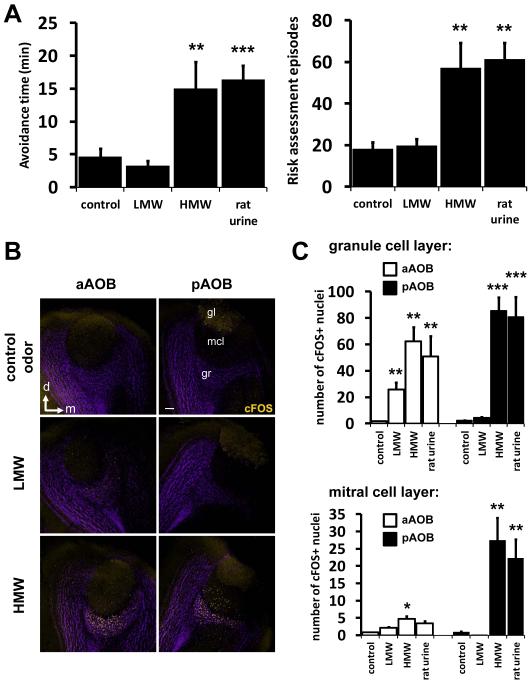
Natural animal secretions are typically odorous and expected to be composed of a complex blend of stimuli. We chose an unbiased method to purify and identify the behavior-inducing kairomone ligand(s). We sequentially fractionated the natural odor source and tracked the relevant bioactivity by behavioral analysis to identify kairomone-containing fractions. We first fractionated total rat urine over size-exclusion ultrafiltration columns and tested these fractions for defensive behavior evoking activity. We found that the low molecular weight fraction (LMW, containing ligands less than 10kDa molecular mass) entirely lacked kairomone activity in our assay, while the high molecular weight fraction (HMW, containing ligands greater than 10kDa molecular mass) was sufficient to induce prolonged avoidance and repeated risk assessment episodes, similar in quality and quantity to total rat urine (Fig 3A).
Figure 3. Partial purification of the behavior-inducing kairomone from rat urine by size exclusion fractionation.
Size exclusion fractionation of whole male rat urine through an ultrafiltration column yields LMW fraction (molecules smaller than 10kDa), which lacks kairomone activity, and HMW fraction (larger than 10kDa) containing defense promoting bioactivity. (A) Mouse avoidance and risk assessment behavior-inducing activity in rat urine is present in the HMW fraction. (B) HMW fraction induces cFos activity in the AOB. (C) Quantification of cFos positive nuclei in the granule and mitral cell layers of the AOB. White bars = aAOB, black bars = pAOB. Note that the pAOB is almost exclusively activated by HMW fraction, while the LMW fraction mostly activates the anterior AOB. n=8; *P<0.05; **P<0.01; ***P<0.001; ANOVA followed by Tukey-Kramer HSD post-hoc analysis. Mean ± SEM. Control odor is PBS-soaked gauze. See also Fig. S3.
When we analyzed the AOB of animals exposed to these two fractions, we found limited cFos response in the aAOB to the LMW fraction and extensive activation in the granule cell layer of the aAOB to the HMW fraction, which additionally strongly activated the pAOB (Fig 3B-C). Interestingly, only whole urine and the HMW fraction stimulated detectable cFos immunoactivity in the mitral cell layer that contains the output neurons of the pAOB (Fig. 3B-C), while very little cFos was detected in this region in response to urine stimuli that did not elicit defensive behavior (Fig. S3). VNs that project to the pAOB are known to be activated by peptides and proteins (Munger et al., 2008), consistent with the large molecular mass of our kairomone-containing HMW fraction. Overall, both fractions contain ligands that activate the accessory olfactory system, however, the kairomone activity is found exclusively in the HMW fraction and the functional significance of those in the LMW fraction remain unknown. This finding underscores our strategy of utilizing behavioral analysis to purify ligands of known biological relevance.
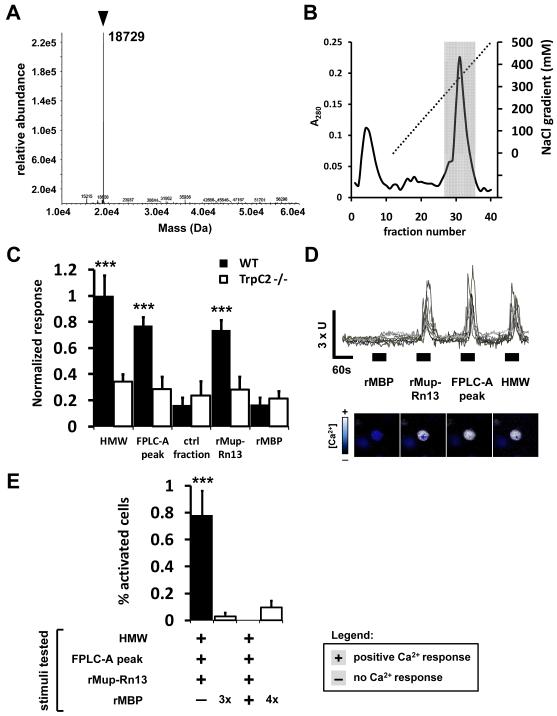
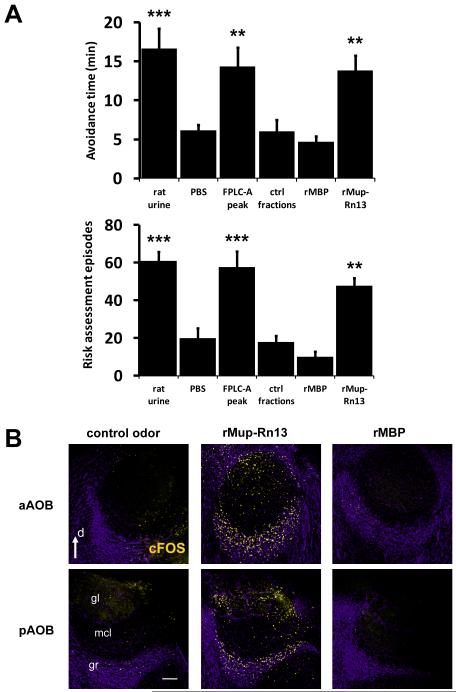
To further determine the complexity and identity of potential ligands, we subjected the HMW fraction stimulus to electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) and found both the HMW fraction and whole rat urine to contain one prominent protein with a molecular mass of 18,729 kDa as well as many less abundantly expressed proteins (Fig 4A). We used anion-exchange fast protein liquid chromatography (FPLC) to separate the HMW fraction components into 40 fractions over a 0-1 M NaCl gradient (Fig. 4B). To streamline the identification of the bioactivity we first screened the FPLC fractions for the ability to activate VNs as indicated by calcium imaging. Only those fractions containing the prominent protein which eluted between 300-450mM NaCl induced calcium transients in VNs (FPLC-A, 3723 VNs sampled, Fig. 4C), which was statistically indistinguishable from that initiated by the entire HMW activity. This activity did not occur in response to other FPLC control fractions and is similarly abolished in TrpC2−/− VNs (Fig 4C, S4A). When we analyzed the response at the single cell level, the FPLC-A fractions were found to induce calcium transients in the same neurons as those activated by the HMW fraction (Fig. 4D-E, S4B). Importantly, these fractions were fully sufficient to stimulate robust defensive behaviors when presented to predator odor naïve mice and no behavior-promoting activity was present in other tested FPLC fractions (Fig. 5A).
Figure 4. Activation of subsets of vomeronasal sensory neurons by purified putative kairomones.
(A) ESI-MS analysis identifying the major protein constituent in the HMW of rat urine (arrowhead). (B) Further fractionation of the HMW by anion exchange FPLC. Fractions from the shaded area were combined to form “FPLC-A peak” and bioactivity was compared to fractions gathered from the non-shaded areas (ctrl fractions). (C) Quantification of response to fractions of rat HMW, recombinant rat Mup13 (rMup-Rn13) and recombinant maltose-binding protein (rMBP) in dissociated VNs isolated from wild-type (black bars) or TrpC2−/− (white bars) male mice and assayed by calcium imaging. The ordinate shows the normalized response compared to the rat HMW activation level. n=4-16 expts; ***P<0.001; ANOVA followed by Tukey-Kramer HSD post-hoc analysis against no stimulus control (0.509% ± 0.177; 0.256 normalized response). (D) Representative calcium transients from 8 isolated VNs, sequentially stimulated with rMBP, rMup-Rn13, the FPLC-A peak, and the HMW fractions of rat urine. Axis bars: X = 60s; Y= 3x(F340/380nm). Images of a representative responding cell are presented below the traces, pseudocolored dark-to-light to indicate calcium influx. (E) Comparative percent activity of dissociated VNs stimulated with rMBP, rMup-Rn13, the FPLC-A peak, and the HMW fraction as assayed by calcium imaging. Each bar denotes the percentage of all imaged cells exhibiting a calcium spike in response to the stimuli marked with a plus sign and not exhibiting a response to the stimuli marked with a minus sign. All cells were exposed to all four stimuli, except for control cells, which were exposed to the indicated number of repetitive pulses of rMBP (white bars). Note the population of cells activated by all three rat stimuli (first bar), which is significantly above the number of cells responding to three pulses of control rMBP (second bar) and no cells responded to rat and rMBP stimuli (third bar). n=10-11; *** P< 0.001; ANOVA followed by Tukey-Kramer HSD post-hoc analysis against respective rMBP control. Mean ± SEM. See also Fig. S4.
Figure 5. Purified inter-species proteins activate the vomeronasal system and induce responses similar to native kairomones.
(A) Behavior inducing activity was found in the FPLC-A fraction and is accounted for by recombinant Mup13 (rMup-Rn13). Recombinant maltose-binding protein (rMBP) and control FPLC fractions did not initiate defensive behavior. (B) rMup-Rn13 protein exposure induces AOB activation (see quantification in Fig. S5C). n=8-12; gr, granule cell layer of the AOB; mcl, mitral cell layer of the AOB; gl, glomerular layer of the AOB; d, dorsal; m, medial; bar=100μm. **P<0.01; ***P<0.001; ANOVA followed by Tukey-Kramer HSD post-hoc analysis against PBS-soaked control gauze. Mean ± SEM. See also Fig. S5.
To identify the FPLC-A activity we used nano-liquid chromatography MS/MS to obtain the sequence of the prominent protein in the behavior promoting fractions. Interestingly, the resulting peptides identified the protein as an alpha-2u-globulin (data not shown). On comparison with the rat genome, we resolved its sequence to the product of a specific gene: Mup13, a homolog of mouse Mup pheromones (Logan et al., 2008). The central hydrophobic binding pocket of all Mups creates a high affinity for small organic ligands which are themselves known to have chemosignaling functions (Flower, 1996; Leinders-Zufall et al., 2000). To determine if the kairomone activity was produced by the presence of these protein-associated ligands, we incubated the HMW fraction with menadione, which competitively displaces potential rat Mup-bound ligands from the native Mup protein (Chamero et al., 2007). Naïve mice responded with complete defensive behavior towards menadione-displaced Mups, indicating that native small molecules which may be associated with the active protein fraction do not function as kairomones (Fig S5A). When considered with our fractionation data, it suggests that rat Mup13 protein found in urine is transmitted between species and generates neural activation of the vomeronasal system to signal fear. To validate these findings we cloned, expressed, and purified a recombinant fusion protein between maltose binding protein (MBP) and rat Mup13 in Escherichia coli (rMup-Rn13). Remarkably, we found the recombinant rMup-Rn13 sufficient to induce intracellular calcium transients in a similar number of VNs as the HMW fraction and the FPLC-A (Fig 4C), fully accounting for the native activity. Ligand-induced calcium transients were not observed from VNs in the presence of recombinant maltose binding protein alone (Fig. 4C). rMup-Rn13 was unable to activate TrpC2−/− mutant neurons or MOE neurons, confirming its function as a ligand that directly activates VNO sensory neurons (Figs. 4C, S6N). We analyzed the sensitivity of VNs to rMup-Rn13 by generating a dose response curve and found relatively high levels of the ligand to be necessary to initiate intracellular calcium transients (Fig. S4C). This concentration is likely to be within the range of biological significance, since native rat Mup13 is secreted at 0.5-1.5 mg/ml in rat urine (D.L. data not shown) (Mao et al., 1998). rMup-Rn13 induces calcium transients in the same subsets of VNs activated by the HMW and FPLC-A fractions (Figs. 4D-E, S4B), confirming that our recombinant protein accounts for the native source of significant VN activation. Furthermore, the cFos response to rMup-Rn13 in the AOB was statistically indistinguishable from total rat urine, both quantitatively and spatially (Figs. 5B, S5C). Importantly, a singular presentation of rMup-Rn13 to naïve mice promoted defensive behavior (Fig. 5A) and a significant increase in ACTH (Fig. S5B). Together, these analyses demonstrate the sufficiency of the rat Mup as a kairomone signal.
Cat Mup functions as a kairomone
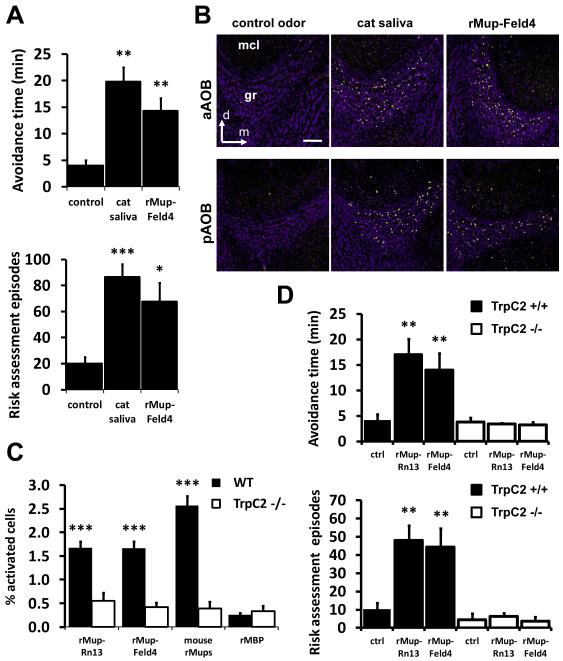
We found it notable that our lab mice innately respond to odors from three different species through sensory neurons of the VNO. Prey species could achieve a similar behavioral response to a variety of potential predators simply by detecting a single ligand, common to all carnivorous animals (Fendt, 2006). In order to identify the molecular logic of how prey species respond with defensive behavior to a variety of other species, we aimed to isolate a second predator kairomone. Unfortunately, odor stimuli analyzed in our study are obtained by briefly swabbing medical gauze on a cat’s neck or isolating recently shed snake skin and we found neither of these preparations to be readily amenable to fractionation. However, we did find cat saliva, a potential source of fur chemosignals, sufficient to induce cFos expression in the AOB and initiate defensive behavior (experimental logic in “stimuli” of Suppl. Exp. Procedures, Fig. 6A-B). The submandibular salivary gland is known to secrete copious amounts of Feld4, the cat homolog of the rat and mouse Mups (Smith et al., 2004). Feld4 is a prominent cat allergen, indicating that it is stable and transmissible between species (Smith et al., 2004). Therefore, we considered this Mup protein as the potential source of the cat kairomone bioactivity. Interestingly, a native odor sample that failed to elicit defensive behavior (rabbit urine; Fig. 1E) does not appear to contain Mup proteins (Fig. S6P-Q). To directly test if cat Feld4 is detected as a kairomone, we expressed and purified a recombinant fusion protein between maltose binding protein (MBP) and Feld4 in Escherichia coli (rMup-Feld4). When assayed, rMup-Feld4 predominantly accounted for the native kairomone activity (Fig. 6A). We used calcium imaging analysis to identify the responding sensory neurons. rMup-Feld4 failed to produce calcium transients in MOE neurons (Fig. S6N), however, the recombinant protein was sufficient to directly initiate calcium transients in a subpopulation of VNs and generate a cFos response in the AOB (Fig. 6B-C, S6A-K). This activity is dependent on VN signaling as TrpC2−/− VNs failed to produce calcium transients or cFos induction in the AOB to rMup-Feld4 (Fig. 6C, S6D-M). Importantly, while TrpC2−/− animals do not display significant defensive responses towards recombinant Feld4 (Fig. 6D), this ligand is sufficient to promote significant defensive behaviors and ACTH release in wild-type mice (Fig. 6A, S6O). Our analysis indicates that detection of cat Feld4 through the VNO sensory system induces defensive behavior.
Figure 6. Isolation and characterization of a second purified kairomone.
(A) Avoidance and risk assessment behaviors induced by cat saliva kairomones is accounted for by recombinant Mup (rMup-Feld4). (B) cFos immunostaining of the anterior and posterior AOB following exposure to cat saliva and rMup-Feld4. (C) Quantification of calcium imaging to recombinant kairomones, rMup-Rn13 and rMup-Feld4, and recombinant Mup pheromones (a pool of mouse rMup3, 8, 17, 24 and 25, each of which is expressed in C57/BL/6J male urine) in dissociated VNs isolated from wild-type (black bars) or TrpC2−/− (white bars) male mice. Recombinant maltose binding protein (rMBP) is used as a control. n=6-24 expts. (D) Defensive behavior to recombinant Mup protein kairomones depend on VNO function. n=8-12; gr, granule cell layer of the AOB; mcl, mitral cell layer of the AOB; d, dorsal; m, medial; bar=100μm. *P<0.05; **P<0.01; ***P<0.001; ANOVA followed by Tukey-Kramer HSD post-hoc analysis. Mean ± SEM. Control odor is PBS-soaked gauze. See also Fig. S6.
Intra- and inter-species Mups are functionally distinct
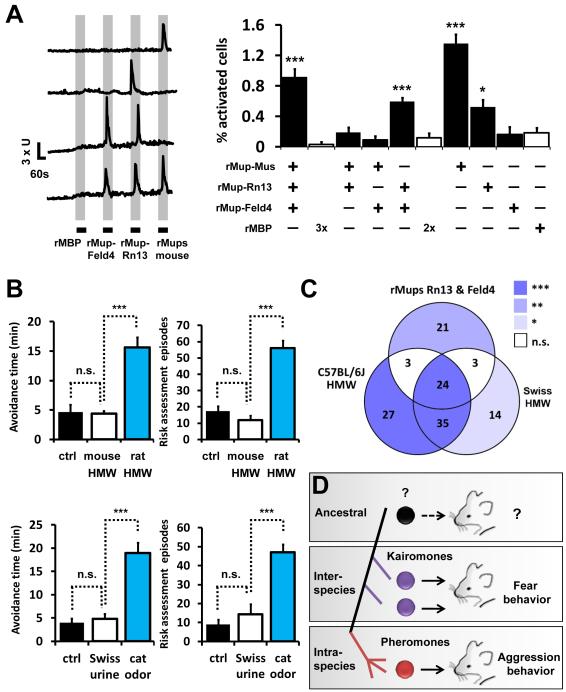
Given that mouse Mups have a different biological significance to mice than rat or cat Mups, we analyzed the neuronal and behavioral responses to Mups from different species. We compared the response of dissociated VN neurons to each of these cues by calcium imaging to determine if they activate identical populations of neurons. We found four independent populations of responding cells, some that detect multiple Mup variants and some that were reproducibly and specifically activated by individual recombinant ligands (Fig. 7A, S7H). Among these, there were ensembles of neurons that displayed calcium transients solely to either mouse Mup variants (Fig. 7A, 7th bar) or Mups from cat and rat (Fig. 7A, 5th bar). These VN responses are likely driven by sensory receptor tuning to sequence variance of the individual Mups (Fig. S7A, C). We expect these populations to be biologically relevant because of the significant number of activated cells compared to our negative control stimulus, recombinant maltose binding protein (rMBP, Fig. 7A), as well as the reproducibility of the specific responses when a single stimulus was repetitively pulsed (Fig. S7B). This analysis reveals that each Mup stimulus has the potential to encode a different quality of information (Fig. S7H). However, on its own, VN activation profiles do not reveal the underlying neural code that generates behavior.
Figure 7. Kairomones and pheromones encode different functions.
(A) Left: Representative calcium transients from isolated VNs, sequentially stimulated with recombinant maltose-binding protein (rMBP), rMup-Feld4, rMup-Rn13 and recombinant mouse Mup pheromones (a pool of mouse rMup3, 8, 17, 24 and 25). Axis bars: X = 60s; Y= 3x(F340/380nm). Boxes indicate application and duration of stimulus. Right: comparative percent activity of dissociated VNs stimulated with recombinant rat and cat kairomones and mouse Mups as assayed by calcium imaging. Each bar denotes the percentage of all imaged cells exhibiting a calcium spike in response to the stimuli marked with a plus sign and not exhibiting a response to the stimuli marked with a minus sign. All cells were exposed to all four stimuli, except for control cells, which were exposed to the indicated number of repetitive pulses of rMBP (white bars). Note the presence of populations of cells responsive to kairomones only (fifth bar) and responsive to all Mups (first bar), which are significantly above controls exposed to pulses of rMBP. n=10-11 expts. (B) Avoidance and risk assessment behaviors are triggered only in the presence of the rat-derived HMW fraction (blue bars, top panels) and cat swab (blue bars, bottom panels), inter-species Mups, but not in the presence of C57BL/6 mouse HMW fraction (white bars, top panels) or Swiss strain urine (white bars, bottom panels), which contain mouse Mups. n=11-12. (C) Venn diagram showing populations of cells responsive to kairomones (rMup-Rn13 and rMup-Feld4) and/or Mup-containing HMW fractions from C57BL/6J and Swiss mouse urine, as assayed by calcium imaging (n=10-11 expts; see also Fig. S7E for complete documentation of % activated VNs). Statistical significance of each population (represented by each intersect), against respective rMBP control pulses, is color coded. (D) Model for the proposed co-option of semiochemicals. Left; schematic representation of chronograph of Mup ligand evolution. Center; following stabilization of detection of ancestral ligand, genomic duplication and drift enabled Mups to be detected as kairomones (purple) or pheromones (red). Right; Mups have undergone neofunctionalization to instruct different behaviors. *P<0.05; **P<0.01; ***P<0.001; ANOVA followed by Tukey-Kramer HSD post-hoc analysis. n.s.= non-significant. Mean ± SEM. Control odor (ctrl in B) is PBS-soaked gauze. See also Fig. S7.
Next, we took advantage of the fact that the defensive behavior in response to kairomones is context-independent: the response occurs when stimuli are solely presented on a cotton gauze. In contrast, aggressive behavior promoted by mouse urine pheromones is context-dependent: initiated only when coincidentally detected with another mouse. Behavior in response to mouse Mups out of context has not been evaluated. We analyzed freely-moving behaving animals for avoidance time and risk assessment episodes in response to exposure to native mouse Mups presented on cotton gauze. As expected, removed from the context of another mouse, mouse Mup pheromones did not initiate aggressive behavior. Interestingly, they equally showed no signs of initiating defensive behavior (Fig. 7B). To control for the possibility of mouse Mup habituation or contextual learning of self-expressed Mups, we additionally assayed the response to Mups from a different mouse strain (heterogenic Swiss), which excretes different Mup variants (Cheetham et al., 2009). Cues from Swiss mice activate subsets of VNs tuned to strain differences (Fig. 7C, S7E) and we found this pheromone stimulus to be equally unable to induce defensive behaviors or the release of ACTH (Fig. 7B, S7D). We assayed the ability of the pheromone and kairomone responding VNs to additionally detect our control complex natural stimulus, the HMW fraction of rabbit urine, and found it not to activate kairomone responsive VNs (Fig. S7F-G). This VN response is consistent with the lack of defensive behavior observed in response to rabbit urine (Fig. 1E). Taken together, our analyses indicate that there is a functional difference between cat and rat Mups that are detected as kairomones and mouse Mups that are detected as pheromones. This difference is likely initiated by Mup-specific activation of VN ensembles.
Discussion
Accessory olfaction function is not limited to pheromones
Multiple olfactory subsystems are present in the mammalian nasal cavity, including the MOE, VNO, septal organ, and Grueneberg ganglion. The functional significance of this anatomical segregation has not been determined in mammals (Munger et al., 2008). Since its discovery almost two centuries ago, it has been speculated that the VNO serves to detect pheromones (Tirindelli et al., 2009). More recent studies using genetic tools, electrophysiological recordings and calcium imaging assays have confirmed this function (Chamero et al., 2007; Holy et al., 2000; Leinders-Zufall et al., 2000; Leypold et al., 2002; Luo et al., 2003; Stowers et al., 2002). The estimated number of VNO sensory receptors (>250) vastly exceeds both currently identified pheromone ligands and the expected range of social behaviors mediated by pheromones, leaving potential coding space for other types of olfactory cues (Shi and Zhang, 2007; Young et al., 2005; Young and Trask, 2007). We now show that mouse VNs detect non-pheromonal ligands and that the accessory olfactory system is functionally necessary to initiate innate, stereotypic defensive behaviors and endocrine surges in response to odors from other species.
Chemical detection of threatening environments
We were able to purify kairomone ligands from two different species and found them both to be Mup homologs. Mups are endowed with several characteristics that serve as good protein kairomones (Wyatt, 2003). First, the receiving animal must detect a ligand that is fixed in the genome of the signaler. Mups have been retained in the genomes of all sequenced placental mammals (except for humans), suggesting that they likely possess an advantageous ancestral function (Logan et al., 2008). The primary function of Mups in the signaling animal is not known; however, recent reports indicate that at least one Mup has beneficial metabolic effects by decreasing hyperglycemia and glucose intolerance in type 1 and type 2 diabetic mice (Zhou et al., 2009). Second, they must be easily detected by the receiver. In all known cases, Mups are secreted or excreted into the environment where they are extremely stable, resistant to degradation, and easily transmissible between individuals. This is demonstrated by the fact that many major respiratory allergens are either Mups or related lipocalins (Virtanen et al., 1999). For example, over sixty percent of humans who are allergic to cats test positive for Feld4-specific IgE (Smith et al., 2004). Third, genome analysis has shown that Mups have undergone multiple species-specific evolutionary expansions followed by selective constraint (Logan et al., 2008; Mudge et al., 2008). The organization of olfactory receptor genes in genomic clusters susceptible to duplication (Lane et al., 2002) and the anatomical organization of the olfactory bulb into modular glomerular units (Mombaerts, 2001) provides a system suitable for expansion of olfactory detectors that can encode a novel function (neofunctionalization).
Co-option of semiochemicals: one mechanism, multiple functions
How does a detection system that responds defensively to a variety of species upon first exposure evolve? Isolation of Mup homologs of distinct behavioral consequences from two different species provides great insight. We have previously shown that one of the VNO’s functions is the detection of aggression-promoting Mup pheromones (Chamero et al., 2007). We have now determined that Mups also function to communicate between species via the VNO. Though we do not know the ancestral function of Mups, their detection may have become stabilized to sense one’s own production of Mups, to protectively detect other species, or to communicate within a species (Fig. 7D, top panel). Once the ancestral ligand/receptor detection pairing was constrained in the genome, duplication followed by neofunctionalization in an evolutionary mouse lineage may have, for example, enabled sensation of additional species emitting Mups of more divergent sequence (Conant and Wolfe, 2008; McLennan, 2008), initiating an inter-species defense system that increased fitness and futher stabilized Mup detection (Fig. 7D, middle panel). Finally, the Mup gene cluster expanded and cognate olfactory receptors diversified to provide for intra-species communication (Fig. 7D, bottom panel). In this scenario, Mup detection is co-opted from kairomone to pheromone (Fig. 7D).
Interestingly, defensive behavior can entail freezing, fighting, or fleeing depending on the context of the stimuli (Eilam, 2005). Though pheromone-mediated aggression (fighting) and kairomone-promoted defensive fleeing are mutually exclusive behaviors, the controlling neural circuits may share common mechanisms. Our current findings initiate several fundamental questions. How does the receiving animal differentiate those Mups emitted from a conspecific, which do not elicit defensive behavior, from those from species that do? Is the activity from all kairomones integrated into a common neural circuit that serves as a master control of defensive behavior? The purification and identification of salient ligands with intrinsic activity now provides the molecular tools to detect and manipulate the precise neural code that governs behavior.
Experimental Procedures
Mice
Wild-type animals were eight-week old male C57Bl6J mice, unless otherwise noted. Female mice showed identical responses as analyzed by cFos expression and behavior (data not shown). TrpC2+/+ and −/− littermates were obtained from heterozygous mating couples, which were produced by backcrossing the TrpC2−/− knockout line (Stowers et al., 2002) into the C57Bl/6J background for at least 4 generations. To ensure the identification of innate behavior, animals had no previous exposure to odors from other animal species. All procedures were approved by the Institutional Animal Care and Use Committee (IUCAC).
Behavioral assays
Individually caged mice were habituated for 2 hours in the dark over 2 consecutive days and assayed on day 3. See Suppl. Exp. Proc for collection and preparation of predator odor and control stimuli. Mice were assayed and filmed for 1 h in the dark. Movies were scored blindly for approach and avoidance times during the first 30 min of exposure, risk assessment episodes were quantified for the first 15 min of assay (see details in Suppl. Exp. Proc. section and Movie S1). Either unpaired t-tests or one-way ANOVA were applied. The number of risk assessment episodes was additionally scored for 10 subsequent 3 min sessions and statistically compared to controls by one-way ANOVA. Error bars indicate SEM.
Calcium imaging
Transient increases in free Ca2+ concentration in dissociated VNO neurons were determined by ratiometric Fura-2 fluorescence as described (Chamero et al., 2007). The HMW and FPLC-A fractions of rat urine and recombinant rat and cat Mups were imaged at 3.33μg/ml (Fig. 4C) or 10μg/ml (elsewhere) in imaging buffer unless otherwise specified (see Suppl. Exp. Procedures for details on rat urine fractionation and production of recombinant Mups). Control fractions were diluted to the same extent as the FPLC-A fraction, irrespective of actual protein concentration in the fraction. Pooled mouse Mups were imaged at a total of 27.7μg/ml as described (Chamero et al., 2007). Protein concentrations were calculated by Bradford assay and adjusted for recombinant maltose-binding protein (rMBP) content. The rMBP control was imaged at 6.66μg/ml (Fig. 4C) or 20μg/ml (elsewhere) in imaging buffer. Statistical significance was tested using one-way ANOVA followed by the Tukey-Kramer HSD post-hoc analysis. Further details on stimuli used, number of experiments, imaged cells per experiment and percentages of activated cells are given in the Suppl. Exp. Procedures section and Table S1.
Supplementary Material
Acknowledgements
We thank F Langone, GAG Pereira, FM Costa, P Arruda, AT Yamada, MJ da Silva, MG Paniago, JA Yunes and STO Saad for resources. K Spencer, A Roberts, C Levy, K Lloyd, TS Nakahara, FB Beato, E Kiyota, and KA Flanagan for technical support. Our volunteer cats Mitsy, Chewy, Cringer, Holiday, and Dolce. C Zuker, A Patapoutian, and K Baldwin commented on the manuscript. This work was supported by a Young Investigator Grant from FAPESP (FP), Skaggs Foundation (DWL, LS), NIH-NIDCD (LS). FP and LS initiated the study, FP and DWL performed the experiments, all authors wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional methods can be found in the Suppl. Exp. Procedures section.
References
- Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Buron G, Hacquemand R, Pourie G, Lucarz A, Jacquot L, Brand G. Comparative behavioral effects between synthetic 2,4,5-trimethylthiazoline (TMT) and the odor of natural fox (Vulpes vulpes) feces in mice. Behav Neurosci. 2007;121:1063–1072. doi: 10.1037/0735-7044.121.5.1063. [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Cheetham SA, Smith AL, Armstrong SD, Beynon RJ, Hurst JL. Limited variation in the major urinary proteins of laboratory mice. Physiol Behav. 2009;96:253–261. doi: 10.1016/j.physbeh.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Conant GC, Wolfe KH. Turning a hobby into a job: how duplicated genes find new functions. Nat Rev Genet. 2008;9:938–950. doi: 10.1038/nrg2482. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Carrive P, McGregor IS. The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects. Brain Res. 2001;897:228–237. doi: 10.1016/s0006-8993(01)02227-2. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- Eilam D. Die hard: a blend of freezing and fleeing as a dynamic defense--implications for the control of defensive behavior. Neurosci Biobehav Rev. 2005;29:1181–1191. doi: 10.1016/j.neubiorev.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Fendt M. Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. J Chem Ecol. 2006;32:2617–2627. doi: 10.1007/s10886-006-9186-9. [DOI] [PubMed] [Google Scholar]
- Finlayson JS, Asofsky R, Potter M, Runner CC. Major urinary protein complex of normal mice: origin. Science. 1965;149:981–982. doi: 10.1126/science.149.3687.981. [DOI] [PubMed] [Google Scholar]
- Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318(Pt 1):1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M, Frumin N. Roles of the vomeronasal and olfactory systems in prey attack and feeding in adult garter snakes. Physiol Behav. 1979;22:1183–1189. doi: 10.1016/0031-9384(79)90274-9. [DOI] [PubMed] [Google Scholar]
- Holy TE, Dulac C, Meister M. Responses of vomeronasal neurons to natural stimuli. Science. 2000;289:1569–1572. doi: 10.1126/science.289.5484.1569. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Robertson DHL, Tolladay U, Beynon RJ. Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Anim Behav. 1998;55:1289–1297. doi: 10.1006/anbe.1997.0650. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Lane RP, Cutforth T, Axel R, Hood L, Trask BJ. Sequence analysis of mouse vomeronasal receptor gene clusters reveals common promoter motifs and a history of recent expansion. Proc Natl Acad Sci U S A. 2002;99:291–296. doi: 10.1073/pnas.012608399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DW, Marton TF, Stowers L. Species specificity in major urinary proteins by parallel evolution. PLoS ONE. 2008;3:e3280. doi: 10.1371/journal.pone.0003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Mao Y, Moore RJ, Wagnon KB, Pierce JT, Debban KH, Smith CS, Dill JA, Fuciarelli AF. Analysis of alpha2u-globulin in rat urine and kidneys by liquid chromatography-electrospray ionization mass spectrometry. Chem Res Toxicol. 1998;11:953–961. doi: 10.1021/tx9800405. [DOI] [PubMed] [Google Scholar]
- Marchlewska-Koj A, Cavaggioni A, Mucignat-Caretta C, Olejnicz P. Stimulation of estrus in female mice by male urinary proteins. J Chem Ecol. 2000;26:2355–2365. [Google Scholar]
- McLennan DA. The concept of co-option: why evolution often look miraculous. Evo Edu Outreach. 2008;1:247–258. [Google Scholar]
- Miller LR, Gutzke WH. The role of the vomeronasal organ of crotalines (Reptilia: Serpentes: Viperidae) in predator detection. Anim Behav. 1999;58:53–57. doi: 10.1006/anbe.1999.1126. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. How smell develops. Nat Neurosci. 2001;4(Suppl):1192–1198. doi: 10.1038/nn751. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Mucignat-Caretta C, Caretta A, Cavaggioni A. Acceleration of puberty onset in female mice by male urinary proteins. J Physiol. 1995;486(Pt 2):517–522. doi: 10.1113/jphysiol.1995.sp020830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge JM, Armstrong SD, McLaren K, Beynon RJ, Hurst JL, Nicholson C, Robertson DH, Wilming LG, Harrow JL. Dynamic instability of the major urinary protein gene family revealed by genomic and phenotypic comparisons between C57 and 129 strain mice. Genome Biol. 2008;9:R91. doi: 10.1186/gb-2008-9-5-r91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger SD, Leinders-Zufall T, Zufall F. Subsystem Organization of the Mammalian Sense of Smell. Annu Rev Physiol. 2008 doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci. 2008;28:6407–6418. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Sam M, Vora S, Malnic B, Ma W, Novotny MV, Buck LB. Neuropharmacology. Odorants may arouse instinctive behaviours. Nature. 2001;412:142. doi: 10.1038/35084137. [DOI] [PubMed] [Google Scholar]
- Shi P, Zhang J. Comparative genomic analysis identifies an evolutionary shift of vomeronasal receptor gene repertoires in the vertebrate transition from water to land. Genome Res. 2007;17:166–174. doi: 10.1101/gr.6040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W, Butler AJ, Hazell LA, Chapman MD, Pomes A, Nickels DG, Thomas WR. Fel d 4, a cat lipocalin allergen. Clin Exp Allergy. 2004;34:1732–1738. doi: 10.1111/j.1365-2222.2004.02090.x. [DOI] [PubMed] [Google Scholar]
- Stowe MK, Turlings TC, Loughrin JH, Lewis WJ, Tumlinson JH. The chemistry of eavesdropping, alarm, and deceit. Proc Natl Acad Sci U S A. 1995;92:23–28. doi: 10.1073/pnas.92.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of Sex Discrimination and Male-Male Aggression in Mice Deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Szoka PR, Paigen K. Regulation of mouse major urinary protein production by the Mup-A gene. Genetics. 1978;90:597–612. doi: 10.1093/genetics/90.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Tirindelli R, Dibattista M, Pifferi S, Menini A. From pheromones to behavior. Physiol Rev. 2009;89:921–956. doi: 10.1152/physrev.00037.2008. [DOI] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Virtanen T, Zeiler T, Mantyjarvi R. Important animal allergens are lipocalin proteins: why are they allergenic? Int Arch Allergy Immunol. 1999;120:247–258. doi: 10.1159/000024277. [DOI] [PubMed] [Google Scholar]
- Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. PNAS. 2007;104:6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Jiang XC, Chen P, Inouchi J, Halpern M. Chemical and immunological analysis of prey-derived vomeronasal stimulants. Brain Behav Evol. 1993;41:246–254. doi: 10.1159/000113846. [DOI] [PubMed] [Google Scholar]
- Wyatt TD. Pheromones and animal behaviour: Communication by smell and taste. First edn Cambridge Univeristy Press; Oxford: 2003. [Google Scholar]
- Young JM, Kambere M, Trask BJ, Lane RP. Divergent V1R repertoires in five species: Amplification in rodents, decimation in primates, and a surprisingly small repertoire in dogs. Genome Res. 2005;15:231–240. doi: 10.1101/gr.3339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, Trask BJ. V2R gene families degenerated in primates, dog and cow, but expanded in opossum. Trends Genet. 2007;23:212–215. doi: 10.1016/j.tig.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang L, Rui L. Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. J Biol Chem. 2009;284:11152–11159. doi: 10.1074/jbc.M900754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.