SUMMARY
The retinoblastoma (Rb) tumor suppressor is often inactivated in cancers. To identify genes that can be used to specifically target such cancers, we carried out a genetic screen in Drosophila. We identified gig (fly TSC2) and found that inactivation of rbf (fly Rb) and gig synergistically induced cell death. Interestingly, inactivation of TSC2 specifically kills Rb mutant cancer cells under stress conditions, which is correlated with an inhibition of tumor growth. We show that cancer cell killing induced by concomitant inactivation of Rb and TSC2 is mediated by increased cellular stress, including oxidative stress. Inactivation of TSC2 and Rb synergistically induce oxidative stress via increased protein synthesis, inhibited de novo lipid synthesis, and decreased ROS scavenger enzyme SOD2 induction.
INTRODUCTION
The success of a targeted cancer therapy depends on its ability to target the unique features of tumor cells that are distinct from those of the normal cells. For example, the efficacy of androgen ablation therapy for early prostate cancers is due to the special dependence of prostate cancer cells on androgen for growth, proliferation, and survival (Balk and Knudsen, 2008). Similarly the effectiveness of imatinib for Philadelphia chromosome-positive chronic myeloid leukemia (CML) is due to the unique dependence of these CML cells on Bcr-Abl kinase activity, which is specifically inhibited by imatinib (Druker, 2002).
The changes acquired by cancer cells that contribute to their uncontrolled proliferation and growth often include both deregulated oncogenic pathways as well as inactivated tumor suppressor pathways (Hanahan and Weinberg, 2000). Current strategies to develop targeted cancer therapies generally aim at components of signaling pathways that are deregulated or required in cancer cells, such as specific kinases. Studies of gefitinib, an inhibitor of EGFR tyrosine kinases, revealed that this inhibitor is only effective on a small subset of cancers that exhibit mutations or amplifications that deregulate EGFR signaling (Lynch et al., 2004; Mulloy et al., 2007; Paez et al., 2004). In addition, cancers resistant to this therapy eventually develop (Engelman and Janne, 2008). These observations suggest the need to have an array of drugs that target different features of cancer cells such that different combinations can be used to specifically target different subsets of cancers and prevent the development of resistant cancers.
In addition to deregulated oncogenic activity, cancer cells often acquire inactivation of tumor suppressors such as the retinoblastoma protein Rb. Although approaches that specifically target loss of Rb function in cancers are potentially useful to a significant fraction of human cancers, there has been little success in developing therapies by targeting loss of Rb function in cancers. This is mainly due to the lack of straightforward approaches to restore the Rb function in all cancer cells and the lack of knowledge of so called synthetic lethal genes that are specifically required for the survival of cancer cells with inactivated Rb tumor suppressors. The lack of knowledge about synthetic lethal genes in conjunction with the lack of suitable approaches to identify such genes has limited the development of drugs that could specifically kill cancer cells based on the inactivated tumor suppressors.
Rb regulates diverse biological processes including cell proliferation, differentiation, and apoptosis. The biological functions of Rb are mediated by its interactions with a large number of proteins, particularly the E2F family of transcription factors. Rb and E2F regulate the expression of genes involved in the cell cycle as well as apoptosis (reviewed in (Bracken et al., 2004; Iaquinta and Lees, 2007). Rb and E2F have been shown to regulate apoptosis through a number of different mechanisms. E2F1 overexpression can induce apoptosis via transcriptional activation of pro-apoptotic genes including Arf, p73, APAF-1, Smac/Diablo, Omi HTRA2, and BH3 only-containing cell death regulators. E2F can also induce the expression of initiator and effector caspases (Nahle et al., 2002). Additionally, Rb and E2F have been shown to control the accumulation of reactive oxygen species (ROS) and thereby regulate cell death through a mechanism involving oxidative stress (Tanaka et al., 2002).
Rb/E2F-induced cell death is modulated by other regulators and signaling pathways such as the growth factor-stimulated activation of PI3K and Akt survival signaling (Hallstrom et al., 2008; Hallstrom and Nevins, 2003). It is interesting to note that in addition to their role in survival signaling, PI3K/Akt also has a major role in the regulation of energy metabolism and the coordination of key metabolic pathways (Robey and Hay, 2009). Akt has been shown to regulate mTOR signaling by direct phosphorylation and inhibition of Tuberin, the gene product of the TSC2 tumor suppressor (Inoki et al., 2002; Potter et al., 2002). Because elevated Akt activity induces increased ROS accumulation, activation of PI3K/Akt can inhibit cell death induced by a variety of stimuli but not by oxidative stress. In fact hyperactivity of Akt sensitizes cells to ROS induced cell death (Nogueira et al., 2008).
The Rb and E2F proteins are highly conserved in Drosophila (reviewed in (Du and Pogoriler, 2006). There are only two E2F (dE2F1 and dE2F2), one DP (dDP), and two Rb family (RBF and RBF2) genes in the Drosophila genome. Interestingly, dE2F1 functions similar to the mammalian activating E2Fs while dE2F2 behaves like the repressive E2F proteins. RBF, which binds to both dE2F1 and dE2F2, is similar to the mammalian Rb protein and regulates cell proliferation, differentiation, as well as apoptosis in Drosophila (Du, 2000; Du and Dyson, 1999; Moon et al., 2006; Steele et al., 2009; Tanaka-Matakatsu et al., 2009).
The high conservation of the Rb pathway between Drosophila and mammalian systems prompted us to carry out a genetic screen to identify genes that can modulate the consequences of Rb inactivation (Steele et al., 2009; Tanaka-Matakatsu et al., 2009).
RESULTS
Mutation of gig and rbf leads to synergistic induction of cell death
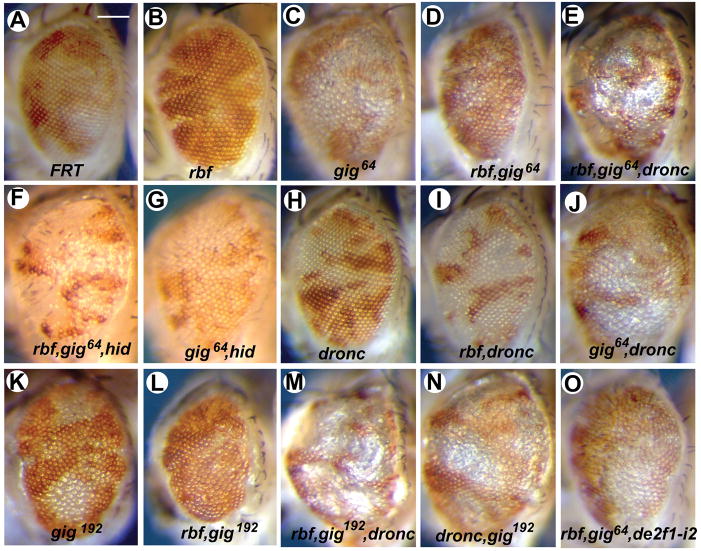
In a genetic screen for mutations that modulate the consequence of rbf inactivation, we identified a mutation, 64. While mutation of 64 alone led to large mutant patches in adult eyes, mutation of 64 in conjunction with rbf led to only very small mutant patches (Fig. 1A–D). In addition, adult eyes with rbf,64 double mutant clones were smaller and displayed a rough appearance.
Figure 1. Inactivation of cell death regulators hid or dronc increases the sizes of gig,rbf double mutant clones.
Images shown are of adult eyes mosaic for mutant (white) and wildtype (red) tissues of the indicated genotypes. Significant amounts of mutant patches were observed in gig single- (C and K) but not in rbf,gig double mutants (D and L). Removing cell death regulators dronc or hid (E–J, M–N) restores rbf,gig mutant patches. The rbf,gig double-mutant phenotype is also reversed in a de2f1-mutant background (O). Scale bar, 100 μm. See also Figure S1.
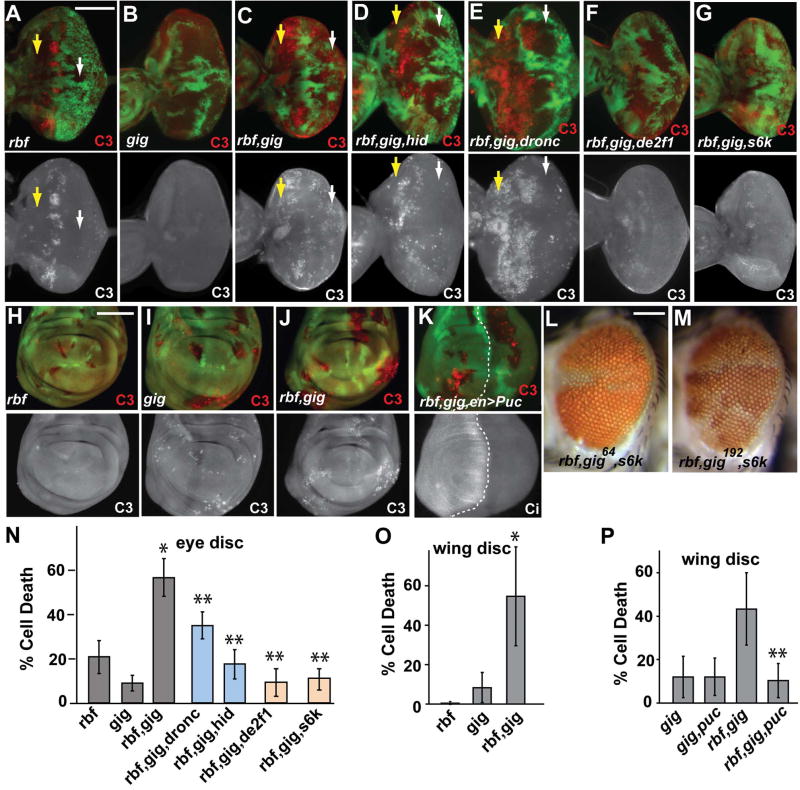
The decreased size of rbf,64 double mutant clones could be due to an effect of the two mutations on cell proliferation or cell death. Examination of DNA replication showed no inhibition of proliferation (data not shown). Therefore activated caspase-3 (C3) was used to examine the level of cell death. Consistent with previous reports (Du, 2000; Moon et al., 2006), mutation of rbf led to increased apoptosis near the MF (Fig. 2A). Although mutation of 64 alone did not cause significant C3 activation in eye discs, mutation of 64 in conjunction with rbf mutation significantly expanded the observed apoptosis to both anterior as well as posterior clones in eye discs (Fig. 2A–C, arrows) and increased the overall level of cell death in clones throughout the eye disc (Fig. 2N). Furthermore, the synergistic induction of cell death by rbf and 64 mutations is not limited to the eye disc. In wing discs, C3 staining was slightly increased in 64 mutant clones but not in rbf single mutant clones. Significantly increased C3 staining was observed in rbf,64 double mutant clones (Fig. 2H–J, O). These results indicate that inactivation of rbf and 64 leads to synergistic induction of cell death in both wing and eye discs.
Figure 2. Synergistic induction of cell death by mutations of rbf and gig.
Mutant clones of the indicated genotypes are marked by the absence of GFP. Activated caspase-3 (C3) staining shows the pattern of cell death in rbf (A), gig64 (B), and rbf,gig64 (C) mutant clones. Mutation of gig significantly enhances the phenotype caused by loss of rbf in clones both anterior (yellow arrows) and posterior (white arrows) to the MF. Synergistic death requires both hid (D) and dronc (E) in posterior but not anterior cells. The cell death phenotype of rbf,gig double-mutants requires de2f1 (F) and ds6k (G). (L–M) images of adult eyes with rbf,gig64,ds6k or rbf,gig192,ds6k triple mutant clones. Enhanced cell death is also observed in rbf,gig clones during wing development (H–J). Quantification of cell death within mutant clones is shown for eye (N) and wing (O) tissues. (K, top panel) Expression of Puc in the posterior of wing disc using the engrailed-Gal4 driver significantly decreased cell death, which is quantified in (P). (K, bottom panel), Ci, which is expressed in the anterior of wing discs, is used to define anterior posterior boundary. Asterisks indicate statistically-significant differences between double- and single-mutant clones (*) or double- vs. triple-mutant clones (**). Error bars indicate ± SD. All discs are oriented with anterior to the left. Scale bar, 100 μm. See also Figure S2.
Deficiency mapping revealed that the 64 mutation lies between 76F-77B. The following evidence showed that the 64 mutation is an allele of gig, the Drosophila TSC2 homolog: 1) 64 mutation failed to complement a previously identified gig allele, gig192; 2) the mutant phenotypes of 64 and rbf,64 clones in adult eyes are very similar to those of the gig192 and rbf,gig192 clones (Fig. 1C–D and K–L); 3) sequencing of the gig gene in 64 mutant identified a C to T mutation that gives rise to a stop codon after amino acid 431 (Fig. S1); and 4) rbf,gig192 double mutant clones also show synergistic induction of cell death in both the developing eye and wing discs (Fig. S2). Therefore we renamed the 64 mutant gig64. Since gig64 does not encode the functional domains of gigas, it is likely that gig64 constitutes a null allele.
rbf,gig-induced cell death in the anterior and posterior parts of the eye disc exhibit differential requirements for Hid and Dronc
Cell death induced by rbf single mutant clones is mediated by induction of hid (Moon et al., 2005; Tanaka-Matakatsu et al., 2009). Inactivation of hid completely abolishes cell death in rbf mutant clones (Tanaka-Matakatsu et al., 2009). Interestingly, although mutation of hid significantly decreased rbf,gig induced cell death, some level of cell death was still observed in rbf,gig,hid triple mutant clones, particularly in the anterior of the eye disc (yellow arrows in Fig. 2D, N, Fig. S2G–H). These observations indicate that cell death induced by mutation of rbf,gig involve both hid-dependent and hid-independent mechanisms.
Similarly, rbf induced cell death is highly dependent on Dronc function (Steele et al., 2009). While dronc mutation significantly decreased the death of rbf,gig mutant cells in the posterior (the differentiating part of the eye disc), inactivation of dronc has little effect on cell death of rbf,gig clones in the anterior, the proliferating part of the eye disc (Fig. 2E, Fig. S2G–H). Therefore anterior and posterior rbf,gig cells displayed a marked difference in their dependence on dronc for cell death.
Consistent with the observations that inactivation of hid or dronc partially inhibited cell death in rbf,gig mutant clones, inactivation of hid or dronc in conjunction with rbf and gig mutations significantly increased the amount of mutant tissue in adult eyes as well as their overall sizes (Fig. 1, D–F, L–M). These data also support the idea that that the decreased size of rbf,gig mutant patches in adult eyes is due to the synergistic induction of cell death by rbf and gig.
Cell death induced by rbf,gig mutations requires E2F, S6K, and potentially involves JNK signaling
Cell death induced by rbf mutation is E2F-dependent (Du, 2000; Moon et al., 2006). de2f1i2 is a de2f1 mutant that encodes a truncated protein missing the C-terminal transactivation and RBF binding domains. dE2F1i2 protein can still dimerize with dDP and bind DNA but is unable to activate transcription or bind RBF (Bosco et al., 2001). Significantly reduced levels of cell death of rbf,gig mutant clones were observed in the de2f1i2 background (Fig. 2F, N). In addition, much larger rbf,gig double mutant clones were observed in adult eyes (Fig. 1O). These results indicate that dE2F1 activity is required for the synergistic induction of death of rbf,gig mutant cells.
TSC2 forms a complex with TSC1 to promote GTP hydrolysis by the small GTPase Rheb. TOR (target of rapamycin) encodes a large serine/threonine protein kinase that can be found in two complexes, TORC1 and TORC2. Mutation of gig leads to the accumulation of the GTP-bound Rheb, which induces the activation of TORC1/S6K activity and promotes protein synthesis, metabolism, and cell proliferation. Although mutation of s6k does not block cell death induced by loss of rbf in the MF area, mutation of s6k significantly reduced cell death of rbf,gig mutant cells in both the anterior and the posterior of the developing eye disc (Fig. 2G, N) and increased sizes of rbf,gig mutant clones in adult eyes (Fig. 2L, M). These observations show that increased S6K activity is also required for the synergistic induction of cell death of rbf,gig mutants.
The c-Jun N-terminal kinase (JNK) pathway is often involved in eliminating aberrant cells from Drosophila developing tissues (Igaki, 2009). Puckered (puc), a dual specificity phosphatase, is both a target as well as a negative regulator of JNK signaling in flies. Inhibition of JNK signaling by expressing Puc significantly decreased cell death in rbf,gig double mutant cells (Fig. 2K, P), suggesting the potential involvement of JNK stress signaling in the synergistic induction of death of rbf,gig mutant cells.
Knockdown of TSC2 in human cancer cells leads to increased cell death depending on Rb status
Both the Rb/E2F and the TSC2/TOR signaling pathways are highly conserved between flies and mammalian systems. Since Rb is often inactivated in human cancer cells, the observed synergistic cell death induction by inactivation of Rb and TSC2 homologs in Drosophila prompted us to determine if inactivation of TSC2 can specifically induce cell death in Rb mutant cancer cells.
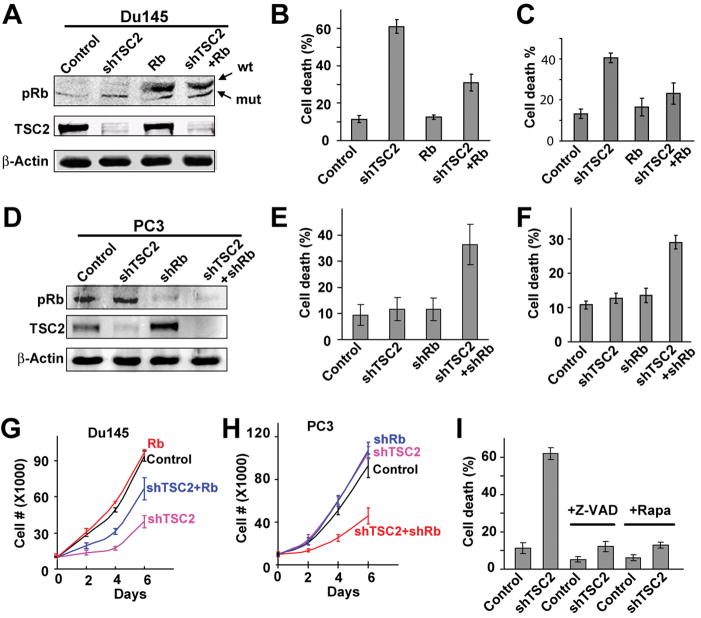
shRNA against the C terminus of TSC2 (shTSC2) was shown to strongly reduce the level of TSC2 (Sun et al., 2008). Consistent with this, lentivirus containing the shTSC2 construct significantly reduced the endogenous TSC2 levels in both the Rb-mutant DU145 and Rb-WT PC3 prostate cancer cells (Fig. 3A, D). Annexin V and Propidium iodide (PI) staining were used to determine the effect of shTSC2 on cell death. While shTSC2 did not significantly affect cell death under normal culture conditions, significantly elevated levels of cell death were observed in DU145shTSC2 cells but not in control DU145 or PC3shTSC2 cells when cells were cultured under hypoxic conditions (Fig. 3B, E). In addition, the ability of different shTSC2 constructs to induce cell death in DU145 cells was correlated with their ability to decrease the level of TSC2 (Fig. S3A–B). Furthermore, the increased death of DU145shTSC2 cells is not restricted to hypoxic conditions. Significantly elevated levels of cell death were also observed in DU145shTSC2 but not in DU145control or PC3shTSC2 cells when cells were cultured in low serum (Fig. 3C, F). Consistent with these observations, shTSC2 significantly inhibited proliferation in DU145 cells but not in PC3 cells (Fig. 3G–H). Additionally, increased levels of death in DU145shTSC2 cells were also observed when they were cultured in soft agar (Fig. S3H–I). Therefore knockdown of TSC2 significantly increased the sensitivity of Rb mutant DU145 cells to death under a variety of stress conditions.
Figure 3. TSC2 knockdown induces cell death in Rb-mutant cancer cells under stress conditions.
(A) Rb-mutant DU145 prostate cancer cells were infected with lentivirus expressing shTSC2 or/and functional human Rb. (B and C) Cell death in DU145 cells with different treatments was measured under the conditions of hypoxia (B) or 2.5% FBS (C). (D) Rb-positive PC-3 prostate cancer cells were infected with lentivirus expressing shTSC2 and/or shRb. (E and F) Cell death in PC-3 cells with different treatments was measured under the condition of hypoxia (E) or 2.5% FBS (F). (G and H) Cell growth in 2.5% FBS was measured. Different cell types and treatments are as indicated. (I) The mTORC1 inhibitor rapamycin (10nM) and pan-caspase inhibitor Z-VAD (100 μM) inhibited shTSC2-induced cell death in DU145 cells under hypoxic conditions. In this and in all the subsequent figures, pools of lentivirus-infected cells were used unless indicated otherwise. Error bars indicate ± SD. See also Figure S3.
To demonstrate that shTSC2-induced death in cancer cells is dependent on the absence of Rb function, we determined the effect of expressing WT Rb in DU145shTSC2 cells. Expression of WT Rb protein did not affect the shTSC2-induced decrease in TSC2 protein level (Fig. 3A) but did significantly decrease shTSC2-induced death in DU145 cells (Fig. 3B, C) and partially restored cell proliferation (Fig. 3G). Furthermore, knockdown of Rb using shRb in conjunction with shTSC2 in PC3 cells significantly increased cell death (Fig. 3E, F) and inhibited cell proliferation (Fig. 3H). Taken together, these results demonstrate that cell death induced by shTSC2 is dependent on the absence of Rb function.
Rapamycin was used to determine if shTSC2-induced cell death in DU145 cells depends on TORC1 signaling. Inhibition of TORC1 activity by rapamycin significantly decreased shTSC2-induced cell death (Fig. 3I and Fig. S3L-M). These results indicate that shTSC2-induced cell death is dependent on increased TORC1 activity. In addition, significantly decreased levels of cell death were observed when Z-VAD was used to inhibit caspase activation (Fig. 3I and Fig. S3J–K). Therefore shTSC2-induced cell death is largely caspase-dependent.
To determine if shTSC2 can also specifically kill other cancer cells depending on their Rb status, we examined the effect of shTSC2 on Saos-2 (Rb mutant) and MG-63 (Rb WT) osteosarcoma cells as well as MDA-MB-468 (Rb mutant) and MDA-MB-231 (Rb WT) breast cancer cells. shTSC2 significantly reduced TSC2 levels in these different cancer cells (Fig. S3C). Interestingly, shTSC2 significantly increased death in Rb-mutant Saos-2 and MDA-MB-468 cancer cells but not in Rb-WT MG-63 and MDA-MB-231 cancer cells (Fig. S3D–G). Therefore, knockdown of TSC2 can induce death in a variety of cancer cells depending on Rb status.
shTSC2 inhibits the growth of Rb mutant cancer cells in soft agar and mouse xenografts
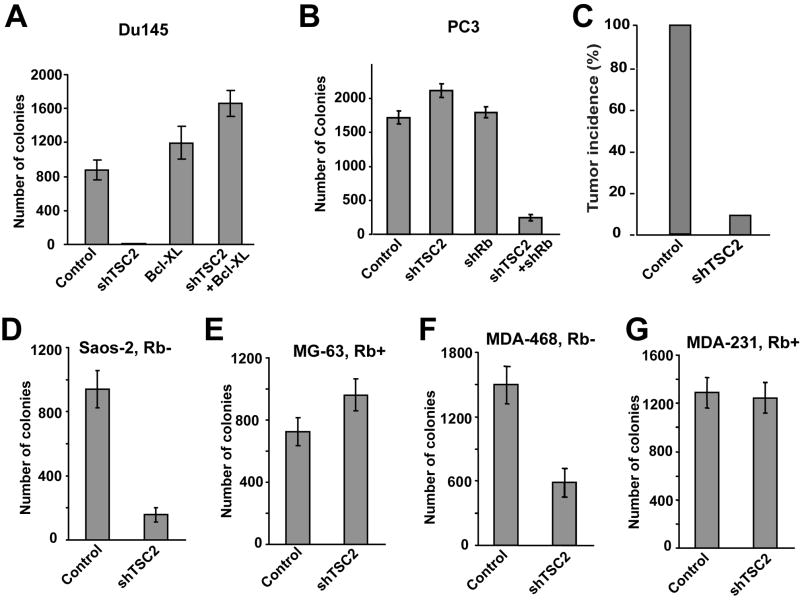
The ability of cancer cells to grow and form colonies in soft agar was used to determine if shTSC2-induced changes in the level of cell death described above correlates with changes in anchorage-independent growth. As shown in Fig. 4, shTSC2 dramatically inhibited the ability of DU145 cells to form colonies in soft agar (Fig. 4A). Similarly, while shTSC2 alone did not inhibit PC3 cells from forming colonies in soft agar, shRb in conjunction with shTSC2 did significantly inhibit colony formation (Fig. 4B). These results showed that shTSC2-induced cell death in these prostate cancer cells is correlated with the inhibition of cancer cell growth in soft agar.
Figure 4. TSC2 knockdown suppresses anchorage-independent growth of Rb-mutant cancer cells in soft agar and reduces tumor formation in xenograft models.
(A) DU145 cells were infected with lentivirus expressing shTSC2 and/or human Bcl-XL. (B) PC-3 cells were infected with lentivirus expressing shTSC2 and/or shRb. (C) Control and DU145shTSC2 cells were injected into nude mice to test their ability to form tumors. (D and E) Osteosarcoma cells were infected with lentivirus expressing shTSC2 or control vector. TSC2 knockdown suppresses the colony formation of Rb-mutant Saos-2 cells but not Rb-WT MG-63 cells. (F and G) Breast cancer cells are infected with lentivirus expressing shTSC2 or control vector. TSC2 knockdown suppresses the colony formation of Rb-mutant MDA-MB-468 cells but not Rb-WT MDA-MB-231 cells. Error bars indicate ± SD. See also Figure S4.
To further assess whether the correlation between shTSC2-induced cell death and inhibition of colony formation can be extended to other cancer cells, the effect of shTSC2 on osteosarcoma and breast cancer cell growth was determined. shTSC2 significantly inhibited colony formation of Rb-mutant cancer cells (Saos-2 and MDA-MB-468) but not Rb-WT cancer cells (MG-63 and MDA-MB-231) (Fig. 4D–G). These results show that shTSC2-induced cell death is correlated with an inhibition of cancer cell growth in soft agar, which is also dependent on the absence of Rb function.
To determine the in vivo significance of TSC2 knockdown on tumor growth, a mouse xenograft model was used to evaluate the effect of TSC2 knockdown. DU145control or DU145shTSC2 cells were injected into athymic nude mice and tumor growth was followed. While all the mice injected with DU145control cells had tumor growth, only one tumor formed in mice injected with DU145shTSC2 cells (Fig. 4C and Fig. S4). Therefore shTSC2 also significantly reduced the incidence of tumor growth in xenograft models. These results, in conjunction with the previous results of increased cell death and inhibition of cell growth in soft agar, suggest that inhibition of TSC2 can potentially be used to specifically target Rb mutant cancer cells.
Overexpression of activated Akt does not inhibit shTSC2-induced cell death
Inactivation of TSC2 leads to the activation of TORC1, which in turn activates S6K (Wullschleger et al., 2006). S6K has been shown to form a negative feedback loop with IRS proteins that leads to inhibition of Akt signaling (Harrington et al., 2004; Shah et al., 2004). Consistent with this, inactivation of TSC2 or TSC1 was shown to activate TORC1 but inhibit TORC2 activity, resulting in the downregulation of Akt signaling (Yang et al., 2006).
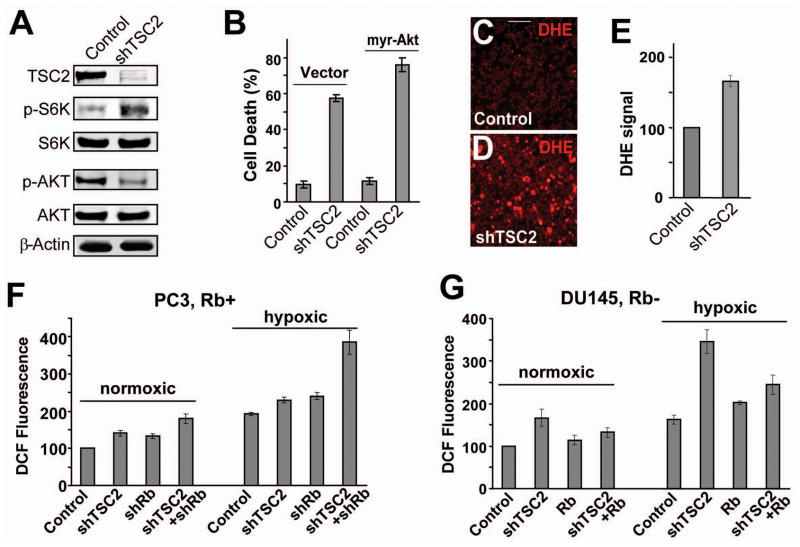
Western blots using antibodies against total or phospho-Akt (Ser473) were carried out to determine the effect of shTSC2 on Akt activation in DU145 cells. shTSC2 led to reduced phospho-Akt and increased phospho-S6K levels without changing the levels of total Akt or S6K (Fig. 5A). Since phosphorylation on Ser473 is required for the full activation of Akt and Akt is known to be an important survival signal, we tested the effect of expressing an activated form of Akt on shTSC2-induced cell death. Expression of activated Akt did not inhibit shTSC2-induced cell death (Fig. 5B). These observations suggest that decreased Akt signaling is not likely to be the main cause of cell death in DU145 cells. Since Akt signaling cannot inhibit cell death induced by ROS (Nogueira et al., 2008; Robey and Hay, 2009), we investigated the involvement of oxidative stress in shTSC2-induced cell death.
Figure 5. TSC2 knockdown induces ROS generation.
(A) Western blots showing the level of TSC2, phospho-S6K (T389), phospho-Akt (S473), total S6K, total Akt, and β-Actin in control DU145 cells and in DU145shTSC2 cells. (B) Overexpression of constitutively activated myr-AKT did not inhibit shTSC2-induced cell death in DU145 cells. (C and D) DU145shTSC2 cells (D) exhibited significantly higher levels of ROS (detected by the fluorescent dye DHE) than control cells (C) grown in soft agar. Scale bar, 100 μm. (E) shTSC2 induces ROS generation in DU145 cells grown in complete media. ROS levels were determined by flow cytometry after staining with DHE. (F) PC-3 cells with shTSC2 and/or shRb were cultured in complete media. ROS levels were determined by flow cytometry after staining with the fluorescent dye CM-H2DCFDA. (G) DU145 cells with shTSC2 and/or re-expressed Rb were cultured in complete media. The ROS levels are determined as described in (F). Error bars indicate ± SD.
Inactivation of Rb and TSC2 synergistically increase oxidative stress
We used DHE, a dye that detects superoxide, to determine if shTSC2 induce oxidative stress in DU145 cells. Highly elevated DHE fluorescence was observed in DU145shTSC2 cells compared to the DU145control cells grown in soft agar (Fig. 5C,D). Similarly, FACS analysis detected significantly elevated DHE fluorescence in DU145shTSC2 cells grown under normal conditions (Fig. 5E). Therefore shTSC2 induces significant level of oxidative stress.
Rb WT PC3 cells were used to further characterize the effect of inactivating TSC2 and Rb on ROS induction. In cells grown under normoxia, we found that knockdown of either TSC2 or Rb led to modest but reproducible increases in ROS levels and that knockdown of Rb in conjunction with TSC2 led to further increased ROS levels (Fig. 5F). Interestingly, a higher level of ROS was observed in all the treatment groups under hypoxia with the most dramatically increased ROS level observed in PC3shRb+shTSC2 cells (Fig. 5F). These observations show that hypoxia increased ROS levels and that shRb and shTSC2 led to a synergistic increase in the level of ROS, which is correlated with increased death of PC3shRb+shTSC2 cells (Compare Fig. 3E, Fig. 4B, and Fig. 5F).
Similarly, shTSC2 led to a significant increase in ROS levels in DU145 cells, particularly under hypoxic conditions (Fig. 5G). Furthermore, while expression of WT Rb alone did not decrease ROS levels, Rb expression significantly decreased shTSC2-induced ROS levels (Fig. 5G). These results show that inactivation of Rb and TSC2 synergistically increase ROS levels and that a high level of ROS under hypoxic conditions is correlated with shTSC2-induced death in Rb mutant cancer cells (compare Fig. 3B,C and Fig. 5G).
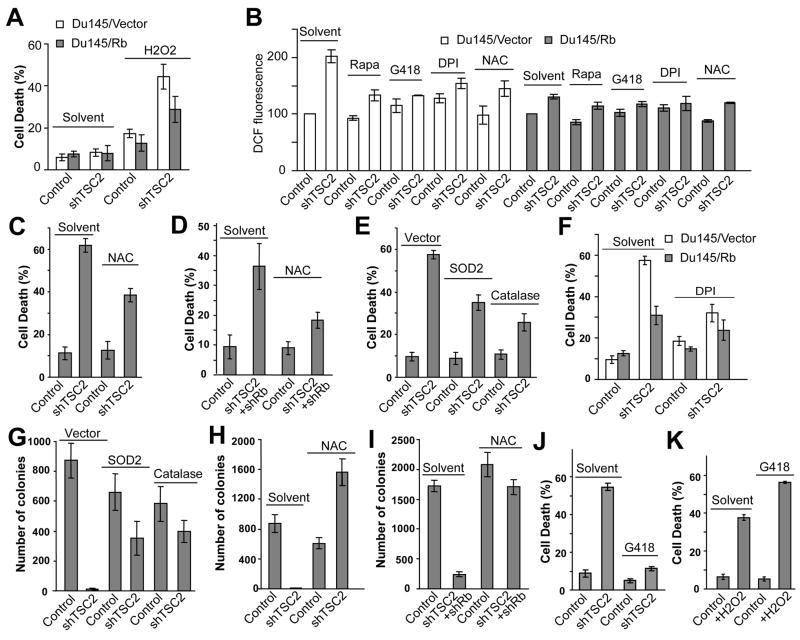
To test the possibility that stress conditions such as hypoxia might increase the level of oxidative stress above a certain threshold to promote shTSC2-induced cell death, we determined the effect of increasing oxidative stress by addition of H2O2 into the culture media. At concentrations of H2O2 that induced a low level of cell death in DU145control cells, high levels of cell death were observed in DU145shTSC2 cells (Fig. 6A). Expression of Rb in DU145shTSC2 cells decreased oxidative stress (Fig. 6B) and also decreased H2O2-induced cell death (Fig. 6A). These results show that DU145shTSC2 cells are much more sensitive to oxidative stress and suggest that stress conditions such as hypoxia contribute to shTSC2-induced cell death, at least in part, by increasing oxidative stress.
Figure 6. shTSC2-induced cell death is mediated by the induction of ROS.
(A) The effect of shTSC2 and Rb on H2O2-induced cell death in DU145 cells. Cells were cultured in complete media and were treated with 800 μM H2O2 for 24h before assaying for cell death. (B) The effect of rapamycin (10 nM), G418 (400 μg/ml), DPI (5 μM) and NAC (5 mM) on ROS levels in control and DU145shTSC2 with or without Rb expression. Cells were cultured under hypoxic conditions for 24 hours and assayed for ROS by Facs. (C) NAC (5 μM) suppressed shTSC2 -induced cell death in DU145 under hypoxic conditions. (D) NAC (5 μM) suppressed the death of PC-3shTSC2+shRb cells under hypoxic conditions. (E) Overexpression of the ROS-scavenging enzymes SOD2 or Catalase inhibited the death of DU145shTSC2 cells under hypoxic conditions. (F) The effect of DPI (5 μM) on cell death in DU145shTSC2 and DU145shTSC2+Rb cells. (G) Overexpression of either SOD2 or Catalase restored the ability of DU145shTSC2 cells to form colonies in soft agar. (H) NAC (10 mM) restored the ability of DU145shTSC2 cells to form colonies in soft agar. (I) NAC (10 mM) restored the ability of PC-3shTSC2+shRb cells to form colonies in soft agar. (J and K) G418 represses death induced by shTSC2 in DU145 cells under hypoxic conditions (J) but increases death in DU145 cells that are induced by H2O2 (K). Error bars indicate ± SD.
Reducing oxidative stress significantly decreases shTSC2-induced cell death and increases cancer cell growth in soft agar
The above observations suggest that cell death induced by inactivation of both Rb and TSC2 are due to synergistically induced oxidative stress. To test this idea, the antioxidant N-acetyl cysteine (NAC) was used to reduce oxidative stress. Addition of NAC significantly reduced ROS levels (Fig. 6B) and significantly reduced death in DU145shTSC2 cells (Fig. 6C). Similarly, NAC treatment also significantly reduced death in PC3shRb+shTSC2 cells (Fig. 6D). In addition, reducing oxidative stress by expressing the ROS scavenger enzymes SOD2 or Catalase also significantly decreased the level of shTSC2-induced death in DU145 cells (Fig. 6E). Interestingly, expression of Rb in DU145 cells significantly reduced shTSC2 induced ROS and cell death, which is not significantly decreased further by antioxidants (Fig. 6B and F). These observations strongly support the idea that oxidative stress is a critical mediator of cell death in DU145 cells and that Rb plays a critical role regulating ROS when TSC2 is inactivated.
Cell growth in soft agar was used to further determine the effect of reducing oxidative stress on shTSC2-induced inhibition of cancer cell growth. While expression of SOD2 or Catalase did not increase colony growth in DU145control cells, SOD2 or Catalase expression in DU145shTSC2 cells significantly increased colony growth in soft agar (Fig. 6G). Similarly, reducing oxidative stress by NAC dramatically increased the growth of DU145shTSC2 but not DU145control cells in soft agar (Fig. 6H). Furthermore, NAC treatment also dramatically increased the colony growth of PC3shRb+shTSC2 cells but not the PC3control cells (Fig. 6I). In conclusion, these results provide further support for the idea that oxidative stress induced by Rb and TSC2 inactivation contributes to increased cancer cell death and growth inhibition.
Inhibition of protein synthesis reduces shTSC2-induced oxidative stress and cell death
Inhibition of TORC1 by rapamycin significantly inhibited shTSC2-induced cell death (Fig. 3I and Fig. S3L-M). Since the above results showed that shTSC2-induced cell death is, at least in part, due to increased oxidative stress, we determined the effect of rapamycin on ROS levels. Indeed, rapamycin significantly reduced ROS levels in DU145shTSC2 cells without significantly affecting the ROS levels in control cells (Fig. 6B). Since a key function of TORC1 is to stimulate protein synthesis, we determined the effect of inhibiting protein synthesis on shTSC2-induced cell death and oxidative stress. G418 interferes with the function of 80S ribosomes and inhibits protein synthesis in eukaryotic cells. Interestingly, G418 significantly reduced ROS levels in DU145shTSC2 cells but not in DU145control cells (Fig. 6B). Furthermore, while G418 treatment significantly reduced shTSC2-induced cell death (Fig. 6J), it did not suppress H2O2-induced cell death in DU145 cells (Fig. 6K). These results show that the ability of G418 to inhibit shTSC2-induced cell death is correlated with its ability to decrease oxidative stress and suggest that increased protein synthesis contributes to shTSC2-induced oxidative stress and cell death.
SOD2 contributes to Rb inactivation-induced ROS levels and cell death
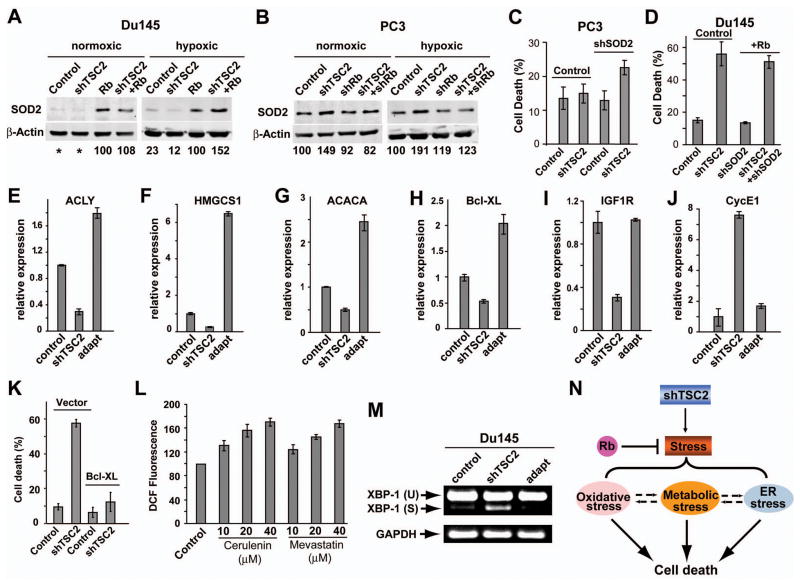
To investigate how inactivation of Rb induces oxidative stress synergistically with shTSC2, we examined the effect of Rb on ROS scavenger enzyme expression. While the level of SOD2 was very low in control and DU145shTSC2 cells, significantly higher levels of SOD2 were detected when WT Rb was expressed (Fig. 7A). Interestingly, under hypoxic conditions shTSC2 led to further increased levels of SOD2 only in the presence of WT Rb (Fig. 7A). Therefore, Rb regulates the basal as well as shTSC2-induced SOD2 levels in DU145 cells. The inability of DU145shTSC2 cells to induce SOD2 under hypoxic conditions was correlated with high level of oxidative stress and increased cell death (Fig. 5G, 3B). We also tested the effect of Rb on SOD2 levels in PC3 cells. shTSC2 led to elevated SOD2, particularly under hypoxic conditions (Fig. 7B). Interestingly, shRb blocked shTSC2-induced increase in SOD2 levels without affecting the basal SOD2 levels (Fig. 7B). The inability of PC3shRb+shTSC2 cells to induce SOD2 is also correlated with high levels of oxidative stress and increased cell death (Fig. 5F and 3E).
Figure 7. Inactivation of both Rb and TSC2 leads to decreased survival signaling and defective ROS control.
(A and B) Western blots showing that shTSC2 induced increased SOD2 levels under hypoxic conditions in an Rb-dependent manner. DU145 cells (A) and PC-3 cells (B) were cultured in normoxic or hypoxic conditions for 24h. (C) PC3 cells infected with lentivirus expressing shTSC2 and/or shSOD2 as indicated. Cell death was determined after culturing under hypoxic conditions for 48h. (D) Similar levels of death are observed in DU145shTSC2 and DU145shTSC2+Rb+shSOD2 cells, indicating shSOD2 blocks the ability of Rb to inhibit death in DU145shTSC2 cells. (E–J) mRNA levels of Bcl-XL, IGF1R, ACLY, HMGCS1, ACACA and CycE1 were determined by Real-time PCR. DU145control, DU145shTSC2, and DU145shTSC2-adapt cells were cultured under hypoxic conditions for 24h and total RNA was extracted for RT-PCR analysis. (K) Overexpression of Bcl-XL reduces shTSC2-induced death in DU145 cells under hypoxic conditions. (L) The inhibition of lipid synthesis induces ROS generation in DU145 cells. Cerulenin is an inhibitor of fatty acid synthase and mevastatin is an inhibitor of HMG reductase. (M) RT-PCR shows increased XBP-1 splicing in DU145shTSC2 cells as compared with DU145control and DU145shTSC2-adapt cells. (N) A model for synergistic cell death induction by Rb and TSC2 inactivation in cancer cells. Error bars indicate ± SD. See also Figure S5.
To further test the idea that SOD2 contributes to shTSC2-induced cell death, we used shRNA to knockdown SOD2 in PC3 cells (Fig. S5A). While shTSC2 or shSOD2 alone did not significantly affect cell death in PC3 cells, shTSC2+shSOD2 significantly increased the level of cell death (Fig. 7C), which is correlated with significantly increased ROS levels (Fig. S5B). Furthermore, while expression of Rb significantly inhibited shTSC2-induced cell death in DU145 cells (Fig. 3B), similar levels of cell death are observed between the DU145shTSC2 and the DU145shTSC2+Rb+shSOD2 cells (Fig. 7D). These results provide strong evidence that SOD2 is a critical target of Rb that contributes to Rb and TSC2 inactivation induced cell death. However, since shRb+shTSC2 induces a higher level of cell death than shSOD2+shTSC2 does in PC3 cells (compare Fig. 3E and 7C), it is likely that additional targets of Rb also contribute to Rb and TSC2 inactivation induced death in these cells.
shTSC2 inhibits de novo lipid synthesis, decreases survival signaling, and induces ER stress
To further characterize the mechanisms that contribute to shTSC2-induced cell death and the acquisition of resistance, we recovered cells from a tumor that developed in a mouse injected with DU145shTSC2 cells. Analysis of these cells showed that the TSC2 protein level was still significantly reduced although not as dramatically as that in DU145shTSC2 cells (Fig. S5C). However, these cells had regained the ability to grow in soft agar and are resistant to cell death under hypoxia (data not shown). Therefore it appears that these cells have acquired resistance to shTSC2-induced cell death and we refer to these cells as DU145shTSC2-adapt cells.
Microarray experiments were carried out to identify genes that were significantly altered in response to shTSC2 but were restored in DU145shTSC2-adapt cells. 463 genes showed significant upregulation or downregulation in response to shTSC2. The expression of 170 genes from these 463 was reversed in the adapted cells. These include genes involved in the cell cycle, lipid metabolism, and cell survival signaling such as Bcl-XL and components of the insulin-like growth factor/EGFR/PI3K signaling (Fig. S5D). The expression of some of the genes was verified by Real-time RT-PCR (Fig. 7E–J). A general agreement was observed between the RT-PCR results and the microarray data. For example, Bcl-XL, the insulin-like growth factor IGF1R, and key enzymes for de novo lipid synthesis such as ACLY, HMGCS1, and ACACA were all significantly reduced in DU145shTSC2 cells but not in DU145shTSC2-adapt cells. In fact, the expression of Bcl-XL, HMGCS1, ACACA, and ACLY were actually higher in the adapted cells (Fig. 7E–I). On the other hand, increased Cyclin E expression was detected in DU145shTSC2 cells but not in DU145shTSC2-adapt cells (Fig. 7J). Expression of Bcl-XL strongly inhibited shTSC2-induced cell death (Fig. 7K) and restored cell growth in soft agar (Fig. 4A), suggesting that changes in the expression of Bcl-XL may contribute to shTSC2-induced cell death in DU145shTSC2 cells and the resistance to death in DU145shTSC2-adapt cells.
Cancer cells generally synthesize most of their lipids de novo. Inhibition of ACL inhibits de novo lipid synthesis and suppresses tumor cell growth (Hatzivassiliou et al., 2005; Pearce et al., 1998). In addition, inhibition of fatty acid or cholesterol synthesis induces cell death in several cancer models (De Schrijver et al., 2003; Demierre et al., 2005). To examine the possibility that the decreased expression of genes involved in lipid synthesis may contribute to shTSC2-induced cell death, we examined the effect of inhibiting fatty acid or cholesterol synthesis on ROS levels. Inhibition of fatty acid synthesis by Cerulenin led to increased ROS levels (Fig. 7L), consistent with a published report (Migita et al., 2009). Similarly, inhibition of cholesterol synthesis by Mevastain also increased ROS (Fig. 7L). Taken together, these results suggest that inhibition of de novo lipid synthesis may also contribute to the shTSC2-induced cell death by increasing oxidative stress.
The significantly decreased expression of genes involved in lipid synthesis in conjunction with increased protein synthesis raise the possibility that shTSC2 will induce additional cellular stress in these cells. Indeed increased XBP-1 splicing is observed in DU145shTSC2 cells as compared to the DU145control or DU145shTSC2-adapt cells (Fig. 7M), suggesting that ER stress is also induced by shTSC2 in DU145 cells and that reduced level of ER stress is correlated with the resistance of DU145shTSC2-adapt cells to shTSC2-induced cell death. Activation of ER stress can induce cell death through multiple pathways (Ron and Walter, 2007). Taken together, our results suggest that inactivation of Rb and TSC2 induces multiple cellular stresses that contribute to synergistic cell death.
Discussion
Since Rb is a tumor suppressor that is often inactivated in cancers, approaches that specifically kill Rb mutant cells can potentially lead to targeted cancer therapies. From a genetic screen in Drosophila, we showed that inactivation of Rb and TSC2 homologs induced cell death synergistically in normal developing tissues. We further showed that inactivation of TSC2 specifically kills Rb mutant cancer cells and inhibited cancer cell growth in soft agar and in xenograft models. These results suggest that TSC2 can potentially be used to specifically target Rb mutant cancers.
Germline mutations of either TSC2 or TSC1 will cause Tuberous Sclerosis Complex, which is an autosomal dominant disorder characterized by benign tumor formation in a variety of organs (reviewed in (Crino et al., 2006). Therefore, inhibition of TSC2 may cause some side effects. However since the onset of TSC2 inactivation induced cell death is rapid, short-term inactivation of TSC2 will be sufficient to induce specific killing of Rb-mutant cancer cells. Therefore it is possible that specific killing of cancer cells without significant side effects can be achieved simply by adjusting the time period of TSC2 inactivation.
How does Rb and TSC2 inactivation induce synergistic cell death?
Our results suggest that Rb and TSC2 inactivation induced synergistic cell death is mediated by the high level of cellular stress (see model in Fig. 7N). Inactivation of TSC2 deregulates TORC1 activity, which promotes protein synthesis and increases mitochondria oxidative phosphorylation. These activities of TORC1 potentially induce a number of cellular stresses, including metabolic stress and oxidative stress. The observed inhibition of lipid synthesis is potentially a consequence of metabolic stress induced by shTSC2 and will lead to even higher levels of oxidative stress (Fig. 7L). In addition, since cancer cells are generally dependent on de novo lipid synthesis (Hatzivassiliou et al., 2005), an inhibition of lipid synthesis in conjunction with increased protein synthesis will induce increased ER stress in cancer cells (Little et al., 2007; Ozcan et al., 2008). Indeed both oxidative stress and ER stress are induced by shTSC2 in Rb mutant cancer cells. Furthermore, TSC2 inactivation inhibits autophagy as well as aggresome formation, which limits the ability of these cells to counteract misfolded protein and ER stress (Kubota, 2009; Zhou et al., 2009). Therefore inactivation of TSC2 in cancer cells induces multiple types of cellular stress. Our results in this study show that oxidative stress plays an important role in Rb and TSC2 inactivation induced cell death.
Induction of cancer cell death by shTSC2 is dependent on the absence of Rb function. It appears that Rb plays a critical role in determining the sensitivity of cells to shTSC2-induced cellular stress. Rb/E2F has been implicated in the expression of several important stress regulators, including SOD2, GRP78, and AMPKα2, which modulates oxidative stresses, ER stress, and energy stress, respectively (Hallstrom et al., 2008; Racek et al., 2008; Tanaka et al., 2002). We show here that the inability of Rb mutant cancer cells to induce SOD2 sensitized these cells to shTSC2-induced cell death. Further studies will be needed to determine if AMPKα2 and GRP78 are regulated by Rb in cancer cells and contribute to shTSC2-induced cancer cell killing. Our observations that ER stress is activated by shTSC2 in Rb mutant cancer cells and that shTSC2 induces significant cell death in Rb mutant cells under a variety of stress conditions suggest multiple types of cellular stress contribute to synergistic cell death.
The mechanism by which cellular stresses induce death is complex and is not entirely clear. ROS is a typical indicator of oxidative stress and can induce cell death through damaging the mitochondria membrane or through inducing JNK signaling or other cellular stresses. On the other hand, oxidative stress can also result from other cellular stresses, such as metabolic stress and ER stress, which are also induced by shTSC2 in Rb mutant cancer cells. In addition ER stress can also induce cell death through CHOP, ER-associated initiator caspases, or JNK signaling independent of ROS. Therefore it is possible that multiple pathways contribute to synergistic cell death within the same cell. This is consistent with the observation that shTSC2 can specifically kill a variety of Rb mutant cancers despite the loss of p53, Pten, or the presence of oncogenic Ras.
Our model that increased cellular stress mediates synergistic cell death induced by Rb and TSC2 inactivation is also consistent with the results obtained in flies. Similar to the observation in human cancer cells, the synergistic cell death in the developing fly tissues is mediated by the Rb/E2F and the TSC2/TOR signaling pathways and requires high level of protein synthesis. Decreasing the level of protein synthesis significantly inhibits the synergistic cell death. Furthermore, inhibition of JNK signaling, which is activated by various cellular stresses, decreased synergistic cell death of the rbf,gig double mutant clones. Therefore the synergistic cell death of rbf,gig double mutant clones also involves cellular stress.
Cancers resistant to TSC2 inactivation
The proposed model also suggests how Rb mutant cancer cells may acquire resistance to TSC2 inactivation-induced cell death. Since oncogenic Ras or PI3K/Akt will promote ROS-mediated cell death, simply activating these survival signals is not likely to be sufficient for resistant cancer to develop. On the other hand, changes in the expression of genes that can reduce cellular stress and genes that can increase survival signaling without increasing oxidative stress may lead to the development of resistant cancers. Indeed, increased expression of key enzymes in lipid synthesis, increased Bcl-XL expression, and restoration of the EGFR and PI3K survival signaling is observed in the resistant cancer cells. It appears that these combined changes inhibit cell death by reducing the level of cellular stress including ER stress and oxidative stress and increasing the level of survival signaling.
The development of resistant cancers is a common problem for targeted therapies. For example, even for successful targeted therapies such as imatinib and gefitinib, resistant cancers eventually develop. It is worth pointing out that very few therapeutic agents are curative in human cancer treatment when delivered alone. Most chemotherapeutic agents are delivered in combination when cures are achieved. Considering the possible involvement of the Bcl-2 family of cell death inhibitors in the development of resistant cancers and the contribution of cellular stress in the killing of cancer cells, it will be interesting to determine the effect of the Bcl-2 family inhibitor such as ABT-737 or agents that can increase cellular stress such as inhibitors of lipid synthesis or activators of AMPK on shTSC2-induced cell death.
Subsets of Rb-deficient cancers that are sensitive to TSC2 inactivation
It is interesting to note that cancer cells with different mechanisms of Rb inactivation exhibit different responses to TSC2 inactivation. Rb is inactivated in cancer cells by mutation, loss of expression, or by functional inactivation. We showed that shTSC2 specifically kills Rb mutant cancer cells. Furthermore, shRNA against Rb sensitized Rb-WT cancer cells to shTSC2-induced cell death. These results indicate that inactivation of TSC2 can be used to kill cancer cells that have either mutated or silenced Rb. On the other hand, MG-63 cells, which have WT Rb but deleted p16INK4a and p14ARF (Park et al., 2002), are resistant to shTSC2-induced cell death. Therefore cancer cells that carry WT Rb that is functionally inactivated by mutation of p16 may be resistant to TSC2 inactivation. It is likely that mutation of p16 does not completely eliminate the Rb function in these cells.
Experimental Procedures
Cell Culture
DU145, PC-3, Saos-2, MG-63, MDA-MB-231 and MDA-MB-468 cells were obtained from the American Type Culture Collection. The DU145shTSC2-adapt cell line was generated by dissociating cells from the tumor developed in a mouse injected with DU145shTSC2 cells. All the cells were maintained in DMEM medium supplemented with 10% FBS (Atlas Biologicals), 50 IU of penicillin/streptomycin (Gemini Bio-Products) and 2 mmol/L of L-glutamine (Invitrogen) in a humidified atmosphere with 5% CO2 at 37°C. Hypoxic studies were carried out at 1% oxygen.
Drosophila genetics and assays
See Supplemental Experimental Procedures for details.
FACS analysis of cell death and ROS
Quantification of cell death was performed using FACScan (BD Biosciences) after cells were stained with Annexin V-FITC and propidium iodide according to manufacture’s specifications. See Supplemental Experimental Procedures for details.
Soft agar growth assay
For colony formation, 104 cells suspended in 0.35% agarose solution were poured over hard-bottomed agar (0.6%) previously solidified in 6-well plates. Cells were cultured in a humidified atmosphere with 5% CO2 at 37°C for 4–6 weeks, and then colonies were counted.
Western blot
After desired treatments as specified in the Results section, cells were washed twice with PBS, lysed in buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium vanadate, 1 μg/ml leupeptin, 1mM phenylmethylsulfonylfluoride). Equal amounts of protein were loaded. Western detection was carried out using a Li-Cor Odyssey image reader. The goat anti-mouse IgG and goat anti-rabbit IgG secondary antibodies were obtained from Li-Cor.
Xenograft Experiments
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Chicago. Male nude mice (Hsd: Athymic Nude-Foxnlnu, aged 4–6 weeks on arrival) were purchased from Harlan. 5×106 cells were injected into the flanks of mice for tumor development.
SIGNIFICANCE
Although cancer cells often exhibit inactivation of tumor suppressors such as Rb, such knowledge has yet to be exploited to develop targeted cancer therapies. Based on the observations from our genetic screen in Drosophila, we demonstrate that inactivation of TSC2 specifically kills Rb mutant cancer cells and inhibits tumor growth. We show that cell death is synergistically induced by the inactivation of Rb and TSC2 and involves increased cellular stress. Our results suggest that TSC2 is a target that can be used to specifically kill Rb deficient cancers, which will be a significant fraction of all human cancers.
Supplementary Material
Acknowledgments
We would like to thank Drs. Kay Macleod and Jian Wu for plasmids, Drs. Richard Hiipakka and John Kokontis for discussions and for reagents. This work is supported by a DOD grant and NIH grants RO1GM074197 and P01 AT004418.
Footnotes
Accession number
The accession number for microarray data is GSE21147.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Du W, Orr-Weaver TL. DNA replication control through interaction of E2F-RB and the origin recognition complex. Nat Cell Biol. 2001;3:289–295. doi: 10.1038/35060086. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: unraveling the biology. Trends Biochem Sci. 2004;29:409–417. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799–3804. [PubMed] [Google Scholar]
- Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- Druker BJ. Inhibition of the Bcr-Abl tyrosine kinase as a therapeutic strategy for CML. Oncogene. 2002;21:8541–8546. doi: 10.1038/sj.onc.1206081. [DOI] [PubMed] [Google Scholar]
- Du W. Suppression of the rbf null mutants by a de2f1 allele that lacks transactivation domain. Development. 2000;127:367–379. doi: 10.1242/dev.127.2.367. [DOI] [PubMed] [Google Scholar]
- Du W, Dyson N. The role of RBF in the introduction of G1 regulation during Drosophila embryogenesis. EMBO J. 1999;18:916–925. doi: 10.1093/emboj/18.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Pogoriler J. Retinoblastoma family genes. Oncogene. 2006;25:5190–5200. doi: 10.1038/sj.onc.1209651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- Hallstrom TC, Mori S, Nevins JR. An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell. 2008;13:11–22. doi: 10.1016/j.ccr.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrom TC, Nevins JR. Specificity in the activation and control of transcription factor E2F-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:10848–10853. doi: 10.1073/pnas.1831408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Iaquinta PJ, Lees JA. Life and death decisions by the E2F transcription factors. Curr Opin Cell Biol. 2007;19:649–657. doi: 10.1016/j.ceb.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaki T. Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling. Apoptosis. 2009;14:1021–1028. doi: 10.1007/s10495-009-0361-7. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Kubota H. Quality control against misfolded proteins in the cytosol: a network for cell survival. J Biochem. 2009;146:609–616. doi: 10.1093/jb/mvp139. [DOI] [PubMed] [Google Scholar]
- Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res. 2007;67:1262–1269. doi: 10.1158/0008-5472.CAN-06-1794. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G, Inazuka F, Grisanzio C, Palescandolo E, Shin E, et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst. 2009;101:519–532. doi: 10.1093/jnci/djp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon NS, Di Stefano L, Dyson N. A gradient of epidermal growth factor receptor signaling determines the sensitivity of rbf1 mutant cells to E2F-dependent apoptosis. Mol Cell Biol. 2006;26:7601–7615. doi: 10.1128/MCB.00836-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon NS, Frolov MV, Kwon EJ, Di Stefano L, Dimova DK, Morris EJ, Taylor-Harding B, White K, Dyson NJ. Drosophila E2F1 has context-specific pro- and antiapoptotic properties during development. Dev Cell. 2005;9:463–475. doi: 10.1016/j.devcel.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Mulloy R, Ferrand A, Kim Y, Sordella R, Bell DW, Haber DA, Anderson KS, Settleman J. Epidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinib. Cancer Res. 2007;67:2325–2330. doi: 10.1158/0008-5472.CAN-06-4293. [DOI] [PubMed] [Google Scholar]
- Nahle Z, Polakoff J, Davuluri RV, McCurrach ME, Jacobson MD, Narita M, Zhang MQ, Lazebnik Y, Bar-Sagi D, Lowe SW. Direct coupling of the cell cycle and cell death machinery by E2F. Nat Cell Biol. 2002;4:859–864. doi: 10.1038/ncb868. [DOI] [PubMed] [Google Scholar]
- Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Park YB, Park MJ, Kimura K, Shimizu K, Lee SH, Yokota J. Alterations in the INK4a/ARF locus and their effects on the growth of human osteosarcoma cell lines. Cancer Genet Cytogenet. 2002;133:105–111. doi: 10.1016/s0165-4608(01)00575-1. [DOI] [PubMed] [Google Scholar]
- Pearce NJ, Yates JW, Berkhout TA, Jackson B, Tew D, Boyd H, Camilleri P, Sweeney P, Gribble AD, Shaw A, Groot PH. The role of ATP citrate-lyase in the metabolic regulation of plasma lipids. Hypolipidaemic effects of SB-204990, a lactone prodrug of the potent ATP citrate-lyase inhibitor SB-201076. Biochem J. 1998;334(Pt 1):113–119. doi: 10.1042/bj3340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- Racek T, Buhlmann S, Rust F, Knoll S, Alla V, Putzer BM. Transcriptional repression of the prosurvival endoplasmic reticulum chaperone GRP78/BIP by E2F1. J Biol Chem. 2008;283:34305–34314. doi: 10.1074/jbc.M803925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RB, Hay N. Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Shah OJ, Wang Z, Hunter T. Inappropriate Activation of the TSC/Rheb/mTOR/S6K Cassette Induces IRS1/2 Depletion, Insulin Resistance, and Cell Survival Deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Steele L, Sukhanova MJ, Xu J, Gordon GM, Huang Y, Yu L, Du W. Retinoblastoma family protein promotes normal R8-photoreceptor differentiation in the absence of rhinoceros by inhibiting dE2F1 activity. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Fang Y, Yoon MS, Zhang C, Roccio M, Zwartkruis FJ, Armstrong M, Brown HA, Chen J. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci U S A. 2008;105:8286–8291. doi: 10.1073/pnas.0712268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Matsumura I, Ezoe S, Satoh Y, Sakamaki T, Albanese C, Machii T, Pestell RG, Kanakura Y. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Mol Cell. 2002;9:1017–1029. doi: 10.1016/s1097-2765(02)00522-1. [DOI] [PubMed] [Google Scholar]
- Tanaka-Matakatsu M, Xu J, Cheng L, Du W. Regulation of apoptosis of rbf mutant cells during Drosophila development. Dev Biol. 2009;326:347–356. doi: 10.1016/j.ydbio.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yang Q, Inoki K, Kim E, Guan KL. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci U S A. 2006;103:6811–6816. doi: 10.1073/pnas.0602282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ikenoue T, Chen X, Li L, Inoki K, Guan KL. Rheb controls misfolded protein metabolism by inhibiting aggresome formation and autophagy. Proc Natl Acad Sci U S A. 2009;106:8923–8928. doi: 10.1073/pnas.0903621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.